Abstract
The sea anemone, Exaiptasia diaphana, is a model of coral-dinoflagellate (Symbiodiniaceae) symbiosis. However, little is known of its potential to form symbiosis with Cladocopium—a key Indo-Pacific algal symbiont of scleractinian corals, nor the host nutritional consequences of such an association. Aposymbiotic anemones were inoculated with homologous algal symbionts, Breviolum minutum, and seven heterologous strains of Cladocopium C1acro (wild-type and heat-evolved) under ambient conditions. Despite lower initial algal cell density, Cladocopium C1acro-anemeones achieved similar cell densities as B. minutum-anemones by week 77. Wild-type and heat-evolved Cladocopium C1acro showed similar colonization patterns. Targeted LC-MS-based metabolomics revealed that almost all significantly different metabolites in the host and Symbiodiniaceae fractions were due to differences between Cladocopium C1acro and B. minutum, with little difference between heat-evolved and wild-type Cladocopium C1acro at week 9. The algal fraction of Cladocopium C1acro-anemones was enriched in metabolites related to nitrogen storage, while the host fraction of B. minutum-anemones was enriched in sugar-related metabolites. Compared to B. minutum, Cladocopium C1acro is likely slightly less nutritionally beneficial to the host under ambient conditions, but more capable of maintaining its own growth when host nitrogen supply is limited. Our findings demonstrate the value of E. diaphana to study experimentally evolved Cladocopium.
Similar content being viewed by others
Introduction
Like corals, the sea anemone Exaiptasia diaphana is a cnidarian that establishes symbiosis with dinoflagellates in the family Symbiodiniaceae. In this symbiosis, the algae translocate products of carbon fixation and nitrogen assimilation as glucose, glycerol, amino acids, alanine and/or organic acids to the host [1,2,3], and gain host-derived inorganic nutrients such as carbon dioxide, ammonium, amino acids, lipids and fatty acids [4]. Symbiodiniaceae loss in corals due to ocean warming (i.e., coral bleaching) has significant negative impacts on coral health and the persistence of coral reef ecosystems [5, 6], highlighting the importance of studying the coral-Symbiodiniaceae symbiosis. Compared to corals, however, E. diaphana is fast growing, easy to maintain and to render aposymbiotic (i.e., free of algal symbionts), and it can survive longer when bleached. These characters allow researchers to easily establish an E. diaphana population, manipulate their Symbiodiniaceae community and to examine cellular processes that would otherwise be challenging to study with corals [7]. Hence, E. diaphana is a common a model for coral-Symbiodiniaceae symbiosis.
E. diaphana has been used to examine the flexibility of algal symbiont-host pairings [8, 9], and the physiological [10, 11], transcriptomic [12], metabolic [12, 13] and proteomic [14] consequences of associating with homologous and/or heterologous (i.e., non-native) algal symbionts under ambient and/or elevated temperatures. Eleven Symbiodiniaceae genera (i.e., Breviolum, Cladocopium, Durusdinium, Effrenium, Fugacium, Freudenthalidium, Gerakladium, Halluxium, Miliolidium, Philozoon, Symbiodinium) have been formally described [15,16,17,18]. For Indo-Pacific E. diaphana, their homologous (i.e., native) Symbiodiniaceae are predominately Breviolum minutum (ITS2: B1) [19], although Cladocopium and Durusdinium are occasionally found in low abundance [20]. Atlantic E. diaphana natively associate with Symbiodinium linucheae (ITS2: A4) and B. minutum and occasionally with Cladocopium [19].
Cladocopium spp. are the most widely distributed algal symbionts of Indo-Pacific scleractinian corals [21] and are found in >150 cnidarian species on the Great Barrier Reef (GBR) [22]. Although some heterologous algal symbionts can colonize aposymbiotic E. diaphana [8, 9, 11, 12, 14, 23, 24], colonization success of Cladocopium spp. is generally poor [11, 25] and these are therefore often excluded from E. diaphana experiments [8, 12]. However, most studies only monitored host algal cell density up to a few weeks post inoculation [23]. Nevertheless, a few studies have demonstrated short-term [9] and long-term (>1 year) [23] successful colonization of aposymbiotic E. diaphana by Cladocopium (ITS2: C1).
All E. diaphana studies so far focus on wild-type Symbiodiniaceae (i.e., Symbiodiniaceae that have been growing under long-term ambient conditions), and none have explored the potential of E. diaphana as a model to study experimentally evolved Symbiodiniaceae. Experimental evolution has been shown to enhance growth, photo-physiological performance and/or the upper thermal limit in many marine microalgal species [26]. For example, experimentally evolved Cladocopium C1acro maintained positive growth and produced less reactive oxygen species (ROS) than wild-type Cladocopium C1acro under elevated temperatures (31 °C) [27, 28]. These experimentally evolved heat-tolerant symbionts (hereafter refer to as “heat-evolved” symbionts) can potentially be used to inoculate corals to improve their thermal tolerance [29]. Buerger et al. [28] demonstrated that heat-evolved Cladocopium C1acro can colonize aposymbiotic coral larvae, where three of the ten strains tested conferred enhanced thermal bleaching tolerance on the larvae compared to the wild-type Cladocopium C1acro while seven did not. However, coral larvae and adults differ significantly in physiology, hence more studies are required to examine the potential benefits and drawbacks of associating with heat-evolved Symbiodiniaceae, particularly in the adult host stage. The values of E. diaphana as a coral model would be enhanced significantly if it can form symbiosis with heat-evolved Symbiodiniaceae and this sea anemone serves as a model for these investigations.
Bi-directional exchange of organic and inorganic compounds is critical to the success and stability of the cnidarian-dinoflagellate symbiosis [1]. Different Symbiodiniaceae strains may translocate different types (e.g., glycerol vs. lipids) and quantities of photosynthate to the host [1], affecting the host’s nutritional potential and overall fitness. While E. diaphana can be rendered aposymbiotic and be colonized by heterologous Symbiodiniaceae, heterologous associations can be less beneficial to the host than homologous symbioses [8, 10, 12]. However, the nutritional consequences of E. diaphana associated with heterologous Cladocopium—a key algal symbionts in Indo-Pacific corals, and heat-evolved Cladocopium—a potential candidate to improve cnidarian thermal tolerance, remain unknown.
