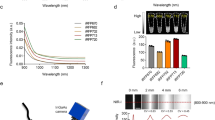
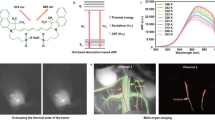
Abstract
Fluorescence imaging in the second near-infrared (NIR-II) window enables deep-tissue imaging with high resolution and improved contrast by taking advantage of the reduced light scattering and tissue autofluorescence in this region of the spectrum. NIR-II fluorescence imaging uses photoluminescent contrast agents — including carbon nanotubes, quantum dots, rare earth-doped nanocrystals, gold nanoclusters, small molecules and their aggregates — and fluorescent proteins, which all exhibit fluorescence in the 1,000–3,000 nm range. After administration of these fluorophores in vivo, live animals can be imaged with specialized detectors and optical instruments, yielding images with contrast and resolution unparalleled by conventional visible and near-infrared fluorescence imaging. This powerful approach enables dynamic imaging of vascular structures and haemodynamics; molecular imaging and image-guided surgery of tumours; and visualization of deep-seated structures, such as the gastrointestinal system. NIR-II fluorescence imaging has revolutionized biomedical imaging over the past 15 years and is poised to make comparable advancements in cardiology, neurobiology and gastroenterology. This Primer describes the principles of NIR-II fluorescence imaging, reviews the most used fluorophores, outlines implementation approaches and discusses specific scientific and clinical applications. Furthermore, the limitations of NIR-II fluorescence imaging are addressed and future opportunities across various scientific domains are explored.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Wang, F. et al. In vivo non-invasive confocal fluorescence imaging beyond 1,700 nm using superconducting nanowire single-photon detectors. Nat. Nanotechnol. 17, 653–660 (2022). This publication showcases the use of the longest excitation and emission wavelengths (up to 2,000 nm) ever employed for in vivo fluorescence imaging with one-photon excitation.
Smith, A. M., Mancini, M. C. & Nie, S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 4, 773–780 (2009). To our knowledge, this paper marks the first example of in vivo fluorescence imaging performed in the NIR-II spectrum.
Hong, G. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 8, 723–730 (2014). To our knowledge, this publication presents the first instance of brain vascular imaging conducted through an intact scalp and skull, without requiring any surgical procedures on the mouse’s head.
Yang, S. J., Del Bonis-O’Donnell, J. T., Beyene, A. G. & Landry, M. P. Near-infrared catecholamine nanosensors for high spatiotemporal dopamine imaging. Nat. Protoc. 16, 3026–3048 (2021).
Hu, Z. et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 4, 259–271 (2020). To our knowledge, this work represents the first human clinical study of NIR-II fluorescence imaging, using the FDA-approved dye ICG as the contrast agent.
Wang, F. et al. Light-sheet microscopy in the near-infrared II window. Nat. Methods 16, 545–552 (2019).
Dang, X. et al. Deep-tissue optical imaging of near cellular-sized features. Sci. Rep. 9, 3873 (2019).
Hong, G. et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 18, 1841–1846 (2012).
Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021). This work demonstrates the use of X-rays as the excitation light source to excite NIR-II fluorescence for in vivo imaging.
Wang, Z. et al. Dynamically monitoring lymphatic and vascular systems in physiological and pathological conditions of a swine model via a portable NIR-II imaging system with ICG. Int. J. Med. Sci. 19, 1864–1874 (2022).
Cai, Z. et al. NIR-II fluorescence microscopic imaging of cortical vasculature in non-human primates. Theranostics 10, 4265–4276 (2020).
Antaris, A. L. et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 15, 235–242 (2016). To our knowledge, this work represents the first example of using small-molecule fluorophores for in vivo NIR-II fluorescence imaging with rapid renal clearance.
Beyene, A. G. et al. Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent catecholamine nanosensor. Sci. Adv. 5, eaaw3108 (2019).
Fan, Y. et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 13, 941–946 (2018).
Brozena, A. H., Kim, M., Powell, L. R. & Wang, Y. Controlling the optical properties of carbon nanotubes with organic colour-centre quantum defects. Nat. Rev. Chem. 3, 375–392 (2019).
