Abstract
Sedimentary ancient DNA (sedaDNA) analyses are increasingly used to reconstruct marine ecosystems. The majority of marine sedaDNA studies use a metabarcoding approach (extraction and analysis of specific DNA fragments of a defined length), targeting short taxonomic marker genes. Promising examples are 18S-V9 rRNA (~121–130 base pairs, bp) and diat-rbcL (76 bp), targeting eukaryotes and diatoms, respectively. However, it remains unknown how 18S-V9 and diat-rbcL derived compositional profiles compare to metagenomic shotgun data, the preferred method for ancient DNA analyses as amplification biases are minimised. We extracted DNA from five Santa Barbara Basin sediment samples (up to ~11 000 years old) and applied both a metabarcoding (18S-V9 rRNA, diat-rbcL) and a metagenomic shotgun approach to (i) compare eukaryote, especially diatom, composition, and (ii) assess sequence length and database related biases. Eukaryote composition differed considerably between shotgun and metabarcoding data, which was related to differences in read lengths (~112 and ~161 bp, respectively), and overamplification of short reads in metabarcoding data. Diatom composition was influenced by reference bias that was exacerbated in metabarcoding data and characterised by increased representation of Chaetoceros, Thalassiosira and Pseudo-nitzschia. Our results are relevant to sedaDNA studies aiming to accurately characterise paleo-ecosystems from either metabarcoding or metagenomic data.
Similar content being viewed by others
Introduction
Sedimentary ancient DNA (sedaDNA) analyses have become increasingly applied to the sub-seafloor for the reconstruction of marine ecosystems. Using sedaDNA, taxa across all three domains of life (archaea, bacteria, eukaryota) have been detected, including non-fossilising species (e.g., [1, 2]). The latter shows the enormous potential of sedaDNA techniques to go beyond standard environmental proxies and facilitate the reconstruction of paleo-ecosystems across the entire marine food web, rather than the small proportion of marine biodiversity detectable from fossils alone.
Amongst the most popular study targets are eukaryotes, especially microscopic phytoplankton, key environmental indicators whose compositional changes reflect changes in past ocean conditions and climate [3,4,5]. Furthermore, DNA sequences from photosynthetic organisms in deep-sea sediments are more likely of ancient origin than from living contaminants because these organisms require light for their survival. Particularly important are the diatoms, which are responsible for ~20% of annual global net primary production [6, 7]. Diatom microfossils have been characterised extensively in sediment cores to predict past ecosystems (e.g., [8, 9]). However, studying marine eukaryotes by means of sedaDNA has remained complicated as only minuscule amounts of their DNA are preserved in the sub-seafloor (~1.5% of total DNA is of eukaryote origin when using the small subunit ribosomal RNA (SSU) taxonomic marker gene as a ref. [10]).
Most sedaDNA studies have used a metabarcoding approach to maximise the genetic signal of eukaryotes. Metabarcoding targets a specific DNA region, such as a taxonomic marker gene, enabling the identification of different species within a sample [11]. These genetic markers are amplified using primers (short sequences matching the start and end of the target gene) in a polymerase chain reaction (PCR) and subsequently sequenced. A frequently used marker gene for marine eukaryotes is the SSU rRNA (18S rRNA) or shorter regions within this gene, such as 18S-V1, 18S-V3, 18S-V7, 18S-V9 [3, 5, 12,13,14]. The hypervariable gene region 18S-V9 is particularly well characterised as a result of global ocean sampling programs focusing on the study of marine eukaryotes [15,16,17,18], providing an extensive number of modern references (e.g., summarised in the protist ribosomal database, PR2 [19]). Furthermore, 18S-V9 is quite short, ranging from 87 to 186 bp (average 121 bp, most sequences ~130 bp [15]).
There are a few reasons, however, why metabarcoding is problematic when applied to sedaDNA. Ancient DNA is typically very fragmented and damaged, often preventing PCR primers from binding to it [20]. Also, target sequences are usually longer than the short ancient DNA fragments (<100 bp [21, 22]), resulting in preferential amplification of better preserved DNA molecules—a bias that can be further enhanced by the random amplification of DNA fragments in the first few PCR cycles (PCR bias, especially when many cycles are applied [23,24,25]). These issues can significantly distort the results, with the final data being heavily biased towards well-preserved sequences, possibly from contaminant taxa. Similarly, previous paleo-microbiome research using the bacterial taxonomic marker gene 16S rRNA has shown that extensive length variations in the 16S-V3 region are a major cause of differential amplification resulting in taxonomic bias in ancient microbiome reconstructions, preventing them from being accurate [26].
A preferred technique in sedaDNA research is to use a metagenomics approach that relies on the extraction and amplification of the ‘total’ DNA (‘shotgun’ approach), facilitating the investigation of potentially all species in a sample and independent of DNA fragment size [11, 26]. Amongst the first metagenomics sedaDNA studies in marine environments were investigations from the Arabian Sea (e.g., [4, 27]). However, if the DNA of the target organisms is rare compared to the total extracted DNA (as for eukaryotes in sedaDNA), very deep sequencing (achieving a high number of reads) is required to recover sufficient genetic information and perform meaningful statistical analyses. Often, the total pool of metagenomic shotgun data is screened for the occurrence of a taxonomic marker gene, such as the SSU and large subunit ribosomal RNA (LSU) whereby only a fraction of the data is kept for downstream analyses, reducing cost-effectiveness.
Recent metagenomics studies using sediments from Australia and Antarctica have shown that marine sedaDNA can be very short, with most sequences being ~70 and ~40–50 bp, respectively [10, 28]. This is even shorter than the minimum fragment length of the 18S-V9 region (87 bp [15]), suggesting that the application of 18S-V9 metabarcoding might lead to similar skewed eukaryote composition reconstructions as is the case for paleo-prokaryotes using 16S-V3 [26]. A more suitable target gene region might be one that is closer to the typical ~40 - 70 bp sedaDNA fragment size, such as the diatom-specific diat-rbcL gene region, a 76 bp region within the gene encoding the large subunit of the enzyme ribulose-1,5-bisphosphate carboxylase-oxygenase (rbcL) [29]. The rbcL gene is relatively conserved (more than the SSU) and thus discriminates well between phytoplankton taxa at species level [30, 31]. Diat-rbcL has been used previously to investigate diatom composition in tropical and Arctic lakes, as well as in Arctic marine sediments [29, 32, 33].