This study aims to examine (1) whether E. diaphana from the GBR, which harbours B. minutum as its homologous symbiont, can form a stable symbiosis with heterologous wild-type and heat-evolved Cladocopium C1acro, and (2) the nutritional consequences of the respective Cladocopium C1acro associations with specific focus on central carbon metabolism. Since glucose is a key form in which Symbiodiniaceae translocate fixed carbon to the cnidarian host [2, 30], particular focus is given to sugar-related metabolites.
Materials and methods
Experimental organisms
GBR-sourced E. diaphana (Genotype AIMS4) were obtained from the National Sea Simulator at the Australian Institute of Marine Science (AIMS) in Townsville (Queensland). Anemones were maintained under 27 °C at 30 µmol photons m−2 s−1 (12:12 h, light: dark) [7] and their homologous algal symbiont is B. minutum [7]. They were fed ad libitum with freshly hatched Artemia nauplii 5 days a week prior to the experiment to establish sufficient biomass, and were fed twice a week during the experiment. Aposymbiotic anemones were produced using a modified menthol-bleaching method [31] (Supplementary Methods 1.1). Eight different monoclonal Symbiodiniaceae strains were used to inoculate the aposymbiotic anemones (Table 1, hereafter referred to as Symbiodiniaceae treatments): the homologous B. minutum (hereafter referred to as B1), wild-type Cladocopium C1acro (WT10; formerly known as Cladocopium goreaui [32]), and six heat-evolved Cladocopium C1acro strains (SS) which were derived from the same monoclonal mother culture as WT10, three of which conferred enhanced bleaching tolerance on coral larvae (SS1, SS7 and SS8, jointly referred to as SS+) and three which did not (SS3, SS5 and SS9, jointly referred to as SS−) [28]. Cultures were grown under their respective temperatures (Table 1) at 30–60 µmol photons m−2 s−1 (12:12 h, light: dark) in 1% IMK culture medium, and were obtained from long-term laboratory cultures established at AIMS in 2011.
Inoculation of aposymbiotic anemones
A total of 480 similar-sized aposymbiotic anemones (3–4 mm oral disk diameter, n = 60 anemones per Symbiodiniaceae treatment) were starved for nine days before Symbiodiniaceae inoculation. One anemone was placed in each well of 12-well plates, and each was inoculated by pipetting 1 mL of 1 × 106 algal cells onto its oral disk, followed by 40 µL of freshly hatched Artemia nauplii to encourage phagocytosis. The anemones were exposed to the algal inocula for 24 h before seawater change. A second inoculation was performed 48 h later following the same procedure. The number of weeks mentioned throughout this study refers to the weeks since first inoculation. Seventy-two anemones were not inoculated and kept as aposymbiotic controls. After inoculation, anemones were fed Artemia twice a week as per previous studies [8, 9, 12, 13, 24].
Symbiodiniaceae cell density and identity
Four anemones per Symbiodiniaceae treatment were sampled at week 2, 3, 4, 5, 6, 9, 21, 24 and 77 for Symbiodiniaceae cell counts using cytometery (Supplementary Methods 1.2). To confirm that the Symbiodiniaceae in anemones post inoculation matched the inoculum and that no cross contamination has occurred, three sets of samples were collected for ITS2 metabarcoding: (1) 1 mL of each of the eight algal inocula (n = 2 per Symbiodiniaceae treatment), (2) aposymbiotic anemones collected at week 21 (n = 6), and (3) 100 µL of Symbiodiniaceae collected from anemones at week 9 (n = 4 per Symbiodiniaceae treatment), all of which were snap frozen and stored in −80 °C until DNA extraction, PCR amplification and library preparation (Supplementary Methods 1.3). Illumina MiSeq v3 sequencing was conducted at the Walter and Eliza Hall Institute and raw sequences were submitted to SymPortal [33] for Symbiodiniaceae community analysis.
Anemone survival, dry weight and metabolite sample processing
Twelve anemones of each of the eight Symbiodiniaceae treatments were assessed for survival at week 1, 2, 3, 4, 5, and 9. At week 9, 12 anemones per Symbiodiniaceae treatment were sampled for targeted metabolomics analysis (12 anemones × 8 treatments = 96 total). To avoid contamination from Artemia proteins and metabolites, anemones were starved for a week before sampling, when they were snap frozen in liquid nitrogen and stored at −80 °C. Two anemones of the same Symbiodiniaceae treatment were combined into a single sample to provide sufficient biomass for the analysis. Samples were homogenized and centrifuged to separate the host and Symbiodiniaceae fractions (Supplementary Methods 1.4). Each fraction was freeze dried to obtain its dry weight, then extracted and analyzed on the Agilent 1290 Infinity II UHPLC with an Agilent 6470 Triple Quadrupole LC-MS system (Supplementary Methods 1.5). The analysis followed the Agilent metabolomics dynamic MRM (dMRM) method where a curated database with retention times and optimized MS/MS acquisition parameters of ~200 central carbon metabolites are specified. Samples were run in two batches (batch one: B1, WT10, SS− (SS5), SS+ (SS8); batch two: SS− (SS3, SS9) and SS+ (SS1, SS7)).
Statistical analysis—Symbiodiniaceae cell density and host dry weight
Symbiodiniaceae cell density and dry weight data were analyzed in R (version 3.6.1) [34] using the car package [35] and visualized with the ggplot2 package [36]. Each dataset was tested for normality with the Shapiro test [37] and homogeneity of variance with Levene’s test [38] and log transformed if necessary to meet the assumptions of an analysis of variance (ANOVA). One-way ANOVA was used to test the difference in Symbiodiniaceae cell density and host dry weight between Symbiodiniaceae treatment groups (B1, WT10, SS+, SS−) and Tukey’s pairwise comparisons were then conducted.
