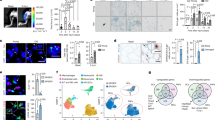
Abstract
Heterochronic blood exchange (HBE) has demonstrated that circulating factors restore youthful features to aged tissues. However, the systemic mediators of those rejuvenating effects remain poorly defined. We show here that the beneficial effect of young blood on aged muscle regeneration was diminished when serum was depleted of extracellular vesicles (EVs). Whereas EVs from young animals rejuvenate aged cell bioenergetics and skeletal muscle regeneration, aging shifts EV subpopulation heterogeneity and compromises downstream benefits on recipient cells. Machine learning classifiers revealed that aging shifts the nucleic acid, but not protein, fingerprint of circulating EVs. Alterations in subpopulation heterogeneity were accompanied by declines in transcript levels of the prolongevity protein α-Klotho (Klotho), and injection of EVs improved muscle regeneration in a Klotho mRNA-dependent manner. These studies demonstrate that EVs play a key role in the rejuvenating effects of HBE and that Klotho transcripts within EVs phenocopy the effects of young serum on aged skeletal muscle.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw data that support the experimental findings are included as Supplementary Information. The image files used for computational analysis are available at https://github.com/ankitbhatia/bioimage_aging. RNA-sequencing data have been deposited to the NCBI Gene Expression Omnibus database with the accession number GSE176478.
Code availability
The code used to perform machine learning-based analyses of EVs is available at https://github.com/ankitbhatia/bioimage_aging. For PC analyses, the OriginLab plugin ‘Principal Component Analysis for Spectroscopy’ was used. The code for RNA-sequencing analysis is available at https://github.com/sruthi-hub/Aging_EV/tree/main.
References
Conboy, M. J., Conboy, I. M. & Rando, T. A. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 12, 525–530 (2013).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Sousa-Victor, P. et al. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage. Nat. Metab. 1, 276–290 (2019).
Li, L. et al. Impairment of chondrocyte proliferation after exposure of young murine cartilage to an aged systemic environment in a heterochronic parabiosis model. Swiss Med. Wkly. 148, w14607 (2018).
Gontier, G. et al. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 22, 1974–1981 (2018).
Pathan, M. et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 47, D516–D519 (2019).
Shah, R., Patel, T. & Freedman, J. E. Circulating extracellular vesicles in human disease. N. Engl. J. Med. 379, 2180–2181 (2018).
Chen, W. W. et al. BEAMing and DropletDigital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol. Ther. Nucleic Acids 2, e109 (2013).
Yang, J., Wei, F., Schafer, C. & Wong, D. T. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS ONE 9, e110641 (2014).
Salih, M., Zietse, R. & Hoorn, E. J. Urinary extracellular vesicles and the kidney: biomarkers and beyond. Am. J. Physiol. Renal Physiol. 306, F1251–F1259 (2014).
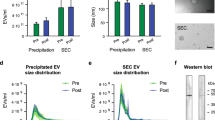
Monguio-Tortajada, M. et al. Extracellular-vesicle isolation from different biological fluids by size-exclusion chromatography. Curr. Protoc. Stem Cell Biol. 49, e82 (2019).
Arraud, N. et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 12, 614–627 (2014).
Revenfeld, A. L. et al. Diagnostic and prognostic potential of extracellular vesicles in peripheral blood. Clin. Ther. 36, 830–846 (2014).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Robbins, P. D. Extracellular vesicles and aging. Stem Cell Investig. 4, 98 (2017).
Alibhai, F. J. et al. Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell 19, e13103 (2020).
Yoshida, M. et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342 (2019).
Picca, A. et al. Mitochondrial dysfunction and aging: insights from the analysis of extracellular vesicles. Int. J. Mol. Sci. 20, 805 (2019).
Dubal, D. B. et al. Life extension factor Klotho enhances cognition. Cell Rep. 7, 1065–1076 (2014).
Sahu, A. et al. Age-related declines in alpha-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859 (2018).
Ahrens, H. E., Huettemeister, J., Schmidt, M., Kaether, C. & von Maltzahn, J. Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skelet Muscle 8, 20 (2018).
Tapscott, S. J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 132, 2685–2695 (2005).
Zammit, P. S. et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 119, 1824–1832 (2006).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016).