Here, we provide the first comparison of metagenomic and metabarcoding derived eukaryote sedaDNA data from the Santa Barbara Basin. We selected both 18S-V9 and diat-rbcL for our metabarcoding approach, testing whether they capture a similar breadth of eukaryote and diatom diversity to shotgun data. We investigated paleo-eukaryote composition and taxon-specific sedaDNA fragment lengths, and whether the latter, and/or potential reference biases, impacted the taxonomic profiles.
Methods
Sediment core sampling
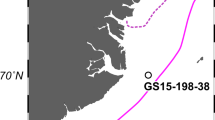
An 18 m long Jumbo Piston Core (MV1012-002P) was collected at ~576.5 m water depth in the Santa Barbara Basin off California, USA, during the CalEchoes MV1012 expedition (28 September 2010, R/V Melville) (Fig. 1). The core was cut into 1.5 m sections, each was capped, vacuum-bagged with nitrogen gas and a commercial oxygen-absorber, sealed and transported to the Scripps Institution of Oceanography Geological Collection (SIO-GC) for storage (4 °C). In 2017, the bottom 25 cm of five sections (Sections 1, 3, 5, 8, 11, each 10.16 cm diameter in PVC liner) were cut off using sterile tools while wearing gloves and face masks to minimize contamination. The 25 cm sections were re-bagged/-sealed, kept at 4 °C and subsampled for sedaDNA analysis at the paleogenomics facilities of the Musée de l’Homme, Paris, France, in 2018.
The exact coordinates are 34.288°N, 120.036°W. ODP Site 893 A is <100 m away from MV1012-002 thus not depicted here. Map created in ODV (Schlitzer, R., Ocean Data View, https://odv.awi.de, 2018).
The bag containing Section 1 (youngest) was slightly damaged, thus was prioritised for sedaDNA sampling to minimise rapid sedaDNA degradation. Afterwards, we worked from bottom to top sections. We decontaminated the lab (Surfa’Safe Premium, ANIOS, France), placed a fresh bench cloth, and changed gloves between cutting and sampling of each section. A Dremel EZ cutter fitted with a SpeedClic adapter and a 38 mm metal cutting disc (replaced after each section) was used for splitting. We scooped ~5 cm3 of sediment from the centre of one core section half into a sterile 15 mL centrifuge tube using a sterile plastic spatula. Duplicate samples were collected per depth interval (Table 1) and frozen at −80 °C.
An independent age model does not exist for Core MV1012-002P, thus we applied an approximate age-depth model from Ocean Drilling Program (ODP) Site 893 A [34, 35], located less than 100 m from Core MV1012-002P (Table 1). Cores collected in this region have similar age-depth models [36], and correlation between cores is generally within the error range of Δ14C radiocarbon-based ages.
sedaDNA extractions
Hoods and equipment were de-contaminated before and after extractions (using Surfa’Safe Premium and UV light). Gloves were frequently changed, and equipment and surfaces were disinfected between processing each sample. One extract was prepared for each sample (i.e., two extracts per depth, Table 1) from ~0.25 g of sediment, working from the oldest to youngest sample. Extractions followed the DNeasy PowerLyzer PowerSoil DNA Isolation Kit (QIAGEN, Germany) protocol, except that DNA was eluted three times in 60 µL elution buffer instead of once in 100 µL to achieve a higher DNA concentration. We added two extraction blank controls (EBCs; extracts 57,58) by treating empty bead-tubes with the same protocol, which provided a total of 12 extracts (10 samples, 2 EBCs). Library preparation and sequencing of the EBCs followed the same procedure as for samples.
Metagenomic (‘shotgun’) library preparation
Libraries were prepared from the 12 raw extracts using the TruSeq Nano DNA Low Throughput Library Prep Kit (Illumina, CA, USA) with TruSeq DNA Single Indexes Set A (Illumina). We followed the manufacturer’s protocol, except that we retained all DNA fragments by not removing large fragments and by adding 200 µL Sample Purification Beads (instead of 30 µL as per Illumina protocol) in the “small fragments removal” step. Instead of purifying our libraries using magnetic beads we ran them on a 1.5% agarose gel and cut out bands between 200 - 300 bp using sterile scalpels. We pooled the gel pieces of our duplicate libraries in one vial and purified them using the NucleoSpin Gel and PCR Clean-up kit and protocol (Macherey Nagel, Germany). We washed and eluted the DNA twice with the same 12 µL elution buffer and quantified the libraries using the Qubit dsDNA HS Assay (Invitrogen, MA, USA). The DNA-content of library of sample 41/42 (4.35 mbsf) was very low, thus we added an ethanol precipitation step (final volume 6 µL), and then pooled the barcoded libraries into an equimolar 10 nM pool (except for sample 41/42, 4.35 mbsf, which was 7.64 nM). The samples were sequenced on a HiSeq 4000 (2 ×150 bp cycle; ~350 Mio paired-end reads total, i.e., ~58Mio/sample, and an approximate sequencing depth of 20X/sample assuming a diatom genome-size of 80–100 Mb) at Fasteris, Switzerland.
Metabarcoding (‘amplicon’) library preparation
We amplified the 18S-V9 region (121 bp) using PCR (25 µL/reaction) containing 1 µL DNA template (1 in 10 dilution), Pfu Buffer (final concentration 1X, 2.5 µL) and Pfu Polymerase (1.25 units, 0.2 µL) (Promega, WI, USA), dNTPs (10 mM each, 0.5 µL), the primer pair 1389 F 5′-TTGTACACACCGCCC-3′ and 1510 R 5′-CCTTCYGCAGGTTCACCTAC-3′ (0.3 µM, 1 µL each) [15], and nuclease-free water (18.8 µL). PCR amplifications (lid-preheat to 105 °C, 30 s at 98 °C; 35 cycles of 10 s at 98 °C, 30 s at 57 °C, 30 s at 72 °C; and 72 °C for 10 min) were performed in triplicates on a Mastercycler (Eppendorff, Germany) and then pooled.
