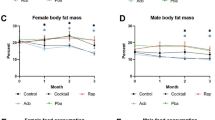
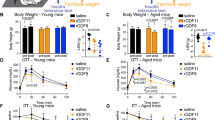
Abstract
Diminished insulin and insulin-like growth factor-1 signaling extends the lifespan of invertebrates1,2,3,4; however, whether it is a feasible longevity target in mammals is less clear5,6,7,8,9,10,11,12. Clinically utilized therapeutics that target this pathway, such as small-molecule inhibitors of phosphoinositide 3-kinase p110α (PI3Ki), provide a translatable approach to studying the impact of these pathways on aging. Here, we provide evidence that dietary supplementation with the PI3Ki alpelisib from middle age extends the median and maximal lifespan of mice, an effect that was more pronounced in females. While long-term PI3Ki treatment was well tolerated and led to greater strength and balance, negative impacts on common human aging markers, including reductions in bone mass and mild hyperglycemia, were also evident. These results suggest that while pharmacological suppression of insulin receptor (IR)/insulin-like growth factor receptor (IGFR) targets could represent a promising approach to delaying some aspects of aging, caution should be taken in translation to humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Source data for all figures and extended data are provided with this manuscript.
References
Friedman, D. B. & Johnson, T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118, 75–86 (1988).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Clancy, D. J. et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292, 104–106 (2001).
Tatar, M. et al. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110 (2001).
Accili, D. et al. Early neonatal death in mice homozygois for a null allele of the insulin receptor gene. Nat. Genet. 12, 106–109 (1996).
Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J. & Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 (1993).
Holzenberger, M. et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421, 182–187 (2003).
Merry, T. L. et al. Impairment of insulin signalling in peripheral tissue fails to extend murine lifespan. Aging Cell 16, 761–772 (2017).
Bokov, A. F. et al. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS ONE 6, e26891 (2011).
Ashpole, N. M. et al. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience 39, 129–145 (2017).
Mao, K. et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 9, 2394 (2018).
Xu, J. et al. Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell 13, 19–28 (2014).
Blüher, M., Kahn, B. B. & Kahn, C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574 (2003).
Softic, S. et al. Lipodystrophy due to adipose tissue-specific insulin receptor knockout results in progressive NAFLD. Diabetes 65, 2187–2200 (2016).
Friesen, M., Hudak, C. S., Warren, C. R., Xia, F. & Cowan, C. A. Adipocyte insulin receptor activity maintains adipose tissue mass and lifespan. Biochem. Biophys. Res. Commun. 476, 487–492 (2016).
Selman, C. et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 22, 807–818 (2008).
Taguchi, A., Wartschow, L. M. & White, M. F. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317, 369–372 (2007).
Selman, C., Lingard, S., Gems, D., Partridge, L. & Withers, D. J. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis.” Science 320, 1012 (2008).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Foukas, L. C. et al. Long-term p110alpha PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol. Med. 5, 563–571 (2013).
Ortega-Molina, A. et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab 15, 382–394 (2012).
Nojima, A. et al. Haploinsufficiency of Akt1 prolongs the lifespan of mice. PLoS ONE 8, e69178 (2013).
Furet, P. et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 23, 3741–3748 (2013).
Venot, Q. et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 558, 540–546 (2018).
Hedges, C. P. et al. Efficacy of providing the PI3K p110alpha inhibitor BYL719 (Alpelisib) to middle-aged mice in their diet. Biomolecules 11, 150 (2021).
Hedges, C. P. et al. Prolonged treatment with a PI3K p110alpha inhibitor causes sex- and tissue-dependent changes in antioxidant content, but does not affect mitochondrial function. Biosci. Rep. 40, BSR20201128 (2020).
Palliyaguru, D. L. et al. Fasting blood glucose as a predictor of mortality: lost in translation. Cell Metab. 33, 2189–2200 (2021).