Statistical analysis—metabolomics
The raw data were: (1) blank corrected, (2) normalized to internal standards and (3) normalized to the host dry weight (for the host fraction), or Symbiodiniaceae dry weight (for the Symbiodiniaceae fraction). The data were then batched-corrected (automated, least distance amongst batches) and zero-treated (missing value = 1/5 of the Limit of Detection) in MetaboAnalyst 5.0 [39]. See Supplementary Methods 1.6, Figs. S1 and S2 for batch correction details. Data analysis was performed in MetaboAnalyst 5.0 separately for the host and Symbiodiniaceae fractions. Log transformation and pareto scaling were applied to each dataset, and data normality and homogeneity were visually confirmed. The analyses were repeated without scaling and the outcomes remained the same. Note that fold changes (FC) reported throughout this study were calculated in MetaboAnalyst 5.0 using the data with no transformation or scaling.
PCAs and PLS-DAs were generated using all the 207 central carbon metabolites. Metabolites that were significantly different between Symbiodiniaceae treatments were identified with a one-way ANOVA and visualized with a heatmap using Euclidean distance and Ward clustering algorithm. Pairwise comparisons were then examined for the host and Symbiodiniaceae fractions (i.e., B. minutum vs Cladocopium C1acro, Cladocopium C1acro WT10 vs SS, and Cladocopium C1acro SS− vs SS+), and t-tested. P values were corrected using the Benjamini–Hochberg method [40] and a metabolite was considered significant when the padj < 0.05 and FC > 1.3. To identify the pathways that the significant metabolites were linked to, MetaboAnalyst’s Pathway Analysis function [41] was used with Plasmodium falciparum 3D7 library (the option most closely related to Symbiodiniaceae [42]). For both the host and Symbiodiniaceae fractions, only the B. minutum vs Cladocopium C1acro comparison yielded a sufficient number of metabolites for meaningful pathway analysis (see Results), hence the analysis was only conducted for this pair.
A metabolite was classified as sugar-related if it was a disaccharide (i.e., two molecules of glucose or one molecule of glucose with, e.g., a galactose), monosaccharide (i.e., single molecular sugar), amino sugar, sugar acid, sugar alcohol or intermediate sugar metabolite (Supplementary Methods 1.7). Anemones with more Symbiodiniaceae biomass may receive more sugar-related metabolites translocated by the symbionts. While B. minutum-anemones had higher algal cell density than Cladocopium C1acro-anemones at the time of sampling for metabolomics (see Results), the B. minutum cells are smaller than Cladocopium C1acro cells [2, pers. observ.], therefore Symbiodiniaceae dry weight was used as a proxy of Symbiodiniaceae biomass. Two different normalization methods were applied for all sugar-related metabolites in the host fraction: (1) normalized to the host dry weight only, and (2) normalized to the host and Symbiodiniaceae dry weight.
Results
Symbiodiniaceae cell density and identity
At week 9 (when the metabolic samples were taken), anemones of different Symbiodiniaceae treatments differed significantly in Symbiodiniaceae cell density (F3,28 = 7.36, p < 0.001). Pairwise comparisons indicated that B. minutum-anemones had higher cell densities than Cladocopium C1acro SS+ and SS− anemones, while cell densities of all Cladocopium C1acro-anemones (WT10, SS+, SS−) were the same (Fig. 1, S3, Table 2, S1). By week 77, however, anemone groups no longer differed in Symbiodiniaceae cell density (F3,28 = 0.66, p = 0.587) (Fig. 1, Table 2). ITS2 metabarcoding data showed the identity of the Symbiodiniaceae in the anemones matched that of the inocula (Fig. S4, Supplementary Results 1.1).
Anemone survival and dry weight
At week 9, anemones of different Symbiodiniaceae treatments had 100% survival (Table S2). Dry weight of the anemone host at week 9 was significantly different between Symbiodiniaceae treatment groups (F3,44 = 7.14, p < 0.001). Pairwise comparisons indicated the host dry weight of the Cladocopium C1acro groups (WT10, SS + , SS−) did not differ from each other, but they were on average ~34% lower than that of the B. minutum-anemones (Fig. S5, Table S3). While dry weight of aposymbiotic anemones was not measured, we observed that Cladocopium C1acro-anenomes and B. minutum-anemones were both noticeably larger in size than aposymbiotic anemones.
Metabolomics—anemone host fraction
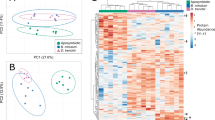
The three Cladocopium C1acro groups (i.e., anemones colonized by heterologous Symbiodiniaceae wild-type WT10, heat evolved SS+ and SS−) overlapped almost completely in the PCA generated from the host fraction for all 207 central carbon metabolites, and most B. minutum-anemone (i.e., anemones colonized by homologous Symbiodiniaceae) replicates did not separate from Cladocopium C1acro-anemones either (Fig. 2a). The PLS-DA did not pass quality control (permutation p = 0.09, Q2 < 0.2; indicating low predictive ability) and is hence not used for interpretation (Fig. S6). ANOVA identified 23 metabolites (~11%) that varied significantly in relative abundance among groups (B1, WT10, SS−, SS+) and t-tests showed this pattern was driven by differences between the B. minutum and Cladocopium C1acro groups (WT10, SS−, SS+) (Table 3, S4, Fig. S7a). Out of these 23 metabolites, 19 and four were enriched in B. minutum- and Cladocopium C1acro-anemones, respectively, with the metabolites d-maltose and cellobiose the most significantly enriched in B. minutum-anemones (Table 3, Fig. S7a). None of the metabolites showed a significant difference between wild-type Cladocopium C1acro (WT10) and heat-evolved Cladocopium C1acro (SS), nor between the heat-evolved SS− and SS+ (Table S4). The heatmap based on the 23 significant host fraction metabolites showed a similar pattern to the PCA, although with a clearer separation between B. minutum and all Cladocopium C1acro groups (Fig. S7a). The 23 significant metabolites are linked to seven pathways, but only one metabolite match was found in each pathway (Table S5). Similar t-test results were found when only batch one data from the host fraction was used (Table S6).
a PCA of the host fraction (normalized to host dry weight) measured at week 9. b PCA of the Symbiodiniaceae fraction (normalized to Symbiodiniaceae dry weight) measured at week 9. B1 B. minutum, WT10 wild-type Cladocopium C1acro, SS− non-conferring heat-evolved Cladocopium C1acro, SS+ conferring heat-evolved Cladocopium C1acro.