We amplified the diat-rbcL region (76 bp) using PCR (25 µL/reaction) containing 1 µL DNA template (1 in 10 dilution), PCR Buffer II (final concentration 1X, 2.5 µL) and MgCl2 (1.5 mM, 1.5 µL) and AmpliTaq Gold Polymerase (1.25 units, 0.125 µL) (Applied Biosystems, MA, USA), dNTPs (10 mM each, 0.5 µL), the primer pair Diat_rbcL_705F (AACAGGTGAAGTTAAAGGTTCATAYTT) and Diat_rbcL_808R (TGTAACCCATAACTAAATCGATCAT), (0.32 µM, 0.8 µL each) [29], and nuclease-free water (17.78 µL). PCR amplifications (lid-preheat to 105 °C, 8 min at 95 °C; 45 cycles of 10 s at 95 °C, 30 s at 43.6 °C, 30 s at 72 °C; and 72 °C for 10 min) were done in triplicates and pooled.
PCRs were set up in the paleogenomics lab, and then run in a physically separated post-PCR lab. Library preparation principally followed the protocol described above for the shotgun libraries, using 10 µL of each sample’s 18S-V9 PCR product mixed with 6.25 µL (to achieve an equimolar concentration) of each sample’s diat-rbcL PCR product diluted with nuclease-free water to a final library volume of 60 µL. DNA bands between 150 and 200 bp were cut from the gel, with the replicates per sample pooled, cleaned up and quantified as described in 2.3. Sequencing was undertaken using a MiSeq Nano V2 2 ×125 bp cycle; ~1 Mio paired-end reads total, with ~166,000/sample shared sequencing run containing both 18S-V9 and diat-rbcL amplicons, providing a sequencing depth of 2 371X for diat-rbcL (assuming 134 diatom species, see Results) and 619X for 18S-V9 (assuming 35 phyla, see Results) at Fasteris.
Bioinformatics and statistical analyses
We received already demultiplexed raw sequencing data (see Supplementary Material for sequencing output), which we processed using the same parameters for shotgun and amplicons, following the marine eukaryote sedaDNA bioinformatic pipeline described in [10] (and Supplementary Material). To investigate the eukaryote composition, we processed both shotgun and amplicon data by comparing to a PR2-derived V9 database, namely V9_PR2 [16]. We subtracted species identified in EBCs (Supplementary Material Table 1) and exported read counts per sample on phylum-level (all nodes). To be able to visualise the data, we selected all eukaryote taxa that occurred with a relative abundance of >0.1% (which together made up >99% of the community) in each of the two datasets (31 and 27 taxa in the shotgun and amplicon data, respectively).
We tested whether a relationship exists between the average V9_PR2 reference sequence length for the more abundant taxa and their over/-underrepresentation in amplicon relative to shotgun data (as per [26] for 16S-V3). For this, we extracted reference sequence length distribution data (‘abundant’ taxa identified in both shotgun and amplicon) from the V9_PR2 database (Supplementary Material) and visualised this in a heatmap with the read counts data per taxon using the R library ggplot2 [37]. We drew the ratio between amplicon and shotgun (A:SG) read counts per abundant taxon per sample. As a few taxa had no read counts in some of the shotgun samples (Acantharea, Annelida, Basidiomycota, Chlorophyta, Chytridiomycota, and Opisthokonta) these taxa were excluded from the ratio, leaving 17 taxa for this analysis. We performed Pearson correlation analyses between the average read lengths (“PR2V9AL”) and the A:SG ratio per taxon per sample (PAST v.4.02 [38]) to test for overamplification of short reads in amplicon data. In addition, we compared read length (length of the aligned query/sample sequence) and coverage (how many bases were covered between query and reference sequence) (both exported from MEGANCE6-18-10) of all sequences assigned to Eukaryota, and also for Bacillariophyta, per sample.
For a detailed investigation of diatoms, we compared both the shotgun and amplicon data to an in-house diat-rbcL database (including 1 472 unique 76 bp long diat-rbcL sequences, Supplementary Material). Alignments and EBC taxa filtering were done as for V9_PR2 (Supplementary Material Table 2). Read length and coverage were extracted from.blastn-files and MEGAN, respectively. Correlation analysis on diat-rbcL reference sequence lengths and over-/underrepresentation of diatoms was not possible as for V9_PR2 due to all diat-rbcL being 76 bp long. However, this data is provided with the Supplementary Material for completeness. Finally, we compared diatom composition as detected by V9_PR2 and diat_rbcL, as well as the representation of the detected diatoms in each database to assess potential reference biases.
Results
Eukaryote composition (V9_PR2)
Using V9_PR2 we were able to assign a total of 15 668 (shotgun) and 90 689 reads for the shotgun and amplicon data, respectively. These reads represented 14%, 54%, 0 and 32% (shotgun), and 0%, 29%, 0 and 71% (amplicon) unassigned cellular organisms, Bacteria, Archaea and Eukaryota, respectively. Within the eukaryotes, we determined 51 and 64 taxa for shotgun and amplicon data, respectively. Abundant taxa (average abundance >0.1% across all samples; 31 and 27 taxa in shotgun and amplicon, respectively) are shown in Fig. 2. The latter includes 23 taxa (including assignments made on “Eukaryota” level) that were shared between shotgun and amplicon, and four taxa only detected in the amplicon data (Fig. 2C).