Xu, J., Wan, C. S., Ktoris, K., Reijnierse, E. M. & Maier, A. B. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology 68, 361–376 (2022).
Smith, G. C. et al. Extended treatment with selective phosphatidylinositol 3-kinase and mTOR inhibitors has effects on metabolism, growth, behaviour and bone strength. FEBS J. 280, 5337–5349 (2013).
Krentz, A. J., Viljoen, A. & Sinclair, A. Insulin resistance: a risk marker for disease and disability in the older person. Diabet. Med. 30, 535–548 (2013).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 (2013).
Miller, R. A. et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight 5, e140019 (2020).
Liang, H. et al. Genetic mouse models of extended lifespan. Exp. Gerontol. 38, 1353–1364 (2003).
Tran, D. et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell 13, 669–678 (2014).
Schulz, T. J. et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293 (2007).
Gems, D. & Partridge, L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu. Rev. Physiol. 75, 621–644 (2013).
Ortega-Molina, A. et al. Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys. Cell Metab. 21, 558–570 (2015).
Lopez-Guadamillas, E. et al. PI3Kalpha inhibition reduces obesity in mice. Aging (Albany NY) 8, 2747–2753 (2016).
Araiz, C. et al. Enhanced beta-adrenergic signalling underlies an age-dependent beneficial metabolic effect of PI3K p110alpha inactivation in adipose tissue. Nat. Commun. 10, 1546 (2019).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Katic, M. et al. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell 6, 827–839 (2007).
Sun, N., Youle, R. J. & Finkel, T. The mitochondrial basis of aging. Mol Cell 61, 654–666 (2016).
Weimer, S. et al. D-glucosamine supplementation extends life span of nematodes and of ageing mice. Nat. Commun. 5, 3563 (2014).
Harrison, D. E. et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282 (2014).
Partridge, L., Fuentealba, M. & Kennedy, B. K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 19, 513–532 (2020).
Harrison, D. E. et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell 18, e12898 (2019).
Miller, R. A. et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477 (2014).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Correia-Melo, C. et al. Rapamycin improves healthspan but not inflammaging in nfkappab1(−/−) mice. Aging Cell 18, e12882 (2019).
Wang, R. et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16, 564–574 (2017).
Pak, H. H. et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat Metab. 3, 1327–1341 (2021).
Balasubramanian, P., Howell, P. R. & Anderson, R. M. Aging and caloric restriction research: a biological perspective with translational potential. EBioMedicine 21, 37–44 (2017).
Held, N. M. et al. Aging selectively dampens oscillation of lipid abundance in white and brown adipose tissue. Sci. Rep. 11, 5932 (2021).
Gohlke, S. et al. Identification of functional lipid metabolism biomarkers of brown adipose tissue aging. Mol. Metab. 24, 1–17 (2019).
Oliverio, M. et al. Dicer1-miR-328-Bace1 signalling controls brown adipose tissue differentiation and function. Nat. Cell Biol. 18, 328–336 (2016).
Darcy, J. & Tseng, Y. H. ComBATing aging-does increased brown adipose tissue activity confer longevity? Geroscience 41, 285–296 (2019).
Li, Y., Knapp, J. R. & Kopchick, J. J. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp. Biol. Med. (Maywood) 228, 207–215 (2003).
Vatner, D. E. et al. Enhanced longevity and metabolism by brown adipose tissue with disruption of the regulator of G protein signaling 14. Aging Cell 17, e12751 (2018).
Abdellatif, M. et al. Fine-tuning cardiac insulin-like growth factor 1 receptor signaling to promote health and longevity. Circulation 145, 1853–1866 (2022).
Naot, D. et al. Reduced bone density and cortical bone indices in female adiponectin-knockout mice. Endocrinology 157, 3550–3561 (2016).
Bouxsein, M. L. et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 (2010).
Masson, S. W. C., Sorrenson, B., Shepherd, P. R. & Merry, T. Beta-catenin regulates muscle glucose transport via actin remodelling and M-cadherin binding. Mol. Metab. 42, 101091 (2020).