Kim, M. et al. Nanosensor-based monitoring of autophagy-associated lysosomal acidification in vivo. Nat. Chem. Biol. 19, 1448–1457 (2023). This publication demonstrates the creation of functional nanosensors with emission in the NIR-II spectrum, allowing for the monitoring of cellular functions such as lysosomal acidification.
Hong, G. et al. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem. Int. Ed. 51, 9818–9821 (2012).
Bruns, O. T. et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 1, 0056 (2017).
Vasilopoulou, M. et al. Efficient colloidal quantum dot light-emitting diodes operating in the second near-infrared biological window. Nat. Photonics 14, 50–56 (2019).
Carr, J. A. et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl Acad. Sci. USA 115, 4465–4470 (2018). This paper showcases the feasibility of repurposing ICG, an FDA-approved fluorescent contrast agent, for NIR-II fluorescence imaging.
Wang, S. et al. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 10, 1058 (2019).
Bandi, V. G. et al. Targeted multicolor in vivo imaging over 1,000 nm enabled by nonamethine cyanines. Nat. Methods 19, 353–358 (2022).
Oliinyk, O. S. et al. Deep-tissue SWIR imaging using rationally designed small red-shifted near-infrared fluorescent protein. Nat. Methods 20, 70–74 (2023). This paper demonstrates the use of off-resonance fluorescence of red-shifted fluorescent proteins for in vivo NIR-II imaging.
Chen, M. et al. Long-term monitoring of intravital biological processes using fluorescent protein-assisted NIR-II imaging. Nat. Commun. 13, 6643 (2022).
Wang, R., Li, X., Zhou, L. & Zhang, F. Epitaxial seeded growth of rare‐earth nanocrystals with efficient 800 nm near‐infrared to 1525 nm short‐wavelength infrared downconversion photoluminescence for in vivo bioimaging. Angew. Chem. Int. Ed. 126, 12282–12286 (2014).
Wang, R., Zhou, L., Wang, W., Li, X. & Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 8, 14702 (2017).
Zhong, Y. et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 37, 1322–1331 (2019).
Chen, Y. et al. Shortwave infrared in vivo imaging with gold nanoclusters. Nano Lett. 17, 6330–6334 (2017).
Liu, H. et al. Atomic-precision gold clusters for NIR-II imaging. Adv. Mater. 31, e1901015 (2019).
Baghdasaryan, A. et al. Phosphorylcholine-conjugated gold-molecular clusters improve signal for lymph nnode NIR-II fluorescence imaging in preclinical cancer models. Nat. Commun. 13, 5613 (2022).
Ma, H. et al. Bioactive NIR-II gold clusters for three-dimensional imaging and acute inflammation inhibition. Sci. Adv. 9, eadh7828 (2023).
Pitruzzello, G. Seeing into deep tissue. Nat. Photonics 17, 376–377 (2023).
Rutz, F. et al. in Electro-Optical and Infrared Systems: Technology and Applications X Vol. 8896 (eds Huckridge, D. A. & Ebert, R.) 81–87 (SPIE, 2013).
Zhang, M. et al. Bright quantum dots emitting at ∼1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl Acad. Sci. USA 115, 6590–6595 (2018).
Zhu, S. et al. Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc. Natl Acad. Sci. USA 114, 962–967 (2017).
Ji, A. et al. Acceptor engineering for NIR-II dyes with high photochemical and biomedical performance. Nat. Commun. 13, 3815 (2022).
Tian, R. et al. A genetic engineering strategy for editing near-infrared-II fluorophores. Nat. Commun. 13, 2853 (2022).
Chen, W. et al. Shortwave infrared imaging with J-aggregates stabilized in hollow mesoporous silica nanoparticles. J. Am. Chem. Soc. 141, 12475–12480 (2019).
Li, Z. et al. In situ orderly self-assembly strategy affording NIR-II-J-aggregates for in vivo imaging and surgical navigation. Nat. Commun. 14, 1843 (2023).
Hu, X. et al. J-aggregation strategy toward potentiated NIR-II fluorescence bioimaging of molecular fluorophores. Adv. Mater. 36, e2304848 (2023).
Zhong, Y. & Dai, H. A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 13, 1281–1294 (2020).