Composition is shown in relative abundances for (A) shotgun, and (B) amplicon data (phylum-level). The surface sample should be considered with caution in both (A) and (B) due to the possibility of contamination (see “Methods”). C Venn diagram showing eukaryote taxa richness (phylum level) in the shotgun and amplicon data after alignment with the V9_PR2 database (diagram areas are proportional to the total number of taxa included, for a list of shared/non-shared taxa see Supplementary Material Fig. 1). Only taxa abundant on average >0.1% are included, as they make up >99% of the eukaryote composition.
Within shotgun, the most abundant eukaryotes were Ascomycota (53%), Telonemia (11%), Eukaryota (not further determined, 8%), Polycystinea (4%), Dinophyceae (3.8%), Streptophyta (3.2%), Amoebozoa (3%), Cercozoa (1.6%), Bacillariophyta (1.6%), Arthropoda (1%). In the amplicon data, the most abundant eukaryotes were Ascomycota (33%), Apicomplexa (30%), Dinophyceae (9.5%), Stramenopiles (6.3%), Eukaryota (4.9%), Polycystinea (3.5%), Foraminifera (3.2%), Cercozoa (1.1%) and Chordata (1%). Thus, a total of 10 and 9 taxa were abundant with >1% (average across all samples) in the shotgun and amplicon data, including only five taxa (Ascomycota, Eukaryota, Dinophyceae, Polycystinea, Cercozoa) that were picked up by both methods (i.e., are amongst the shared taxa in Fig. 2C, Supplementary Material Fig. 1). Taxa detected by one method or the other were slightly rarer species (between 0.1 and 1% average relative abundance across all samples; Supplementary Material Table 3).
The shotgun EBC detected two taxonomic groups, one prokaryotic (Gammaproteobacteria) and one eukaryotic (Poacea). The amplicon EBC detected 46 taxa, of which 12 were prokaryotes and 34 were eukaryotes, including dinoflagellate taxa (Dinophysis and Alexandrium), Calanoida and Bacillariophyta (copepods and diatoms, respectively; Supplementary Material Table 1). While any reads assigned to EBC taxa were removed from samples, including reads assigned to the Bacillariophyta node, reads assigned to Bacillariophyta at lower taxonomic levels (e.g., Bacillariophycidae, Bacillariaceae, etc.) remain summarised under the phylum-level Bacillariophyta node (Fig. 2).
Relationship between Eukaryota composition and V9_PR2 reference sequence length
V9_PR2 reference sequence-lengths for the relatively abundant taxa (>0.1% across all samples, including all taxa that were shared and assigned below eukaryote-level, i.e., 22 taxa, see Supplementary Material Table 3) were around the overall average sequence length of the V9_PR2 database (121 bp) (Fig. 3). However, considerable length variation was observed, with most of the abundant taxa being represented by shorter than average reference sequences in the V9_PR2 database, and a few taxa (e.g., Arthropoda, Opisthokonta and Amoebozoa) with a number of reference sequences longer than average (Fig. 3).
We determined a negative correlation between the average V9_PR2 reference sequence length (V9PR2AL) and the A:SG read counts ratio per taxon for all samples (rV9PR2AL,A:SG_1.2 = −0.27269, rV9PR2AL,A:SG_4.3 = −0.33233, rV9PR2AL,A:SG_7.3 = −0.28064, rV9PR2AL,A:SG_11.8 = −0.32559, rV9PR2AL,A:SG_16.4 = −0.30078). This means that shorter V9_PR2 reference sequences for our abundant taxa were associated with an overamplification of these taxa in the amplicon data (for average V9_PR2 reference sequence length of the abundant taxa and A:SG ratios see Supplementary Material Table 4).
Eukaryota and Bacillariophyta sequence length and coverage post-V9_PR2 alignment
Sequences assigned to Eukaryota in shotgun were on average 112 bp and in amplicon data 161 bp, i.e., shotgun reads were around ~50 bp shorter than amplicon reads (Table 2). Bases covered in shotgun were ~40 bp shorter than in amplicon data (Table 2). Similarly, sequences assigned to Bacillariophyta were on average 124 and 167 bp in shotgun and amplicon data, respectively, so showed an ~40 bp difference. For Eukaryota, there was a difference of ~23 bp and 29 bp between sequence length and coverage in shotgun and amplicon data, respectively. For Bacillariophyta, we found a ~36 and ~37 bp difference between sequence length and coverage in shotgun and amplicon data, respectively.
Bacillariophyta read lengths and coverage were similar to those of Eukaryota, for both shotgun and amplicon data (Table 2). Variation in sequence lengths and coverage was much higher in shotgun than in amplicon data. We found no trend towards shorter (i.e., more fragmented) sequences with increasing subseafloor depth for either Eukaryota or Bacillariophyta in the shotgun data. Eukaryota shotgun read lengths were on average ~9 bp shorter (112 bp) than the average reference sequences in the V9_PR2 database (121 bp).
Diatom composition detected via diat-rbcL and read length characteristics
A total of 60 (shotgun) and 80 674 (amplicon) reads were assigned to diatoms (Fig. 4). In total, 27 taxa were determined in the shotgun, and 140 in the amplicon dataset. When considering the “abundant” taxa (on average >0.1%), 27 and 49 diatoms were determined in the shotgun and amplicon data, respectively (Fig. 4). A total of 10 taxa were shared between the two datasets Bacillariophyta, Bacillariophycidae, Chaetoceros, C. cf. pseudobrevis 2 SEH-2013, Pseudo-nitzschia, P. fryxelliana, Thalassiosiraceae, Thalassiosirales, Thalassiosira and T. oceanica (Fig. 4C, Supplementary Material Fig. 2). Sequences assigned to diatoms via diat-rbcL were shorter (by ~16 bp) in the shotgun than in the amplicon data, with amplicon read lengths and coverage all 76 + 1 bases (Table 3).
Diatom composition is shown as relative abundance for (A) shotgun and (B) amplicon data. The surface sample should be considered with caution in both (A) and (B) due to the possibility of contamination (see “Methods”). C Venn diagram showing diatom taxa richness (species level) in the shotgun and amplicon data after alignment with the diat-rbcL database (diagram areas are proportional to the total number of taxa included, for a list of shared/non-shared taxa see Supplementary Material Fig. 2). Only taxa abundant on average >0.1% are included (in A, B, C).