Santos, J. H., Meyer, J. N., Mandavilli, B. S. & Van Houten, B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol. 314, 183–199 (2006).
Furda, A. M., Bess, A. S., Meyer, J. N. & Van Houten, B. Analysis of DNA damage and repair in nuclear and mitochondrial DNA of animal cells using quantitative PCR. Methods Mol. Biol. 920, 111–132 (2012).
Passonneau, J. V. & Lauderdale, V. R. A comparison of three methods of glycogen measurement in tissues. Anal. Biochem. 60, 405–412 (1974).
Stenkula, K. G. & Erlanson-Albertsson, C. Adipose cell size: importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R284–R295 (2018).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Acknowledgements
We acknowledge B. Smith, G. Sherriff, R. Broadhurst, E. Barnaby (AgResearch), and the Vernon Jenson Unit (University of Auckland) for animal care. We also thank L. Ferrer for her assistance with cytokine assays and J. Dent for assisting with mouse phenotyping. This study was funded by the Health Research Council of New Zealand (17/099) and support from the Maurice Wilkins Centre. C.P.H is supported by a Maurice Paykel Postdoctoral Fellowship, and T.L.M. is supported by a Rutherford Discovery Fellowship. Figure illustrations in Figures 1 a,e,f,g and 2 g,k; and extended data Figures 1 a,e,f,g; 3 a,b,c,d; 4 a,b,c,d; and 5 were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
C.P.H., B.S., S.C.B., C. MacRae, P.K., E.J.B., C. MacIndoe, T.T., B.G.M., S.S., M.A., J.K.J. and T.L.M. executed experiments. C.P.H., J.B., K.M.M., A.J.R.H., P.R.S. and T.L.M. contributed to the study design. T.L.M. wrote the manuscript, and all authors provided edits to the manuscript and assisted with interpretation of results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Adolfo Saiardi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effect of PI3K inhibitor (PI3Ki) on body weight and cognitive and heart function in aging mice.
a, Plasma and liver Alpelisib at 20 months of age. b, Body weights of mice that were alive for the entire first 6 months of treatment. c, Food intake at 15 months. d, Body weights during the last four months before death. e, Relative time spent with the novel object during a novel object test at 18 and 30 months, f, and time spent in the inner and outer zones during an open field test at 18 months. g, Echocardiograph measured cardiac output, end diastolic volume, left ventricular mass and ejection fraction in 15-month-old males. Results are mean ± SE with biological replicates (individual mice, n) shown as individual data points or stated within figure. Significance was determined by RM two-way ANOVA (a) two-way ANOVA (b, d, e), linear regression (c) or unpaired two-sided student t-test (f). P-values within figures for PI3Ki vs vehicle of the same sex.
Extended Data Fig. 2 Effect of PI3K inhibitor (PI3Ki) on glucose homeostasis and insulin signalling with aging.
a, Plasma IGF1 at 20 months. b, Glucose tolerance test at 30 months of age. c, Plasma Fructosamine at 20 months. d, Blood glucose response to 3 hours of ad libitum access to food (chow diet) following an overnight fast at 20 months. Results are mean ± SE with biological replicates (individual mice, n) shown as individual data points or stated within figure. Significance was determined by two-way ANOVA (a-d) or RM two-way ANOVA (b, d) with Sidak post-hoc analysis for within sex effects when a significant sex x treatment interaction was seen. P-values within figures for PI3Ki vs vehicle of the same sex.
Extended Data Fig. 3 Effect of PI3Ki on insulin signaling.
Western blot images and quantification of insulin signalling intermediates in the, a, liver, b, muscle (gastrocnemius), c, white adipose tissue (WAT) and, d, brown adipose tissue (BAT) of 20-month-old mice. All samples blotted (n = 6 per group) are shown except for liver p-AktSer473 where blots are representative of n = 12 per group. Each well represents an independent mouse. Results are mean ± SE with biological replicates (individual mice, n) shown as individual data points. Significance was determined by an unpaired student two-sided t-test. P-values within figures for PI3Ki vs vehicle of the same sex.