The 207 central carbon metabolites in this study comprise 20 sugar-related metabolites and one sugar-related pathway (Supplementary Methods 1.7). There was no difference between wild-type and heat-evolved Cladocopium C1acro-anemones, nor between the homologous B. minutum- and Cladocopium C1acro-anemones in this pathway (Table S5). When these 20 sugar-related metabolites in the host fraction were normalized to both host and Symbiodiniaceae dry weight, six out of the 20 sugar-related metabolites were enriched in B. minutum-anemones compared to Cladocopium C1acro-anemones (by ~2 to 9-fold, Table 3). However, when these 20 sugar-related metabolites were normalized to both host and Symbiodiniaceae dry weight, only three out of the 20 sugar-related metabolites were enriched in B. minutum-anemones (by ~3 to 4-fold), and one sugar-related metabolite (D-Mannose) was enriched in Cladocopium C1acro-anemones (by ~5-fold) (Table 3).
Metabolomics—Symbiodiniaceae fraction
In contrast to the host fraction, PCA of the Symbiodiniaceae fraction showed complete separation between the B. minutum and Cladocopium C1acro groups (WT10, SS+, SS−) (Fig. 2b). Heat-evolved Cladocopium C1acro (SS− and SS+) overlapped with each other, and a few of the WT10 replicates clustered with the heat-evolved SS− and SS+ (Fig. 2b, S7b). The PLS-DA did not pass quality control (permutation p = 0.45, Q2 ~ 0.3; indicating low predictive ability) and is therefore not discussed further (Fig. S8). A total of 24 metabolites showed significant differences in relative abundance among groups (B1, WT10, SS−, SS+) based on ANOVA (Fig. S7b). When WT10, SS− and SS+ were combined as Cladocopium C1acro and compared with B. minutum, t-tests suggested 27 metabolites were significantly different (Table 4). In contrast to the host fraction for which metabolites were mostly enriched in B. minutum-anemones, in the Symbiodiniaceae fraction most of the significantly different metabolites (17 out of 19) were enriched in Cladocopium C1acro (Table 4). Uric acid was the metabolite with the greatest difference and was >10 times more abundant in Cladocopium C1acro algal fraction than in B. minutum (Table 4). These 27 metabolites were linked to 13 pathways, three of which had two metabolite matches (i.e., purine metabolism, glyoxylate and dicarboxylate metabolism, and pyrimidine metabolism) (Table S7), and all were enriched in Cladocopium C1acro groups compared with B. minutum (Fig. S9). Three amino acids (n-formyl-l-tyrosine, o-phospho-l-serine, and l-arginine) were significantly enriched in heat-evolved Cladocopium C1acro compared to wild-type, two of which (o-phospho-l-serine, and l-arginine) had a high FC of >300 (Table S8). T-tests results of the Symbiodiniaceae fraction using batch one data only were similar to those based on both batches, although a smaller number of significant metabolites was found (Table S9).
Discussion
Heterologous wild-type and heat-evolved Cladocopium C1acro can establish a functional symbiosis with E. diaphana
Despite being heterologous algal symbionts, our results show that wild-type and heat-evolved Cladocopium C1acro can establish a functional symbiosis with E. diaphana. While Symbiodiniaceae cell density in all Cladocopium C1acro-anemones was lower than in B. minutum-anemones initially, all anemones achieved the same cell density by week 77. With a few exceptions [12], the homologous B. minutum is generally more successful in colonizing aposymbiotic anemones than heterologous Symbiodiniaceae [8, 9, 11, 12, 14, 24, 43, 44]. For example, B. minutum had faster colonization and higher cell densities in Indo-Pacific anemones than the heterologous S. microadriaticum (ITS2: A1), Durusdinium trenchii (ITS2: D1a), Effrenium voratum (ITS2: E1) and Cladocopium spp. (ITS2: C3) [11]. Medrano et al. [24] reported a fourfold higher cell density in anemones colonized by B. minutum compared to D. trenchii, although Matthews et al. [12] found similar cell density between the two by week 5 post inoculation.
Successful long-term symbiosis between E. diaphana and the heterologous Cladocopium is rare. Of the few known cases of Cladocopium uptake, Chen et al. [23] reported successful colonization of bleached anemones with C. goreaui for >1 year, and the Cladocopium cells were transmitted to their asexual offspring produced via pedal laceration. Another Cladocopium species (ITS2: C3) was observed to successfully colonize anemones initially, but their cell density declined rapidly to ~2.0 × 104 cells mg−1 protein by week 8 post inoculation [11]. Cladocopium was only able to colonize the oral disk and tentacles of the anemones initially (as opposed to the entire body as with the homologous B. minutum), and was restricted to the oral disk only by week 8 [11]. Successful short-term (30 days) colonization of Cladocopium C1acro in GBR E. diaphana has previously been demonstrated, although the cell density was fluctuating over time [9].
There are several possible explanations for the colonization success of E. diaphana by the heterologous wild-type and heat-evolved Cladocopium C1acro in this study. Firstly, the heterologous Symbiodiniaceae inocula used in previous studies generally originated from different parts of the world compared to the host [8, 11, 14, 25], except in Tortorelli et al. [9], while both the host and Symbiodiniaceae originated from the central GBR in our study. Allopatric divergence in traits required for symbiosis maintenance (e.g., cell–cell recognition) may have hindered the heterologous algal colonization success, although successful colonization by a heterologous Symbiodiniaceae inoculum originated from regions different to that of the host has been reported in some cases [12, 23]. The field of Symbiodiniaceae-host recognition is still in its infancy, but the role of glycan–lectin interactions [45, 46] and the potential involvement of d-galactose, l-fucose, d-xylose and d-galacturonic acid in the establishment of this mutualism have been shown [45]. Future studies comparing the recognition molecules of Symbiodiniaceae and host from different regions will shed light on the topic of allopatric divergence and Symbiodiniaceae-host compatibility.