No diatoms were detected in the shotgun EBC, however, 45 taxa were determined in the amplicon EBC with most reads assigned to Chaetoceros spp. (especially, Chaetoceros debilis, C. socialis and C. radicans), several Thalassiosira and Pseudo-nitzschia species, as well as others (Supplementary Material Table 2).
Comparison of V9_PR2 vs. diat-rbcL derived diatom composition
In the shotgun data, 79 and 60 sequences were assigned to diatoms using V9_PR2 and diat-rbcL as the reference database, respectively, and composition differed considerably (Fig. 5). Using V9_PR2, diatoms were mostly assigned on relatively high taxonomic levels (e.g., Bacillariophyta) with few taxa being differentiated sporadically in the different samples (Fig. 5A, Supplementary Material Fig. 3). Using diat-rbcL, Chaetoceros, Thalassiosira and Pseudo-nitzschia were more prominent (Fig. 5B).
Relative abundance of diatoms (genus level) in the shotgun data after aligning to (A) V9_PR2 and (B) diat-rbcL. Relative abundance of diatoms (genus level) in the amplicon data after aligning to (C) V9_PR2 and (D) diat-rbcL. The surface sample should be considered with caution in (A–D) due to the possibility of contamination (see “Methods”). Venn diagrams of shared and non-shared diatom taxa after alignment to the V9_PR2 (18S-V9) and diat-rbcL databases for the shotgun (E) and amplicon (F) data (species level, diagram areas are proportional to the total number of species included). For a complete species list and their read counts per sample see Supplementary Material Fig. 3, Supplementary Material Table 5.
In the amplicon data, 329 sequences were assigned to diatoms using V9_PR2, and 80 674 using diat-rbcL. Using V9_PR2, few taxa were detected in the two top samples (Leptocylindrus and Fragilariaceae at 1.2 mbsf, Bacillariophycidae and Bacillariaceae at 4.3 mbsf) while the lowermost samples were more diverse (Fig. 5C). Using diat-rbcL, most reads were assigned to Thalassiosira, Chaetoceros, and Pseudo-nitzschia, with other taxa sporadically occurring at different depths (Fig. 5D). For a complete species list and their read counts see Supplementary Material Fig. 3, and Supplementary Material Table 5.
We found large differences in the number of shared vs. non-shared taxa between shotgun and amplicon data, and V9_PR2 and diat-rbcL alignments (Fig. 5E, F). Database inspections showed that all taxa detected via V9_PR2 were also represented in the diat-rbcL database, except Rhizosoleniaceae. However, out of the 22 taxa exclusively detected via diat-rbcL in shotgun (Fig. 5E, F), 10 are only represented in the diat-rbcL database (Pseudo-nitzschia caciantha, P. dolorosa, Chaetoceros cf. contortus 1 SEH-2013, C. cf. lorenzianus 2 SEH-2013, C. cf. pseudobrevis 2 SEH-2013, Thalassiosirales, Thalassiosiraceae, Coscinodiscus wailesii, Arcocellulus mammifer, Meuniera membranacea, Supplementary Material Fig. 3). Similarly, out of the 134 taxa exclusively detected via diat-rbcl in amplicon, 84 were in this database only, noticeably including several species and strains of Chaetoceros, Pseudo-nitzschia, Thalassiosira and Cylindrotheca (eg., additions SHE-2013, BOF in species names), amongst others (see Supplementary Material Fig. 3, Supplementary Material Table 5).
Discussion
While previous studies have compared marine sedaDNA with microfossil records (e.g., [1, 39]), this is, to our knowledge, the first in-depth comparative analysis of shotgun and amplicon data derived from marine sedaDNA. We selected two very short gene regions (18S-V9 ~121–130 bp, diat-rbcL ~76 bp) for our amplicon approach, anticipating that they would capture a similar breadth of eukaryote and diatom diversity as our shotgun data. However, taxonomic profiles differed considerably between the two approaches and with choice of alignment database (V9_PR2, diat-rbcL).
Technical notes
Recently, protocols have been optimised for the extraction of marine eukaryote sedaDNA, achieving high yields of sedaDNA while also preserving the very small fragments typical of ancient DNA [10]. Our DNA extractions preceded these optimisations, using a protocol that may have produced a bias toward the longer spectrum expected for sedaDNA (~112 bp for eukaryotes in the shotgun data). Specifically, our protocol included DNA-binding spin columns, which have been shown to favour larger DNA fragments [40]. However, as we used the same protocol for all extractions, the comparisons between shotgun and amplicon data remain robust.
We determined a relatively high proportion of Fungi in sample 1.25 mbsf in both shotgun and amplicon data. Fungal growth can result from sub-optimal sediment core storage conditions, such as oxygen exposure [41], and it is likely that, in this sample, fungi had grown pre-extraction due to the damage of the core-section wrapping and oxygen exposure. Fungi presence in the other four samples was relatively low, indicating the extensive precautions to preserve the sediments anoxically over 7 years (bagging, flushing with nitrogen gas, adding oxygen absorbers, sealing and refrigeration) were adequate. While growth of anoxic bacteria during storage cannot be excluded [42], we would expect such growth to occur at very slow rates (as in sub-seafloor environments) with a minor impact on the here analysed eukaryote composition. Good preservation may have further contributed to our finding of relatively long sequences (~112 bp in shotgun) relative to other marine sedaDNA studies [10, 28].
Eukaryote composition in shotgun and amplicon data
Eukaryote composition differed considerably between shotgun and amplicon data. We analysed relative compositional patterns at the phylum-level, with most (23) taxa detected by both datasets. However, the relative abundance of these shared taxa varied greatly. Based on sedaDNA fragment length, heatmap and correlation analyses, we showed that this difference was associated with the read lengths that are favoured by either of the two approaches (shotgun—variable, amplicon—prescribed).
