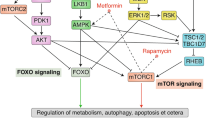
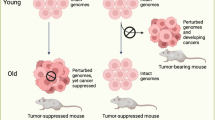
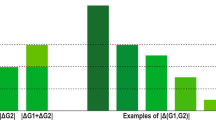
Abstract
Insight on the underlying mechanisms of aging will advance our ability to extend healthspan, treat age-related pathology and improve quality of life. Multiple genetic and pharmacological manipulations extend longevity in different species, yet monotherapy may be relatively inefficient, and we have limited data on the effect of combined interventions. Here we summarize interactions between age-related pathways and discuss strategies to simultaneously retard these in different organisms. In some cases, combined manipulations additively increase their impact on common hallmarks of aging and lifespan, suggesting they quantitatively participate within the same pathway. In other cases, interactions affect different hallmarks, suggesting their joint manipulation may independently maximize their effects on lifespan and healthy aging. While most interaction studies have been conducted with invertebrates and show varying levels of translatability, the conservation of pro-longevity pathways offers an opportunity to identify ‘druggable’ targets relevant to multiple human age-associated pathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Magalhaes, J. P. & Toussaint, O. GenAge: a genomic and proteomic network map of human ageing. FEBS Lett. 571, 243–247 (2004).
Barardo, D. G. et al. Machine learning for predicting lifespan-extending chemical compounds. Aging 9, 1721–1737 (2017).
Parkhitko, A. A., Filine, E., Mohr, S. E., Moskalev, A. & Perrimon, N. Targeting metabolic pathways for extension of lifespan and healthspan across multiple species. Ageing Res. Rev. 64, 101188 (2020).
Moskalev, A. et al. Targeting aging mechanisms: pharmacological perspectives. Trends Endocrinol. Metab. 33, 266–280 (2022).
Hoffman, J. M. et al. Effects of age, sex, and genotype on high-sensitivity metabolomic profiles in the fruit fly, Drosophila melanogaster. Aging Cell 13, 596–604 (2014).
Parkhitko, A. A., Jouandin, P., Mohr, S. E. & Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 18, e13034 (2019).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Golubev, A. G. An essay on the nominal vs. real definitions of aging. Biogerontology 22, 441–457 (2021).
Liu, Y. J., McIntyre, R. L., Janssens, G. E. & Houtkooper, R. H. Mitochondrial fission and fusion: a dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 186, 111212 (2020).
Riuzzi, F. et al. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J. Cachexia Sarcopenia Muscle 9, 1255–1268 (2018).
He, Y. et al. Cellular senescence in sarcopenia: possible mechanisms and therapeutic potential. Front. Cell Dev. Biol. 9, 793088 (2021).
Phillips, P. C. The language of gene interaction. Genetics 149, 1167–1171 (1998).
Gems, D., Pletcher, S. & Partridge, L. Interpreting interactions between treatments that slow aging. Aging Cell 1, 1–9 (2002).
Kang, J. et al. Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 6, 323 (2021).
Ferre, M., Amati-Bonneau, P., Tourmen, Y., Malthiery, Y. & Reynier, P. eOPA1: an online database for OPA1 mutations. Hum. Mutat. 25, 423–428 (2005).
Berdynski, M. et al. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 12, 103 (2022).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Mannick, J. B. & Lamming, D. W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging https://doi.org/10.1038/s43587-023-00416-y (2023).
Tatar, M., Bartke, A. & Antebi, A. The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351 (2003).
Chen, D. et al. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep. 5, 1600–1610 (2013).
Lan, J. et al. Translational regulation of non-autonomous mitochondrial stress response promotes longevity. Cell Rep. 28, 1050–1062 (2019).
Hansen, M. et al. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging cell 6, 95–110 (2007).
Greer, E. L. & Brunet, A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127 (2009).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Giannakou, M. E., Goss, M. & Partridge, L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell 7, 187–198 (2008).
Min, K. J., Yamamoto, R., Buch, S., Pankratz, M. & Tatar, M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell 7, 199–206 (2008).
Brown-Borg, H. M., Borg, K. E., Meliska, C. J. & Bartke, A. Dwarf mice and the ageing process. Nature 384, 33 (1996).
Bartke, A. et al. Extending the lifespan of long-lived mice. Nature 414, 412 (2001).
Bonkowski, M. S., Rocha, J. S., Masternak, M. M., Al Regaiey, K. A. & Bartke, A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl Acad. Sci. USA 103, 7901–7905 (2006).