Secondly, colonization success of Cladocopium C1acro in E. diaphana may not have been fully reflected in previous studies due to the relatively short observation periods. At week 9, Symbiodiniaceae cell density observed in our study was consistent with most published works (i.e., lower in the heterologous algae). However, the Symbiodiniaceae cell densities in B. minutum-anemones plateaued by week 21–24, whereas they continued to increase in Cladocopium C1acro-anemones and had achieved the same level as B. minutum by week 77. The symbiosis established by wild-type and heat-evolved Cladocopium C1acro was functional and healthy, as indicated by the 100% host survival. Conversely, associations with unsuitable heterologous Symbiodiniaceae can result in significant host mortality [9]. Our findings highlight the potential of E. diaphana as a model to study cnidarian-dinoflagellate symbiosis with Cladocopium C1acro, a common and widespread species in Indo-Pacific reef-building corals, as well as with experimentally evolved Cladocopium C1acro that may enhance holobiont thermal bleaching tolerance [28].
Wild-type and heat-evolved Cladocopium C1acro had similar colonization rates and sugar translocation ability
Multiple studies have demonstrated that experimental evolution can enhance certain traits of marine microalgae, although trade-offs are sometimes observed [26]. Earlier studies [27, 28] showed that the heat-evolved Cladocopium C1acro used in this study were able to maintain positive growth and secreted less ROS under elevated temperature (31 °C) in vitro than their wild-type counterparts. At the same time, heat-evolved Cladocopium C1acro grew slower than the wild-type in vitro under ambient conditions [28]. We observed no evidence of trade-offs with in vitro growth and ROS secretion in terms of the symbionts’ ability to colonize and translocate sugar to the host, based on the 207 central carbon metabolites examined under ambient condition. It is not known whether the three amino acids enriched in heat-evolved Cladocopium C1acro in symbiosis with E. diaphana may be related to their enhanced thermal tolerance in vitro [28]. Arginine, as an proteinogenic amino acid with the highest nitrogen to carbon ratio, is an ideal organic nitrogen storage in plants [47]. Heat-evolved Cladocopium C1acro may have greater ability for organic nitrogen storage, which may enable them to maintain growth under elevated temperatures [48]. However, whether this is beneficial to their host is debatable [49, 50] and requires further testing.
Heterologous Cladocopium C1acro may be slightly less nutritionally beneficial to the E. diaphana host than homologous B. minutum under ambient conditions
Anemones associated with heterologous algal symbionts have been shown to have lower photosynthesis rates, less growth, pedal laceration [11], carbon translocation [10], and an upregulation of innate immunity responses and lipid catabolism [12]. 13C labelling showed that all sugar compounds investigated (i.e., maltose, fructose, sucrose and xylose) were solely present in anemones with the homologous B. minutum [51]. None of these sugar compounds were detected in D. trenchii-anemones, and these also had a lower amount and diversity of 13C-labelled carbohydrates and lipogenesis precursors. The authors therefore concluded that homologous algal symbionts provide greater fitness benefits to the host than heterologous algal symbionts via increased de novo glucose synthesis and translocation. Such strong contrast was not observed in our data.
When the relative abundance of sugar-related metabolites was normalized to the host dry weight alone, a few of the sugar-related metabolites were enriched in B. minutum-anemones compared to Cladocopium C1acro-anemones at week 9. This may be a consequence of the higher Symbiodiniaceae densities in B. minutum-anemones at the week 9 time point, which may also be responsible for the higher host dry weight at that time point. When the relative abundance of these sugar-related metabolites was normalized to both the host and Symbiodiniaceae dry weight, interestingly, fewer metabolites were enriched in B. minutum-anemones compared to Cladocopium C1acro-anemones, and one sugar-related metabolite was even enriched in Cladocopium C1acro-anemones. This indicates that sugar translocation per algal biomass of Cladocopium C1acro was likely not much lower than that of B. minutum. Heterologous Cladocopium C1acro, therefore, seems slightly less nutritionally beneficial to the host than homologous B. minutum under ambient conditions, but likely more nutritionally beneficial to the host than heterologous D. trenchii. Several studies have shown that coral dominated by Durusdinium grow more slowly [52, 53] and have lower stored lipids [54] and δ13C values [55] than conspecifics dominated by Cladocopium in the field; and that Cladocopium translocated more carbon to its host compared to Durusdinium under ambient conditions [56]. Nevertheless, a few studies observed no difference in the host and symbiont metabolite pool [57], as well as in heterotrophic nutrition [55] between Durusdinium and Cladocopium dominated corals.
Heterologous Cladocopium C1acro and homologous B. minutum differ in nitrogen storage ability
In the Symbiodiniaceae fraction, the two significantly enriched metabolites in the purine metabolism pathways (i.e., guanine and xanthine) and the most significantly enriched metabolite (i.e., uric acid) in the heterologous Cladocopium C1acro compared to the homologous B. minutum are all linked to nitrogen storage ability. Purine metabolism plays an important role as an ongoing source of nitrogen in plant growth. In the purine degradation pathway, guanase converts guanine to xanthine, and xanthine oxidase catalyzes the oxidation of xanthine to form uric acid as the end product (Fig. 3) [58, 59]. Six genes encoding xanthine oxidase/dehydrogenase have been found in a Symbiodiniaceae (Fugacium kawagutii) genome, supporting the ability of these algae to form uric acid [60]. Uric acid functions as a nitrogen reserve in plants and uric acid crystals have been found in Symbiodiniaceae [61] (Fig. 3). When Exaiptasia spp. were treated with an inhibitor of xanthine oxidase, the uric acid crystals of their Symbiodiniaceae disappeared within seven days, hence these nitrogen reserves can be mobilized rapidly when required [61].