Previously [26], showed that targeted amplification of the prokaryotic 16S-V3 gene region in ancient microbiome samples led to confounded taxonomic profiles. This result is due to the doubling-up of two systematic amplification biases; firstly, as gene regions are targeted that are longer than most sequences in the DNA extracts (assessable via shotgun data), and secondly, as shorter sequences (occurring at high abundance in ancient DNA samples) overamplify while longer sequences under-amplify relative to shotgun data. We found a negative correlation between average V9_PR2 reference sequence length of our abundant eukaryotes and the A:SG read counts ratio for all samples. While these negative correlations were not significant, they were consistent with the results reported by [26] for 16S-V3, and suggest a systematic amplification bias in our 18S-V9 amplicon data. It is possible that the ‘non-significance’ in our analyses was associated with V9_PR2 reference sequences being much shorter (89–135 bp, ~45 bp range, for the abundant taxa) than 16S-V3 used by [26] (~145–215 bp, ~70 bp), providing a smaller bp range to influence correlation strength.
Read lengths were much shorter in the shotgun than in the amplicon data. This was expected, and most ancient sequences have been shown to be <100 bp [21, 22]. Amplicons define a specific DNA fragment size to be amplified, here being ~121–130 bp and 76 bp for 18S-V9 and diat-rbcL, respectively. One would assume that the closer an amplicon target gene region length is to the average DNA fragment lengths in a shotgun sample, the more similar the taxonomic profiles generated from shotgun and amplicon would be. This hypothesis was neither confirmed nor rejected by our data. Our raw filtered shotgun data (pre-alignment) provided an average sequence length of ~116 bp, which matched neither the length of 18S-V9 (~121–130 bp) nor that for diat-rbcL (76 bp). Amplicons achieved better coverage than shotgun data, which would generally be a clear advantage. However, if the compositional data is skewed due to amplification biases then this improvement is redundant. In the future, we recommend pursuing a metagenomic shotgun approach with high sequencing depth to avoid the biases, possibly coupled with hybridisation capture [43]. If a metabarcoding approach is used, exploratory shotgun analyses should precede this to determine average DNA fragment size and to guide target gene region length choices. However, the latter would not allow authentication of the ancient data in amplicons, as the DNA damage patterns underlying bioinformatic authentication assessments are read over during the amplification process [20].
Diatom composition in shotgun and amplicon data
We expected some differences in species resolution between 18S-V9 and diat-rbcL, and possibly higher resolution in diat-rbcL due to rbcL’s demonstrated capability to distinguish phytoplankton at species level [30, 31]. Comparing diatom composition post V9_PR2 and diat-rbcL alignments revealed that about 1/2 to 1/3rd of taxa was only represented in the diat-rbcL database, explaining the much higher species resolution via diat-rbcL in both shotgun and amplicon data (rather than species resolution due to the different markers per se). It also explained the overrepresentation of Chaetoceros, Pseudo-nitzschia, and Thalassiosira in diat-rbcL relative to V9_PR2 data, exacerbated in amplicon data (134 diatoms were only detected via diat-rbcL in amplicon, compared to 22 taxa in the shotgun data - noting that the shotgun diatom results are based on very few assigned reads (<80 each)). While a detailed comparison of our genetic data with existing Santa Barbara Basin diatom microfossil records exceeds the scope of this study, the shotgun data appeared to have broadly captured relative abundances of diatoms expected as per such records [8, 9, 44].
All diatoms detected via V9_PR2 (except Rhizosoleniaceae) were also represented in the diat-rbcL database. Yet, some of these diatoms (8 taxa in shotgun, 10 in amplicon) were not detected via diat-rbcL. Potential reasons why these diatoms were not detected by diat-rbcL might include the overrepresentation of nuclear (here, 18S-V9) relative to chloroplast DNA (here, diat-rbcL), for example, due to faster degradation of chloroplast DNA, as has been shown for the phytoplankton Euglena gracilis [45]. It is also possible that chloroplast DNA was low in our samples, at least for some species, as its amount depends on species-specific chloroplast-size [46]. However, very little is known about marine eukaryote sedaDNA degradation (chloroplast and nuclear) with time, sediment properties, species specificity, and this requires further research. In any case, the continued improvement of reference databases through sequence additions is crucial to generate comprehensive sedaDNA taxonomic profiles.
Extraction blank controls
We detected few contaminant taxa in the shotgun data, whereas the high number of eukaryotes and diatoms determined in the amplicon EBCs (34 and 45 taxa, respectively) was concerning. Amongst these amplicon contaminants were common modern ocean protist species often used as environmental indicators (e.g., Alexandrium—eutrophication, Dinophysis—tropicalisation [47], Chaetoceros—open ocean and upwelling conditions [48]). Diatoms identified in our diat-rbcL EBCs, including various Chaetoceros and Thalassiosira species, were also detected in controls by [33], who used the same diat-rbcL marker to investigate Fram Strait paleo-diatoms over 30,000 years. These matches even included the exact same sequences for some species (e.g., Chaetoceros cf. contortus 1 SEH-2013, Actinocyclus sp. 1 MPA-2013). This demonstrates the importance of EBC inclusion to track common contaminants and assess which species might have been identified based on PCR artefacts. We acknowledge that processing EBC’s will incur additional costs. However, it will significantly improve the interpretation of results.
Conclusion
Our comparison of paleo-eukaryote, especially diatom, composition via metabarcoding and shotgun metagenomics showed considerable differences in taxonomic profiles (including EBC profiles), which were related to differences in sequence length distributions, and influenced by the choice of reference database (18S-V9, diat-rbcL). We conclude that deep metagenomic sequencing remains the most suitable and unbiased approach to study marine eukaryote sedaDNA. If metabarcoding is the chosen technique for a given study, then this should be combined with shotgun metagenomics, at least of a few samples, to determine the bias expected from the difference in target gene region length and average length as per shotgun metagenomics.
Data availability
The raw data (shotgun and amplicon) are publicly available via the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA766251 (“Santa Barbara Basin sedaDNA”, Sep 21).