Extended Data Fig. 4 Effect of PI3Ki on AktThr308, autophagy, AMPK and FOXO1 signalling.
Western blot images and quantification of, a, white adipose tissue (WAT) AktThr308 phosphorylation (n = 6 per group), b, liver AMPKTyr172 and FOXO1Ser256, c-d, liver and WAT autophagy markers of 20-month-old mice. All samples blotted are shown. Results are mean ± SE with biological replicates (sample size, n) shown as individual data points. Significance was determined by an unpaired two-sided student t-test. P-values within figures for PI3Ki vs vehicle of the same sex.
Extended Data Fig. 5 Liver mRNA micro-array.
Genes in the liver that expression was significantly altered by PI3Ki treatment at 20 months (by mRNA micro-array, n = 3 per group pooled from 2–3 independent mice).
Extended Data Fig. 6 Effect of PI3K inhibitor (PI3Ki) on white adipose tissue (WAT) during aging.
a, Plasma leptin at 20 months. b-c, Subcutaneous (s)WAT and visceral (v)WAT area under adipocyte diameter curve (AUC; Fig. 3c). d, Male sWAT genes that expression was significantly altered by PI3Ki at 20 months of age (mRNA by micro-array, n = 4 per group pooled from 2–3 independent mice) and, e, associated top 10 gene ontology biological processes. f, Female sWAT genes that expression was significantly altered by PI3Ki at 20 months of age (mRNA by micro-array, n = 3 per group pooled from 2–3 independent mice) and, g, associated top 10 gene ontology biological processes. i, Significantly altered genes that overlap in male and female sWAT. Results are mean ± SE with biological replicates (sample size, n) shown as individual data points or stated within figure. Significance was determined by two-way ANOVA with sidak post-hoc analysis for within sex effects when a significant sex x treatment interaction was seen (a-c). P-values within figures for PI3Ki vs vehicle of the same sex.
Extended Data Fig. 7 Effect of PI3K inhibitor (PI3Ki) on brown adipose tissue (BAT) during aging.
a, Male and, b, female BAT genes that expression was significantly altered by PI3Ki at 20 months of age (mRNA by micro-array, n = 3 per group pooled from 2–3 independent mice). c, Male and female plasma triglycerides, d, male isolated subcutaneous (s)WAT and visceral (v)WAT de novo lipogenesis at 20 months of age. e, Female Respiratory exchange ratio (RER) from 6 month-old-mice, and, f, mitochondrial oxygen consumption capacity of BAT from 20-month-old male mice. Results are mean ± SE with biological replicates (individual mice, n) shown as individual data points or stated within figure. Significance was determined by unpaired student t-test (c-d), mixed linear model (e) RM two-way ANOVA (f).
Extended Data Fig. 8
Study design.
Extended Data Fig. 9 Extended method details.
a) Details of antibodies used in immunoblotting, b) Gene targets and primer pairs for LA-qPCR, c) Thermal cycler conditions for amplification of nuclear and mitochondrial fragments.
Supplementary information
Source data
Source Data Fig. 1
Figure1_source_data.
Source Data Fig. 2
Figure2_source_data.
Source Data Fig. 3
Figure3_source_data.
Source Data Microarrays
Source data for all figures that include microarray data: Fig. 3d,f,g,h and Extended Data Figs. 5, 6d–h and 7a,b.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source data for Extended Data Fig. 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hedges, C.P., Shetty, B., Broome, S.C. et al. Dietary supplementation of clinically utilized PI3K p110α inhibitor extends the lifespan of male and female mice. Nat Aging 3, 162–172 (2023). https://doi.org/10.1038/s43587-022-00349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-022-00349-y
This article is cited by
-
Hallmarks of cardiovascular ageing
Nature Reviews Cardiology (2023)