The red font represents metabolites enriched in the Cladocopium C1acro groups (WT10, SS+, SS−) compared to B. minutum (B1) and their relative abundances are shown on the heatmap. The locations of uric acid crystals within a Symbiodiniaceae cell as observed in Clode et al. [61] are indicated. The colour scale indicates log2 fold change relative to the mean.
Nitrogen is vital to the synthesis of amino acids, proteins, nucleotides, nucleic acids, chlorophyll, Rubisco and other enzymes that are involved in carbohydrate production - all of which are critical for cell division and growth [62]. Compared to free-living Symbiodiniaceae, Symbiodiniaceae in hospite have higher carbon-to-nitrogen ratios and upregulate multiple transcripts involved in the purine degradation pathway [43]. This suggests Symbiodiniaceae in hospite are nitrogen limited [43]; and a coral host can control the growth and therefore population density of its Symbiodiniaceae by limiting nitrogen supply [63, 64]. Under elevated temperatures, Symbiodiniaceae in hospite may grow more initially due to higher carbon fixation [50], yet they are unable to use that carbon for their own growth if they are nitrogen limited. When the coral Plesiastrea versipora was starved (i.e., no feeding), nitrogen deficiency became apparent in ≥ 4 weeks in their associated Symbiodiniaceae and their photosynthetic rates declined drastically by ~80% [65]. The enhanced nitrogen storage ability of the Cladocopium C1acro compared to B. minutum suggests that they are likely more capable of maintaining photosynthesis for their own growth.
One E. diaphana study showed that heterologous Durusdinium cells were more 15N-enriched than homologous Breviolum cells, yet they provided less carbon to the host under ambient conditions [44]. The extra nitrogen availability to Durusdinium may have been a consequence of lower carbon translocation of the algae, that resulted in lesser host usage of its nitrogen re-assimilated from catabolism [44]. The implications of Symbiodiniaceae nitrogen storage ability on the maintenance of their mutualist relations with the host remains unclear and is an important field for future research.
Conclusions and directions for future research
Several implications relevant to reef restoration and future research have emerged from this study. Firstly, better colonization success and nutrient provisioning may occur when the host and algal symbiont are from the same geographic region. Hence, reef restoration practices that utilize algal symbionts should consider the importance of co-evolution and source algal symbionts from locations near the targeted hosts when possible. Secondly, lower initial host colonization is expected when heterologous algal symbionts are used for reef restoration practices. However, their colonization success will likely improve over time and longer-term monitoring (i.e., >1 year) is important. While the Cladocopium C1acro used is this study is a heterologous symbiont of E. diaphana, it is the most common Symbiodiniaceae genus in Indo-Pacific scleractinian corals [21] and a homologous symbiont for a wide variety of coral species [22]. Therefore, heat-evolved Cladocopium C1acro would likely colonize many coral species at a much faster rate than E. diaphana, and potentially conferring bleaching tolerance to the coral hosts.
Evidence so far from the literature and this study suggests that homologous Symbiodiniaceae symbionts are likely more nutritionally beneficial to the host than heterologous symbionts under ambient conditions, yet this dynamic may change under ocean warming which should be a focus of future research. Under elevated temperatures, thermally tolerant heterologous symbionts (e.g., the heat-evolved Cladocopium C1acro) may become advantageous and more nutritionally beneficial to the host [66, 67]. Due to the significant biomass requirement, omics studies (e.g., metabolomics, proteomics, transcriptomics) on E. diaphana are generally limited to ambient temperature only [12, 14, 24], or limited to one E. diaphana-Symbiodiniaceae combination when multiple temperatures are applied [13]. In addition to omics studies, future studies on heat-evolved Symbiodiniaceae can also apply tacking methods such as 13C labelling, which would provide direct evidence on photosynthate translocation from the algal symbionts to the host. Future studies investigating the physiology and metabolite profiles of anemones associated with homologous and different thermally tolerant heterologous algal symbionts under elevated temperatures will provide invaluable insights for reef restoration initiatives.
Data availability
Metabolic data that have been (1) blank corrected, (2) normalized to internal standards, (3) normalized to the host dry weight (for the host fraction), or Symbiodiniaceae dry weight (for the Symbiodiniaceae fraction), (4) batched-corrected and (5) zero-treated are supplied (host data: Supplementary data S1, Symbiodiniaceae data: Supplementary data S2). Anemone host and Symbiodiniaceae dry weight data (Supplementary data S3), as well as cell density data (Supplementary data S4) are also supplied. Raw sequences of the ITS2 Symbiodiniaceae data are available in GenBank (SAMN22563740- SAMN22563800; project accession no.: PRJNA774436).
References
Davy SK, Allemand D, Weis VM. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev. 2012;76:229–61.
Burriesci MS, Raab TK, Pringle JR. Evidence that glucose is the major transferred metabolite in dinoflagellate–cnidarian symbiosis. J Exp Biol. 2012;215:3467–77.
Kopp C, Domart-Coulon I, Escrig S, Humbel BM, Hignette M, Meibom A. Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. mBio. 2015;6:e02299–14.
Imbs AB, Yakovleva IM, Dautova TN, Bui LH, Jones P. Diversity of fatty acid composition of symbiotic dinoflagellates in corals: evidence for the transfer of host PUFAs to the symbionts. Phytochemistry. 2014;101:76–82.
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, et al. Global warming and recurrent mass bleaching of corals. Nature. 2017;543:373–7.
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359:80.
Dungan AM, Hartman LM, Tortorelli G, Belderok R, Lamb AM, Pisan L, et al. Exaiptasia diaphana from the Great Barrier Reef: a valuable resource for coral symbiosis research. Symbiosis. 2020;80:195–206.
Gabay Y, Parkinson JE, Wilkinson SP, Weis VM, Davy SK. Inter-partner specificity limits the acquisition of thermotolerant symbionts in a model cnidarian-dinoflagellate symbiosis. ISME J. 2019;13:2489–99.