Starosolski, Z. et al. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS ONE 12, e0187563 (2017).
Fang, Y. et al. Design, synthesis, and application of a small molecular NIR-II fluorophore with maximal emission beyond 1200 nm. J. Am. Chem. Soc. 142, 15271–15275 (2020).
Yang, Y. et al. Fluorescence-amplified nanocrystals in the second near-infrared window for in vivo real-time dynamic multiplexed imaging. Nat. Nanotechnol. 18, 1195–1204 (2023).
Hong, G. et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nat. Commun. 5, 4206 (2014).
Antaris, A. L. et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 8, 15269 (2017).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Wang, F. et al. In vivo NIR-II structured-illumination light-sheet microscopy. Proc. Natl Acad. Sci. USA 118, e2023888118 (2021).
Zhu, S. et al. Repurposing cyanine NIR-I dyes accelerates clinical translation of near-infrared-II (NIR-II) bioimaging. Adv. Mater. 30, e1802546 (2018).
Hong, G. Seeing the sound. Science 369, 638 (2020).
Chen, C. et al. Creating visible-to-near-infrared mechanoluminescence in mixed-anion compounds SrZn2S2O and SrZnSO. Nano Energy 68, 104329 (2020).
Yang, F., Cui, H., Wu, X., Kim, S.-J. & Hong, G. Ultrasound-activated luminescence with color tunability enabled by mechanoluminescent colloids and perovskite quantum dots. Nanoscale 15, 1629–1636 (2023).
Jiang, S., Wu, X., Yang, F., Rommelfanger, N. J. & Hong, G. Activation of mechanoluminescent nanotransducers by focused ultrasound enables light delivery to deep-seated tissue in vivo. Nat. Protoc. 18, 3787–3820 (2023).
Lu, L. et al. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 11, 4192 (2020).
International Commission on Non-Ionizing Radiation Protection (ICNIRP). Revision of guidelines on limits of exposure to laser radiation of wavelengths between 400 nm and 1.4 μm. Health Phys. 79, 431–440 (2000).
Zhou, Z., Song, J., Nie, L. & Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 45, 6597–6626 (2016).
Liebel, F., Kaur, S., Ruvolo, E., Kollias, N. & Southall, M. D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Invest. Dermatol. 132, 1901–1907 (2012).
Hale, G. M. & Querry, M. R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 12, 555–563 (1973).
Yang, Y. et al. Counterion‐paired bright heptamethine fluorophores with NIR‐II excitation and emission enable multiplexed biomedical imaging. Angew. Chem. Int. Ed. 61, e202117436 (2022).
Chen, P. et al. Bandgap modulation and lipid intercalation generates ultrabright D–A–D‐based zwitterionic small‐molecule nanoagent for precise NIR‐II excitation phototheranostic applications. Adv. Funct. Mater. 32, 2208463 (2022).
Li, B. et al. Organic NIR-II molecule with long blood half-life for in vivo dynamic vascular imaging. Nat. Commun. 11, 3102 (2020).
Wei, R. et al. Rigid and photostable shortwave infrared dye absorbing/emitting beyond 1200 nm for high-contrast multiplexed imaging. J. Am. Chem. Soc. 145, 12013–12022 (2023).
Qu, Y. et al. High-power InAlGaAs/GaAs and AlGaAs/GaAs semiconductor laser arrays emitting at 808 nm. IEEE Photonics Technol. Lett. 16, 389–391 (2004).
Wu, X. et al. Tether-free photothermal deep-brain stimulation in freely behaving mice via wide-field illumination in the near-infrared-II window. Nat. Biomed. Eng. 6, 754–770 (2022).
Wang, W. et al. Organic fluorophores for 1064 nm excited NIR-II fluorescence imaging. Front. Chem. 9, 769655 (2021).
Lifante, J. et al. In vivo grading of lipids in fatty liver by near-infrared autofluorescence and reflectance. J. Biophotonics 16, e202200208 (2023).
Zichi, J. et al. Optimizing the stoichiometry of ultrathin NbTiN films for high-performance superconducting nanowire single-photon detectors. Opt. Express 27, 26579–26587 (2019).