Yu, D. et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for lifespan extension. Cell Rep. 29, 236–248 (2019).
Dhillon, R. S. et al. SIRT3 deficiency decreases oxidative metabolism capacity but increases lifespan in male mice under caloric restriction. Aging Cell 21, e13721 (2022).
Hofer, S. J., Davinelli, S., Bergmann, M., Scapagnini, G. & Madeo, F. Caloric restriction mimetics in nutrition and clinical trials. Front. Nutr. 8, 717343 (2021).
Madeo, F., Pietrocola, F., Eisenberg, T. & Kroemer, G. Caloric restriction mimetics: towards a molecular definition. Nat. Rev. Drug Discov. 13, 727–740 (2014).
Parkhitko, A. A., Favorova, O. O., Khabibullin, D. I., Anisimov, V. N. & Henske, E. P. Kinase mTOR: regulation and role in maintenance of cellular homeostasis, tumor development, and aging. Biochemistry 79, 88–101 (2014).
Selvarani, R., Mohammed, S. & Richardson, A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience 43, 1135–1158 (2021).
Bjedov, I. et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46 (2010).
Fok, W. C. et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 68, 108–116 (2013).
Fok, W. C. et al. Combined treatment of rapamycin and dietary restriction has a larger effect on the transcriptome and metabolome of liver. Aging Cell 13, 311–319 (2014).
Fok, W. C. et al. Short-term rapamycin treatment in mice has few effects on the transcriptome of white adipose tissue compared to dietary restriction. Mech. Ageing Dev. 140, 23–29 (2014).
Bratic, A. & Larsson, N. G. The role of mitochondria in aging. J. Clin. Investig. 123, 951–957 (2013).
Feng, J., Bussiere, F. & Hekimi, S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1, 633–644 (2001).
Dillin, A. et al. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 (2002).
Copeland, J. M. et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. 19, 1591–1598 (2009).
Yang, W. & Hekimi, S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell 9, 433–447 (2010).
Kayser, E. B., Sedensky, M. M. & Morgan, P. G. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mech. Ageing Dev. 125, 455–464 (2004).
Youle, R. J. & van der Bliek, A. M. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
Liu, Y. J. et al. Mitochondrial translation and dynamics synergistically extend lifespan in C. elegans through HLH-30. J. Cell Biol. https://doi.org/10.1083/jcb.201907067 (2020).
Navarro-Gonzalez, C. et al. Mutations in the Caenorhabditis elegans orthologs of human genes required for mitochondrial tRNA modification cause similar electron transport chain defects but different nuclear responses. PLoS Genet. 13, e1006921 (2017).
Suzuki, T. & Suzuki, T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 42, 7346–7357 (2014).
Arantes-Oliveira, N., Berman, J. R. & Kenyon, C. Healthy animals with extreme longevity. Science 302, 611 (2003).
Hsin, H. & Kenyon, C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399, 362–366 (1999).
Tu, M. P., Epstein, D. & Tatar, M. The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell 1, 75–80 (2002).
Clancy, D. J. et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (2001).
Oliver, B., Perrimon, N. & Mahowald, A. P. The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes Dev. 1, 913–923 (1987).
Sgro, C. M. & Partridge, L. A delayed wave of death from reproduction in Drosophila. Science 286, 2521–2524 (1999).
Antebi, A. Steroid regulation of C. elegans diapause, developmental timing, and longevity. Curr. Top. Dev. Biol. 105, 181–212 (2013).
Larsen, P. L., Albert, P. S. & Riddle, D. L. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139, 1567–1583 (1995).
Perez-Jimenez, M. M. et al. Steroid hormones sulfatase inactivation extends lifespan and ameliorates age-related diseases. Nat. Commun. 12, 49 (2021).
Kregel, K. C. & Zhang, H. J. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R18–R36 (2007).
Snell, T. W., Fields, A. M. & Johnston, R. K. Antioxidants can extend lifespan of Brachionus manjavacas (Rotifera), but only in a few combinations. Biogerontology 13, 261–275 (2012).
Wu, J. Z. et al. Pyrroloquinoline quinone enhances the resistance to oxidative stress and extends lifespan upon DAF-16 and SKN-1 activities in C. elegans. Exp. Gerontol. 80, 43–50 (2016).
Sasakura, H. et al. Lifespan extension by peroxidase and dual oxidase-mediated ROS signaling through pyrroloquinoline quinone in C. elegans. J. Cell Sci. 130, 2631–2643 (2017).