Tortorelli G, Belderok R, Davy SK, McFadden GI, van Oppen MJH. Host genotypic effect on algal symbiosis establishment in the coral model, the anemone Exaiptasia diaphana, from the Great Barrier Reef. Front Mar Sci. 2020;6:833.
Starzak DE, Quinnell RG, Nitschke MR, Davy SK. The influence of symbiont type on photosynthetic carbon flux in a model cnidarian–dinoflagellate symbiosis. Mar Biol. 2014;161:711–24.
Gabay Y, Weis VM, Davy SK. Symbiont identity influences patterns of symbiosis establishment, host growth, and asexual reproduction in a model cnidarian-dinoflagellate symbiosis. Biol Bull. 2018;234:1–10.
Matthews JL, Crowder CM, Oakley CA, Lutz A, Roessner U, Meyer E, et al. Optimal nutrient exchange and immune responses operate in partner specificity in the cnidarian-dinoflagellate symbiosis. PNAS. 2017;114:13194–9.
Hillyer KE, Tumanov S, Villas-Bôas S, Davy SK. Metabolite profiling of symbiont and host during thermal stress and bleaching in a model cnidarian–dinoflagellate symbiosis. J Exp Biol. 2016;219:516–27.
Sproles AE, Oakley CA, Matthews JL, Peng L, Owen JG, Grossman AR, et al. Proteomics quantifies protein expression changes in a model cnidarian colonised by a thermally tolerant but suboptimal symbiont. ISME J. 2019;13:2334–45.
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol. 2018;28:2570–80.e6.
LaJeunesse TC, Wiedenmann J, Casado-Amezúa P, D’Ambra I, Turnham KE, Nitschke MR, et al. Revival of Philozoon Geddes for host-specialized dinoflagellates, ‘zooxanthellae’, in animals from coastal temperate zones of northern and southern hemispheres. Eur J Phycol. 2021;0:1–15.
Nitschke MR, Craveiro SC, Brandão C, Fidalgo C, Serôdio J, Calado AJ, et al. Description of Freudenthalidium gen. nov. and Halluxium gen. nov. to formally recognize clades Fr3 and H as genera in the family Symbiodiniaceae (Dinophyceae). J Phycol. 2020;56:923–40.
Pochon X, LaJeunesse TC. Miliolidium n. gen, a new Symbiodiniacean genus whose members associate with soritid foraminifera or are free-living. J Eukaryot Microbiol. 2021;68:e12856.
Thornhill DJ, Xiang Y, Pettay DT, Zhong M, Santos SR. Population genetic data of a model symbiotic cnidarian system reveal remarkable symbiotic specificity and vectored introductions across ocean basins. Mol Ecol. 2013;22:4499–515.
Núñez-Pons L, Bertocci I, Baghdasarian G. Symbiont dynamics during thermal acclimation using cnidarian-dinoflagellate model holobionts. Mar Environ Res. 2017;130:303–14.
LaJeunesse TC. “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the miocene-pliocene transition. Mol Biol Evol. 2005;22:570–81.
Tonk L, Bongaerts P, Sampayo EM, Hoegh-Guldberg O. SymbioGBR: a web-based database of Symbiodinium associated with cnidarian hosts on the Great Barrier Reef. BMC Ecol. 2013;13:7.
Chen W-NU, Hsiao Y-J, Mayfield AB, Young R, Hsu L-L, Peng S-E. Transmission of a heterologous clade C Symbiodinium in a model anemone infection system via asexual reproduction. PeerJ. 2016;4:e2358.
Medrano E, Merselis DG, Bellantuono AJ, Rodriguez-Lanetty M. Proteomic basis of symbiosis: a heterologous partner fails to duplicate homologous colonization in a novel cnidarian—Symbiodiniaceae mutualism. Front Microbiol. 2019;10:1153.
Belda-Baillie CA, Baillie BK, Maruyama T. Specificity of a model cnidarian-dinoflagellate symbiosis. Biol Bull. 2002;202:74–85.
Chan WY, Oakeshott JG, Buerger P, Edwards OR, Oppen MJHvan. Adaptive responses of free-living and symbiotic microalgae to simulated future ocean conditions. Glob Change Biol. 2021;27:1737–54.
Chakravarti LJ, Beltran VH, van Oppen MJH. Rapid thermal adaptation in photosymbionts of reef-building corals. Glob Change Biol. 2017;23:4675–88.
Buerger P, Alvarez-Roa C, Coppin CW, Pearce SL, Chakravarti LJ, Oakeshott JG, et al. Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci Adv. 2020;6:eaba2498.
van Oppen MJH, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. PNAS. 2015;112:2307–13.
Molina VH, Castillo-Medina RE, Thomé PE. Experimentally induced bleaching in the sea anemone Exaiptasia supports glucose as a main metabolite associated with its symbiosis. J Mar Biol. 2017;2017:e3130723.
Matthews JL, Sproles AE, Oakley CA, Grossman AR, Weis VM, Davy SK. Menthol-induced bleaching rapidly and effectively provides experimental aposymbiotic sea anemones (Aiptasia sp.) for symbiosis investigations. J Exp Biol. 2016;219:306.
Beltrán VH, Puill-Stephan E, Howells E, Flores-Moya A, Doblin M, Núñez-Lara E, et al. Physiological diversity among sympatric, conspecific endosymbionts of coral (Cladocopium C1acro) from the Great Barrier Reef. Coral Reefs. 2021;40:985–97.
Hume BCC, Smith EG, Ziegler M, Warrington HJM, Burt JA, LaJeunesse TC, et al. SymPortal: a novel analytical framework and platform for coral algal symbiont next-generation sequencing ITS2 profiling. Mol Ecol Resour. 2019;19:1063–80.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
Fox J, Weisberg S. An R companion to applied regression, Third. Thousand Oaks CA: Sage Publications; 2019.
Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer; 2016.
Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52:591–611.