Pala, F. et al. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci. 131, jcs212977 (2018).
Paradies, G., Paradies, V., De Benedictis, V., Ruggiero, F. M. & Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 1837, 408–417 (2014).
Cecchini, G. Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 72, 77–109 (2003).
Gamez-Valero, A. et al. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci. Rep. 6, 33641 (2016).
Andreu, Z. & Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 5, 442 (2014).
Moon, S. Enrichment of exosome-like extracellular vesicles from plasma suitable for clinical vesicular miRNA biomarker research. J. Clin. Med. 8, 1995 (2019).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
Mastoridis, S. et al. Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front. Immunol. 9, 1583 (2018).
Gualerzi, A. et al. Raman spectroscopy uncovers biochemical tissue-related features of extracellular vesicles from mesenchymal stromal cells. Sci. Rep. 7, 9820 (2017).
Movasaghi Z., Rehman S., & Rehman I.U., Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 42, 493–541 (2007).
de Cavanagh, E. M. V., Inserra, F., & Ferder, L., Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am. J. Physiol. Heart Circ. Physiol. 309, H15–H44 (2015).
Murphy, E. & Eisner, D. A. Regulation of intracellular and mitochondrial sodium in health and disease. Circ. Res. 104, 292–303 (2009).
Brookes, P. S., Yoon, Y., Robotham, J. L., Anders, M. W. & Sheu, S. S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287, C817–C833 (2004).
Garlid, K. D. & Paucek, P. Mitochondrial potassium transport: the K+ cycle. Biochim. Biophys. Acta 1606, 23–41 (2003).
Garth, J. et al. The effects of the anti-aging protein Klotho on mucociliary clearance. Front. Med. 6, 339 (2019).
Tang, G., Shen, Y., Gao, P., Song, S. S. & Si, L. Y. Klotho attenuates isoproterenol-induced hypertrophic response in H9C2 cells by activating Na(+)/K(+)-ATPase and inhibiting the reverse mode of Na+/Ca2+-exchanger. In Vitro Cell Dev. Biol. Anim. 54, 250–256 (2018).
Shumilina, E. et al. Altered regulation of cytosolic Ca2+ concentration in dendritic cells from klotho hypomorphic mice. Am. J. Physiol. Cell Physiol. 305, C70–C77 (2013).
Strutz-Seebohm, N., Wrobel, E., Schulze-Bahr, E. & Seebohm, G. Klotho: a new trafficking modifier of Kv7.1/KCNE1 channels. Channels 8, 285 (2014).
Ohnishi, M. & Razzaque, M. S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 24, 3562–3571 (2010).
Wehling-Henricks, M. et al. Klotho gene silencing promotes pathology in the mdx mouse model of Duchenne muscular dystrophy. Hum. Mol. Genet. 25, 2465–2482 (2016).
Cheikhi, A. et al. Klotho: an elephant in aging research. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1031–1042 (2019).
Gonzales, P. A. et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 20, 363–379 (2009).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Zomer, A. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015).
de la Cuesta, F. et al. Extracellular vesicle cross-talk between pulmonary artery smooth muscle cells and endothelium during excessive TGF-beta signalling: implications for PAH vascular remodelling. Cell Commun. Signal. 17, 143 (2019).
Kuro-o, M. et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Li, S. et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 46, D106–D112 (2018).
Egerman, M. A. et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 22, 164–174 (2015).
Choi, J. S. et al. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control. Release 222, 107–115 (2016).
Eldh, M. et al. Exosomes communicate protective messages during oxidative stress: possible role of exosomal shuttle RNA. PLoS ONE 5, e15353 (2010).
Fry, C. S., Kirby, T. J., Kosmac, K., McCarthy, J. J. & Peterson, C. A. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20, 56–69 (2017).
Villarroya-Beltri, C. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 (2013).
de Moura, M. B. & Van Houten, B. Bioenergetic analysis of intact mammalian cells using the Seahorse XF24 Extracellular Flux analyzer and a luciferase ATP assay. Methods Mol. Biol. 1105, 589–602 (2014).
Zhang, C. et al. Arsenic promotes NF-kappaB-mediated fibroblast dysfunction and matrix remodeling to impair muscle stem cell function. Stem Cells 34, 732–742 (2016).