Schriner, S. E. et al. Decreased mitochondrial superoxide levels and enhanced protection against paraquat in Drosophila melanogaster supplemented with Rhodiola rosea. Free Radic. Res 43, 836–843 (2009).
Schriner, S. E., Avanesian, A., Liu, Y., Luesch, H. & Jafari, M. Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defenses. Free Radic. Biol. Med. 47, 577–584 (2009).
Bayliak, M. M. & Lushchak, V. I. The golden root, Rhodiola rosea, prolongs lifespan but decreases oxidative stress resistance in yeast Saccharomyces cerevisiae. Phytomedicine 18, 1262–1268 (2011).
Wiegant, F. A. et al. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 10, 27–42 (2009).
Schriner, S. E. et al. Extension of Drosophila lifespan by Rhodiola rosea through a mechanism independent from dietary restriction. PLoS ONE 8, e63886 (2013).
Van Raamsdonk, J. M. & Hekimi, S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 5, e1000361 (2009).
Yen, K. & Mobbs, C. V. Evidence for only two independent pathways for decreasing senescence in Caenorhabditis elegans. Age 32, 39–49 (2010).
Sagi, D. & Kim, S. K. An engineering approach to extending lifespan in C. elegans. PLoS Genet. 8, e1002780 (2012).
Sagi, D. The addition of a developmental factor, unc-62, to already long-lived worms increases lifespan and healthspan. Biol. Open 6, 1796–1801 (2017).
Hou, L. et al. A systems approach to reverse engineer lifespan extension by dietary restriction. Cell Metab. 23, 529–540 (2016).
Davidsohn, N. et al. A single combination gene therapy treats multiple age-related diseases. Proc. Natl Acad. Sci. USA 116, 23505–23511 (2019).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Brooks, W. W. & Conrad, C. H. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J. Mol. Cell. Cardiol. 32, 187–195 (2000).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Kulkarni, A. S., Gubbi, S. & Barzilai, N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. https://doi.org/10.1016/j.cmet.2020.04.001 (2020).
Anisimov, V. N. et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp. Gerontol. 40, 685–693 (2005).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 (2013).
Strong, R. et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884 (2016).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Harrison, D. E. et al. Acarbose improves health and lifespan in aging HET3 mice. Aging cell 18, e12898 (2019).
Strong, R. et al. Lifespan benefits for the combination of rapamycin plus acarbose and for captopril in genetically heterogeneous mice. Aging Cell 21, e13724 (2022).
Jiang, Z. et al. Short term treatment with a cocktail of rapamycin, acarbose and phenylbutyrate delays aging phenotypes in mice. Sci. Rep. 12, 7300 (2022).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Lamming, D. W. et al. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell 12, 712–718 (2013).
Weiss, R., Fernandez, E., Liu, Y., Strong, R. & Salmon, A. B. Metformin reduces glucose intolerance caused by rapamycin treatment in genetically heterogeneous female mice. Aging 10, 386–401 (2018).
Spindler, S. R., Mote, P. L. & Flegal, J. M. Combined statin and angiotensin-converting enzyme (ACE) inhibitor treatment increases the lifespan of long-lived F1 male mice. Age 38, 379–391 (2016).
Evason, K., Huang, C., Yamben, I., Covey, D. F. & Kornfeld, K. Anticonvulsant medications extend worm life-span. Science 307, 258–262 (2005).
Evason, K., Collins, J. J., Huang, C., Hughes, S. & Kornfeld, K. Valproic acid extends Caenorhabditis elegans lifespan. Aging cell 7, 305–317 (2008).
Admasu, T. D. et al. Drug synergy slows aging and improves healthspan through igf and srebp lipid signaling. Dev. Cell 47, 67–79 (2018).
Castillo-Quan, J. I. et al. Lithium promotes longevity through GSK3/NRF2-dependent hormesis. Cell Rep. 15, 638–650 (2016).
Slack, C. et al. The Ras-Erk-ETS-signaling pathway is a drug target for longevity. Cell 162, 72–83 (2015).
Castillo-Quan, J. I. et al. A triple drug combination targeting components of the nutrient-sensing network maximizes longevity. Proc. Natl Acad. Sci USA 116, 20817–20819 (2019).
Danilov, A. et al. Selective anticancer agents suppress aging in Drosophila. Oncotarget 4, 1507–1526 (2013).
Bustos, V. & Partridge, L. Good ol’ fat: links between lipid signaling and longevity. Trends Biochem. Sci. 42, 812–823 (2017).