Welsher, K., Sherlock, S. P. & Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl Acad. Sci. USA 108, 8943–8948 (2011).
Hong, G., Diao, S., Antaris, A. L. & Dai, H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 115, 10816–10906 (2015).
Qian, H., Zhu, M., Wu, Z. & Jin, R. Quantum sized gold nanoclusters with atomic precision. Acc. Chem. Res. 45, 1470–1479 (2012).
Gil, H. M. et al. NIR-quantum dots in biomedical imaging and their future. iScience 24, 102189 (2021).
Chang, B. et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat. Biomed. Eng. 6, 629–639 (2022).
Shen, Y. et al. Perspectives for Ag2S NIR-II nanoparticles in biomedicine: from imaging to multifunctionality. Nanoscale 11, 19251–19264 (2019).
del Rosal, B., Ruiz, D. & Chaves‐Coira, I. In vivo contactless brain nanothermometry. Adv. Funct. Mater. 28, 1806088 (2018).
Zhou, J., Del Rosal, B., Jaque, D., Uchiyama, S. & Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 17, 967–980 (2020).
Gu, Y. et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photonics 13, 525–531 (2019).
Chen, Z.-H. et al. An extended NIR‐II superior imaging window from 1500 to 1900 nm for high‐resolution in vivo multiplexed imaging based on lanthanide nanocrystals. Angew. Chem. Int. Ed. 62, e202311883 (2023).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Yang, Q. et al. Donor engineering for NIR-II molecular fluorophores with enhanced fluorescent performance. J. Am. Chem. Soc. 140, 1715–1724 (2018).
Jia, S. et al. Water-soluble chromenylium dyes for shortwave infrared imaging in mice. Chem 9, 3648–3665 (2023).
Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van de Velde, C. J. H. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 10, 507–518 (2013).
Koch, M. & Ntziachristos, V. Advancing surgical vision with fluorescence imaging. Annu. Rev. Med. 67, 153–164 (2016).
Tao, Z. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 52, 13002–13006 (2013).
Wan, H. et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 9, 1171 (2018).
Tian, R. et al. Albumin-chaperoned cyanine dye yields superbright NIR-II fluorophore with enhanced pharmacokinetics. Sci. Adv. 5, eaaw0672 (2019).
Li, Y. et al. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 11, 1255 (2020).
Shen, H. et al. Rational design of NIR-II AIEgens with ultrahigh quantum yields for photo- and chemiluminescence imaging. J. Am. Chem. Soc. 144, 15391–15402 (2022).
Zhuang, J. et al. Efficient NIR-II type-I AIE photosensitizer for mitochondria-targeted photodynamic therapy through synergistic apoptosis–ferroptosis. ACS Nano 17, 9110–9125 (2023).
Cui, S. et al. Ultra-homogeneous NIR-II fluorescent self-assembled nanoprobe with AIE properties for photothermal therapy of prostate cancer. Nanoscale 13, 15569–15575 (2021).
Li, D. et al. Molecular engineering of NIR‐II AIE luminogen excited at 1700 nm for ultradeep intravital brain two‐photon fluorescence imaging. Adv. Funct. Mater. 33, 2303967 (2023).
Chen, J., Chen, L., She, Z., Zeng, F. & Wu, S. A multifunctional nanoaggregate‐based system for detection of rheumatoid arthritis via optoacoustic/NIR‐II fluorescent imaging and therapy via inhibiting JAK‐STAT/NF‐κB/NLRP3 pathways. Aggregate https://doi.org/10.1002/agt2.419 (2023).
He, X. et al. D‐type neuropeptide decorated AIEgen/RENP hybrid nanoprobes with light‐driven ROS generation ability for NIR‐II fluorescence imaging‐guided through‐skull photodynamic therapy of gliomas. Aggregate https://doi.org/10.1002/agt2.396 (2023).
Wei, W. et al. Synthesis, supramolecular aggregation, and NIR-II phosphorescence of isocyanorhodium(I) zwitterions. Chem. Sci. 14, 11490–11498 (2023).
Li, K. et al. J-aggregates of meso-[2.2]paracyclophanyl-BODIPY dye for NIR-II imaging. Nat. Commun. 12, 2376 (2021).