Acknowledgements
These studies were supported by NIA grants R01AG052978 (F.A.), R01AG061005 (F.A.), R01AG066198-01 (F.A. and R.K.) and R33 ES025606-05 (B.V.H.) and UPMC Enterprises (F.A.). We thank the flow cytometry core at the Department of Immunology, University of Pittsburgh for providing resources and expertise to perform ImageStream analysis (National Institutes of Health grant 1S10OD019942-01), as well as the Center of Biologic Imaging, University of Pittsburgh for providing resources to perform confocal imaging (National Institutes of Health grant 1S10OD019973). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
F.A. and A.S. provided the concept, idea and experimental design for the studies. F.A. and A.S. wrote the manuscript. A.S., Z.J.C., S.S., S.N.S. and A.P. collected, analyzed and interpreted data and reviewed the manuscript. A. Bhatia provided computer vision- and machine learning-based analyses. S.P., C.C., and A.G. collected and analyzed data. M.B. interpreted data and reviewed the manuscript. B.V.H. analyzed and interpreted data and reviewed that manuscript. A. Barchowsky provided consultation for data interpretation and review of the manuscript. M.L. provided support for statistical analyses. N.F., I.L., and R.K. provided support for data interpretation. F.A. provided funding for the studies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Aging thanks Dan Lark, Tim Gavin, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Depletion of EVs eliminates the effect of young serum on Pax7 expression of muscle progenitors.
Quantification of Pax7 in aged MPCs treated with aged serum, young serum or EV-depleted aged or young serum. Scale bars, 50 µm (****P < 0.0001, two-tailed Mann–Whitney test comparing depleted young serum and young serum treatments). Data are presented as mean + s.e.m. Data from different cohorts or experimental groups performed on different days are presented within the same graph as black or red circles.
Extended Data Fig. 2 The ability of EVs to modulate target cell Klotho and MyoD protein levels is dependent on Klotho mRNAs.
a, Imaging and quantification of Klotho protein in aged MPCs following culture in the presence of young or aged EVs for 24 h. Scale bars, 50 µm (n = 6 wells per group performed over two independent experiments, **P < 0.01, two-tailed Welch’s t test). b, Representative violin plot of Klotho protein intensity per EV from young and aged serum, using imaging flow cytometry (n = 11,229–11,685 EVs per group for this experimental run. EVs pooled from four young and four aged serum samples, P > 0.05, two-tailed Mann–Whitney test, experiment repeated in triplicate). Violin plot minima, maxima, median, 25th percentile and 75th percentile are 0, 272915.9, 0, 0 and 26.36 (young serum) and 0, 272241.3, 0, 0, and 23.2 (aged serum). c,d, Quantification of MyoD-positive (%), desmin-positive (%) and ki67-positive (%) aged MPCs receiving young serum EVs treated with scramble or siRNA to Klotho (c) or aged serum EVs or aged serum EVs loaded with synthetic Klotho mRNA (d) (MyoD and desmin (scramble, siRNA) or desmin (aged EVs, synthetic Klotho): **P < 0.01, ***P < 0.001 and ****P < 0.0001, two-tailed t test with Welch’s correction, n = 5–6 wells per group; MyoD (aged EVs and synthetic Klotho): **P < 0.01, two-tailed Mann–Whitney test, n = 5 wells and group; ki67 (scramble, siRNA and aged EVs and synthetic Klotho): P > 0.05 (P = 0.1), two-tailed Mann–Whitney test).
Supplementary information
Source data
Rights and permissions
About this article
Cite this article
Sahu, A., Clemens, Z.J., Shinde, S.N. et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat Aging 1, 1148–1161 (2021). https://doi.org/10.1038/s43587-021-00143-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00143-2
This article is cited by
-
Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism
Nature Aging (2024)
-
A Mesenchymal stem cell Aging Framework, from Mechanisms to Strategies
Stem Cell Reviews and Reports (2024)
-
Extracellular Vesicles and Exosomes in the Control of the Musculoskeletal Health
Current Osteoporosis Reports (2024)
-
Plasma exosomes improve peripheral neuropathy via miR-20b-3p/Stat3 in type I diabetic rats
Journal of Nanobiotechnology (2023)
-
The Information Theory of Aging
Nature Aging (2023)