References
Lejzerowicz F, Esling P, Majewski W, Szczuciński W, Decelle J, Obadia C, et al. Ancient DNA complements microfossil record in deep-sea subsurface sediments. Biol Lett. 2013;9:20130283.
Vuillemin A, Wankel SD, Coskun ÖK, Magritsch T, Vargas S, Estes ER, et al. Archaea dominate oxic subseafloor communities over multimillion-year time scales. Sci Adv. 2019;5:1–12.
Hou W, Dong H, Li G, Yang J, Coolen MJ, Liu X, et al. Identification of photosynthetic plankton communities using sedimentary ancient DNA and their response to late-Holocene climate change on the Tibetan Plateau. Sci Rep. 2014;4:6648.
Giosan L, Orsi WD, Coolen M, Wuchter C, Dunlea AG, Thirumalai K, et al. Neoglacial climate anomalies and the Harappan metamorphosis. Clim Past. 2018;14:1669–86.
De Schepper S, Ray JL, Skaar KS, Sadatzki H, Ijaz UZ, Stein R, et al. The potential of sedimentary ancient DNA for reconstructing past sea ice evolution. ISME J. 2019;13:2566–77.
Falkowski PG, Barber R, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–7.
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: integrating terrestrial and oceanic components. Science (80-). 1998;281:237–40.
Hemphill-Haley E, Fourtanier E. A diatom record spanning 114,000 years from Site 893, Santa Barbara Basin. Proc Ocean Drill Program, 146 Part 2 Sci Results. 1995;146:233–49.
Barron JA, Bukry D, Field D. Santa Barbara Basin diatom and silicoflagellate response to global climate anomalies during the past 2200 years. Quat Int. 2010;215:34–44.
Armbrecht L, Herrando-Pérez S, Eisenhofer R, Hallegraeff GM, Bolch C, Cooper A. An optimized method for the extraction of ancient eukaryote DNA from marine sediments. Mol Ecol Resour. 2020;20:906–19.
Taberlet P, Coissac E, Pompanon F, Brochmann C, Willerslev E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol Ecol. 2012;21:2045–50.
Coolen MJL, Boere A, Abbas B, Baas M, Wakeham SG, Sinninghe Damsté JS. Ancient DNA derived from alkenone-biosynthesizing haptophytes and other algae in Holocene sediments from the Black Sea. Paleoceanography. 2006;21:1–17.
Coolen MJ, Orsi WD, Balkema C, Quince C, Harris K, Sylva SP, et al. Evolution of the plankton paleome in the Black Sea from the Deglacial to Anthropocene. Proc Natl Acad Sci U S A. 2013;110:8609–14.
More KD, Orsi WD, Galy V, Giosan L, He L, Grice K, et al. A 43 kyr record of protist communities and their response to oxygen minimum zone variability in the Northeastern Arabian Sea. Earth Planet Sci Lett. 2018;496:248–56.
Amaral-Zettler LA, McCliment EA, Ducklow HW, Huse SM. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA Genes. PLoS One. 2009;4:1–9.
De Vargas C, Audic S, Henry N, Decelle J, Mahé F, Logares R, et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015;348:6237.
Malviya S, Scalco E, Audic S, Vincent F, Veluchamy A, Poulain J, et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc Natl Acad Sci USA. 2016;113:E1516–25.
Alberti A, Poulain J, Engelen S, Labadie K, Romac S, Ferrera I, et al. Viral to metazoan marine plankton nucleotide sequences from the Tara Oceans expedition. Sci Data. 2017;4:1–20.
Guillou L, Bachar D, Audic S, Bass D, Berney C, Bittner L, et al. The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013;41:597–604.
Dabney J, Meyer M, Pääbo S. Ancient DNA damage. Cold Spring Harb Perspect Biol. 2013;5:1–8.
Pääbo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci USA. 1989;86:1939–43.
Weyrich LS, Duchene S, Soubrier J, Arriola L, Llamas B, Breen J, et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544:357–61.
Wagner A, Blackstone N, Cartwright P, Dick M, Misof B, Snow P, et al. Surveys of gene families using polymerase chain reaction: PCR selection and PCR Drift. Syst Biol. 1994;43:250–61.
Polz MF, Cavanaugh CM. Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microbiol. 1998;64:3724–30.
Webster G, Newberry CJ, Fry JC, Weightman AJ. Assessment of bacterial community structure in the deep sub-seafloor biosphere by 16S rDNA-based techniques: a cautionary tale. J Microbiol Methods. 2003;55:155–64.
Ziesemer KA, Mann AE, Sankaranarayanan K, Schroeder H, Ozga AT, Brandt BW, et al. Intrinsic challenges in ancient microbiome reconstruction using 16S rRNA gene amplification. Sci Rep. 2015;5:1–20.
Orsi WD, Coolen M, Wuchter C, He L, More KD, Irigoien X, et al. Climate oscillations reflected within the microbiome of Arabian Sea sediments. Sci Rep. 2017;7:1–12.
Armbrecht L. The potential of sedimentary ancient DNA to reconstruct past ocean ecosystems. Oceanography 2020;33:116–23.
Stoof-Leichsenring KR, Epp LS, Trauth MH, Tiedemann R. Hidden diversity in diatoms of Kenyan Lake Naivasha: A genetic approach detects temporal variation. Mol Ecol. 2012;21:1918–30.
Xu Z, Chen Y, Meng X, Wang F, Zheng Z. Phytoplankton community diversity is influenced by environmental factors in the coastal East China Sea. Eur J Phycol. 2016;51:107–18.
Pujari L, Wu C, Kan J, Li N, Wang X, Zhang G, et al. Diversity and spatial distribution of chromophytic phytoplankton in the bay of bengal revealed by RuBisCO Genes (rbcL). Front Microbiol. 2019;10:1–17.
Dulias K, Stoof-Leichsenring KR, Pestryakova LA, Herzschuh U. Sedimentary DNA versus morphology in the analysis of diatom-environment relationships. J Paleolimnol. 2017;57:51–66.