Johnson, A. A. & Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 18, e13048 (2019).
Hou, N. S. & Taubert, S. Function and regulation of lipid biology in Caenorhabditis elegans aging. Front Physiol. 3, 143 (2012).
Huang, X. et al. Reducing signs of aging and increasing lifespan by drug synergy. Aging Cell 12, 652–660 (2013).
Huang, X., Leggas, M. & Dickson, R. C. Drug synergy drives conserved pathways to increase fission yeast lifespan. PLoS ONE 10, e0121877 (2015).
Hubbard, B. P. & Sinclair, D. A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 35, 146–154 (2014).
Mercken, E. M. et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 13, 787–796 (2014).
Mitchell, S. J. et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 6, 836–843 (2014).
Minor, R. K. et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 1, 70 (2011).
Palliyaguru, D. L. et al. Combining a high dose of metformin with the SIRT1 activator, SRT1720, reduces life span in aged mice fed a high-fat diet. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 75, 2037–2041 (2020).
Shen, Z., Hinson, A., Miller, R. A. & Garcia, G. G. Cap-independent translation: a shared mechanism for lifespan extension by rapamycin, acarbose, and 17α-estradiol. Aging Cell 20, e13345 (2021).
Tiku, V. et al. Small nucleoli are a cellular hallmark of longevity. Nat. Commun. 8, 16083 (2017).
Annibal, A. et al. Regulation of the one carbon folate cycle as a shared metabolic signature of longevity. Nat. Commun. 12, 3486 (2021).
Qiao, H. H. et al. An efficient and multiple target transgenic RNAi technique with low toxicity in Drosophila. Nat. Commun. 9, 4160 (2018).
Port, F. & Bullock, S. L. Augmenting CRISPR applications in Drosophila with tRNA-flanked sgRNAs. Nat. Methods 13, 852–854 (2016).
Parkhitko, A. A. et al. Cross-species identification of PIP5K1-, splicing- and ubiquitin-related pathways as potential targets for RB1-deficient cells. PLoS Genet. 17, e1009354 (2021).
Norris, A. D., Gracida, X. & Calarco, J. A. CRISPR-mediated genetic interaction profiling identifies RNA binding proteins controlling metazoan fitness. eLife https://doi.org/10.7554/eLife.28129 (2017).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, 3156 (2013).
Fahy, G. M. et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 18, e13028 (2019).
Kaeberlein, T. L. et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5, 487–494 (2006).
Hoffmann, M. et al. MICS-1 interacts with mitochondrial ATAD-3 and modulates lifespan in C. elegans. Exp. Gerontol. 47, 270–275 (2012).
Zimmerman, S. M., Hinkson, I. V., Elias, J. E. & Kim, S. K. Reproductive aging drives protein accumulation in the uterus and limits lifespan in C. elegans. PLoS Genet. 11, e1005725 (2015).
Jia, K., Albert, P. S. & Riddle, D. L. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 129, 221–231 (2002).
Snell, T. W., Johnston, R. K., Rabeneck, B., Zipperer, C. & Teat, S. Joint inhibition of TOR and JNK pathways interacts to extend the lifespan of Brachionus manjavacas (Rotifera). Exp. Gerontol. 52, 55–69 (2014).
Roux, A. E., Quissac, A., Chartrand, P., Ferbeyre, G. & Rokeach, L. A. Regulation of chronological aging in Schizosaccharomyces pombe by the protein kinases Pka1 and Sck2. Aging Cell 5, 345–357 (2006).
Shaposhnikov, M. V. et al. Molecular mechanisms of exceptional lifespan increase of Drosophila melanogaster with different genotypes after combinations of pro-longevity interventions. Commun. Biol. 5, 566 (2022).
Acknowledgements
This work was supported by the National Institute of General Medical Sciences R35 GM146869 (to A.A.P.), NIA R00 AG057792 (to A.A.P.), NIA R03 AG075651 (to A.A.P.), NIA P30 AG024827 pilot grant (to A.A.P.), Richard King Mellon Foundation award (to A.A.P.), NIA R01 AG059563 (to M.T.) and NIA R01 AG069639 (to M.T.).
Author information
Authors and Affiliations
Contributions
A.A.P., E.F. and M.T. wrote the manuscript and prepared the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parkhitko, A.A., Filine, E. & Tatar, M. Combinatorial interventions in aging. Nat Aging 3, 1187–1200 (2023). https://doi.org/10.1038/s43587-023-00489-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-023-00489-9