Levene H. Robust tests for equality of variances. In: Contributions to Probability and Statistics. 1960. Palo Alto: Stanford University Press; 1960. p. 278–92.
Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodol). 1995;57:289–300.
Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26:2342–4.
McFadden GI. Mergers and acquisitions: malaria and the great chloroplast heist. Genome Biol. 2000;1: reviews1026.1-reviews1026.4.
Xiang T, Hambleton EA, DeNofrio JC, Pringle JR, Grossman AR. Isolation of clonal axenic strains of the symbiotic dinoflagellate Symbiodinium and their growth and host specificity1. J Phycol. 2013;49:447–58.
Sproles AE, Oakley CA, Krueger T, Grossman AR, Weis VM, Meibom A, et al. Sub-cellular imaging shows reduced photosynthetic carbon and increased nitrogen assimilation by the non-native endosymbiont Durusdinium trenchii in the model cnidarian Aiptasia. Environ Microbiol. 2020;22:3741–53.
Tortorelli G, Rautengarten C, Bacic A, Segal G, Ebert B, Davy SK, et al. Cell surface carbohydrates of symbiotic dinoflagellates and their role in the establishment of cnidarian–dinoflagellate symbiosis. ISME J. 2022;16:190–9.
Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell Microbiol. 2006;8:1985–93.
Winter G, Todd CD, Trovato M, Forlani G, Funck D. Physiological implications of arginine metabolism in plants. Front Plant Sci. 2015;6:534.
Fernández PA, Gaitán-Espitia JD, Leal PP, Schmid M, Revill AT, Hurd CL. Nitrogen sufficiency enhances thermal tolerance in habitat-forming kelp: implications for acclimation under thermal stress. Sci Rep. 2020;10:3186.
Wooldridge SA. Breakdown of the coral-algae symbiosis: towards formalising a linkage between warm-water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences. 2013;10:1647–58.
Baker DM, Freeman CJ, Wong JCY, Fogel ML, Knowlton N. Climate change promotes parasitism in a coral symbiosis. ISME J. 2018;12:921–30.
Matthews JL, Oakley CA, Lutz A, Hillyer KE, Roessner U, Grossman AR, et al. Partner switching and metabolic flux in a model cnidarian–dinoflagellate symbiosis. Proc R Soc B: Biol Sci. 2018;285:20182336.
Little AF, van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492.
Jones A, Berkelmans R. Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types. PLoS ONE. 2010;5:e10437.
Jones AM, Berkelmans R. Tradeoffs to thermal acclimation: energetics and reproduction of a reef coral with heat tolerant Symbiodinium type-D. J Mar Biol. 2011;2011:e185890.
Wall CB, Kaluhiokalani M, Popp BN, Donahue MJ, Gates RD. Divergent symbiont communities determine the physiology and nutrition of a reef coral across a light-availability gradient. ISME J. 2020;14:945–58.
Cantin NE, van Oppen MJH, Willis BL, Mieog JC, Negri AP. Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs. 2009;28:405.
Matthews JL, Cunning R, Ritson-Williams R, Oakley CA, Lutz A, Roessner U, et al. Metabolite pools of the reef building coral Montipora capitata are unaffected by Symbiodiniaceae community composition. Coral Reefs. 2020;39:1727–37.
Schramm VL, Bagdassarian CK Deamination of nucleosides and nucleotides and related reactions. In: Barton SD, Nakanishi K, Meth-Cohn O (eds). In: Comprehensive natural products chemistry. Oxford: Pergamon; 1999. p. 71–100.
Brychkova G, Fluhr R, Sagi M. Formation of xanthine and the use of purine metabolites as a nitrogen source in Arabidopsis plants. Plant Signal Behav. 2008;3:999–1001.
Lin S, Cheng S, Song B, Zhong X, Lin X, Li W, et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science. 2015;350:691–4.
Clode PL, Saunders M, Maker G, Ludwig M, Atkins CA. Uric acid deposits in symbiotic marine algae. Plant Cell Environ. 2009;32:170–7.
Engels C, Marschner H. Plant uptake and utilization of nitrogen. In: Bacon P, editor. Nitrogen Fertilization in the Environment. New York: Marcel Dekker; 1995. p. 41–82.
Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L. Population control in symbiotic corals. BioScience. 1993;43:606–11.
Krueger T, Horwitz N, Bodin J, Giovani M-E, Escrig S, Fine M, et al. Intracellular competition for nitrogen controls dinoflagellate population density in corals. Proc R Soc B: Biol Sci. 2020;287:20200049.
Davy SK, Withers KJT, Hinde R. Effects of host nutritional status and seasonality on the nitrogen status of zooxanthellae in the temperate coral Plesiastrea versipora (Lamarck). J Exp Mar Biol Ecol. 2006;335:256–65.
McIlroy SE, Wong JCY, Baker DM. Competitive traits of coral symbionts may alter the structure and function of the microbiome. ISME J. 2020;14:2424–32.
Baker DM, Andras JP, Jordán-Garza AG, Fogel ML. Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 2013;7:1248–51.
Acknowledgements
This study was funded by Australian Research Council Laureate Fellowship FL180100036 to MJHvO. We thank B. Lust, J. Maire, and A. Dungan for technical supports, R. Sakamoto for the supply of week 77 anemone samples, as well as D. Rudd and D. de Souza for fruitful discussion.
Author information
Authors and Affiliations
Contributions
MvO, PB, WYC, and SJTMC designed the experiment. SJTMC performed the experiment, KEH carried out the metabolomics sample preparation LC-MS work, and SJTMC and AP-G conducted the flow cytometry work. WYC and SJTMC undertook statistical analyses. WYC, MvO and SJTMC wrote the manuscript with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsang Min Ching, S.J., Chan, W.Y., Perez-Gonzalez, A. et al. Colonization and metabolite profiles of homologous, heterologous and experimentally evolved algal symbionts in the sea anemone Exaiptasia diaphana. ISME COMMUN. 2, 30 (2022). https://doi.org/10.1038/s43705-022-00114-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43705-022-00114-7