Zimmermann HH, Stoof-Leichsenring KR, Kruse S, Müller J, Stein R, Tiedemann R, et al. Changes in the composition of marine and sea-ice diatoms derived from sedimentary ancient DNA of the eastern Fram Strait over the past 30000 years. Ocean Sci. 2020;16:1017–32.
Kennett JP. Latest Quaternary Benthic Oxygen and Carbon Isotope Stratigraphy: Hole 893A, Santa Barbara Basin, California. Proceedings of the Ocean Drilling Program, Vol. 146, Part 2 Scientific Results. Kennet, JP, Baldauf, JG, Lyle, M (Eds) 1995.
Kennett J, Baldauf J, Lyle M. Proceedings of the Ocean Drilling Program, College Station, TX, 146, Part 2 Scientific Results 1995.
Schimmelmann A, Lange CB, Roark EB, Ingram BL. Resources for paleoceanographic and paleoclimatic analysis: a 6,700-year stratigraphy and regional radiocarbon reservoir-age (ΔR) record based on varve counting and 14C-AMS dating for the Santa Barbara Basin, offshore California, USA. J Sediment Res. 2006;76:74–80.
Wickham H ggplot2: Elegant graphics for data analysis. Springer Science & Business Media, New York. 2009.
Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9.
Barrenechea Angeles I, Lejzerowicz F, Cordier T, Scheplitz J, Kucera M, Ariztegui D, et al. Planktonic foraminifera eDNA signature deposited on the seafloor remains preserved after burial in marine sediments. Sci Rep. 2020;10:1–12.
Rohland N, Glocke I, Aximu-petri A, Meyer M. Extraction of highly degraded DNA from ancient bones, teeth and sediments for high-throughput sequencing. Methods Mol Biol. 2018;13:2447–61.
Masui N, Morono Y, Inagaki F. Bio-Archive core storage and subsampling procedure for subseafloor molecular biological research. Sci Drill. 2009;8:35–7.
Capo E, Giguet-Covex C, Rouillard A, Nota K, Heintzman PD, Vuillemin A, et al. Lake sedimentary dna research on past terrestrial and aquatic biodiversity: Overview and recommendations. Quaternary. 2021;4:6.
Horn S. Target enrichment via DNA hybridisation capture. In: Shapiro B, Hofreiter M (eds). Ancient DNA, Methods and Protocols. 2012. Springer, New York, Dordrecht, Heidelberg, London, pp 177-88.
Du X, Hendy I, Schimmelmann A. A 9000-year flood history for Southern California: A revised stratigraphy of varved sediments in Santa Barbara Basin. Mar Geol. 2018;397:29–42.
Manning JE, Richards OC. Synthesis and Turnover of Euglena gracilis Nuclear and Chloroplast Deoxyribonucleic Acid. Biochemistry. 1972;11:2036–43.
Bailet B, Apothéloz-Perret-Gentil L, Baričević A, Chonova T, Franc A, Frigerio JM, et al. Diatom DNA metabarcoding for ecological assessment: Comparison among bioinformatics pipelines used in six European countries reveals the need for standardization. Sci Total Environ. 2020;745:140948.
Hallegraeff GM. Harmful algal blooms: a global overview. In: Hallegraeff GM, Andersson DM, Cembella AD (eds). Manual on harmful marine microalgae. 2003. UNESCO, Paris France, pp 25–49.
Suto I, Kawamura K, Hagimoto S, Teraishi A, Tanaka Y. Changes in upwelling mechanisms drove the evolution of marine organisms. Palaeogeogr Palaeoclimatol Palaeoecol. 2012;339–341:39–51.
Acknowledgements
LA was funded by a 2018 Australian Government Endeavour Postdoctoral Research Fellowship, and supported by the Institute for Marine and Antarctic Studies, University of Tasmania, and the School of Biological Sciences, University of Adelaide. RE was funded by the ARC Centre of Excellence for Australian Biodiversity and Heritage CE170100015. CB received funding for this project from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant DIATOMIC; agreement No. 835067), as well as the French Government “Investissements d’Avenir” programs MEMO LIFE (ANR-10-LABX-54), Université de Recherche Paris Sciences et Lettres (Université PSL) (ANR-1253 11-IDEX-0001-02), and OCEANOMICS (ANR-11-BTBR-0008). We thank the science party and crew of the CalECHOES Cruise (2010), which was funded by the UC Ship Funds program. The Oregon State Coring Facility was used in this project under NSF support to RDN. We also thank Ariel Rabines, Angela Zoumplis, Alex Hangsterfer, and Eric Allen at Scripps institution of Oceanography for assistance with collecting and transporting the core sub-samples. We are grateful to Céline Bon for allowing us to perform part of this work at the Plateau Technique du MNHN, site du Musée de l’Homme (Plateforme Paléogénomique et Génétique Moléculaire, Musée de l’Homme, Paris).
Author information
Authors and Affiliations
Contributions
Designed research: LA, CB, JU, RE; Undertook fieldwork and initial core sampling/sectioning: RN, MS, ECS; Performed sedaDNA/laboratory work: LA, JU, LT, RW; Contributed data or analytical tools: LA, CB, RE, JJPK, FR, ECS; Analyzed data: LA, RE, JJPK, RW, FR; Wrote the paper: LA, CB, RE, JJPK, ECS, with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Armbrecht, L., Eisenhofer, R., Utge, J. et al. Paleo-diatom composition from Santa Barbara Basin deep-sea sediments: a comparison of 18S-V9 and diat-rbcL metabarcoding vs shotgun metagenomics. ISME COMMUN. 1, 66 (2021). https://doi.org/10.1038/s43705-021-00070-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43705-021-00070-8
This article is cited by
-
Millennial-scale variations in Arctic sea ice are recorded in sedimentary ancient DNA of the microalga Polarella glacialis
Communications Earth & Environment (2024)
-
Marine ecosystem shifts with deglacial sea-ice loss inferred from ancient DNA shotgun sequencing
Nature Communications (2023)