Sun, C. et al. J-aggregates of cyanine dye for NIR-II in vivo dynamic vascular imaging beyond 1500 nm. J. Am. Chem. Soc. 141, 19221–19225 (2019).
Wang, S. et al. Photostable small-molecule NIR-II fluorescent scaffolds that cross the blood–brain barrier for noninvasive brain imaging. J. Am. Chem. Soc. 144, 23668–23676 (2022).
Chen, H. et al. Bioinspired large Stokes shift small molecular dyes for biomedical fluorescence imaging. Sci. Adv. 8, eabo3289 (2022).
Wang, T. et al. A hybrid erbium(III)–bacteriochlorin near-infrared probe for multiplexed biomedical imaging. Nat. Mater. 20, 1571–1578 (2021).
Shu, X. et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science 324, 804–807 (2009).
Maiti, A. et al. Structural and photophysical characterization of the small ultra-red fluorescent protein. Nat. Commun. 14, 4155 (2023).
Wolfram, J. et al. Safety of nanoparticles in medicine. Curr. Drug. Targets 16, 1671–1681 (2015).
Smith, B. R. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 9, 481–487 (2014).
Diao, S. et al. Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. Int. Ed. 54, 14758–14762 (2015).
Ma, Z., Wang, F., Wang, W., Zhong, Y. & Dai, H. Deep learning for in vivo near-infrared imaging. Proc. Natl Acad. Sci. USA 118, e2021446118 (2021). This publication displays a novel use of deep learning to enhance the resolution of in vivo NIR-II fluorescence imaging beyond the capability of traditional image analysis techniques.
Santos, H. D. A. et al. Ultrafast photochemistry produces superbright short-wave infrared dots for low-dose in vivo imaging. Nat. Commun. 11, 2933 (2020). This paper presents a new approach to synthesizing exceptionally bright NIR-II contrast agents, combining chemical synthesis with ultrafast laser techniques.
Kim, M. et al. Detection of ovarian cancer via the spectral fingerprinting of quantum-defect-modified carbon nanotubes in serum by machine learning. Nat. Biomed. Eng. 6, 267–275 (2022).
Huang, B. et al. Near-infrared-IIb emitting single-atom catalyst for imaging-guided therapy of blood–brain barrier breakdown after traumatic brain injury. Nat. Commun. 14, 197 (2023).
O’Herron, P. et al. Neural correlates of single-vessel haemodynamic responses in vivo. Nature 534, 378–382 (2016).
Chow, B. W. et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature 579, 106–110 (2020).
Sun, Y. et al. Melanin-dot-mediated delivery of metallacycle for NIR-II/photoacoustic dual-modal imaging-guided chemo-photothermal synergistic therapy. Proc. Natl Acad. Sci. USA 116, 16729–16735 (2019).
Xu, Y. et al. Construction of emissive ruthenium(II) metallacycle over 1000 nm wavelength for in vivo biomedical applications. Nat. Commun. 13, 2009 (2022).
Ren, F. et al. Shortwave-infrared-light-emitting probes for the in vivo tracking of cancer vaccines and the elicited immune responses. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-023-01083-5 (2023).
Zhang, X.-D. et al. Traumatic brain injury imaging in the second near-infrared window with a molecular fluorophore. Adv. Mater. 28, 6872–6879 (2016).
Krzywinski, M. & Altman, N. Points of significance: importance of being uncertain. Nat. Methods 10, 809–810 (2013).
Blainey, P., Krzywinski, M. & Altman, N. Points of significance: replication. Nat. Methods 11, 879–880 (2014).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Academic Press, 2013).
Krzywinski, M. & Altman, N. Power and sample size. Nat. Methods 10, 1139–1140 (2013).
Wu, L. et al. Generation of hydroxyl radical-activatable ratiometric near-infrared bimodal probes for early monitoring of tumor response to therapy. Nat. Commun. 12, 6145 (2021).
Krzywinski, M. & Altman, N. Significance, P values and t-tests. Nat. Methods 10, 1041–1042 (2013).
Krzywinski, M. & Altman, N. Points of significance: analysis of variance and blocking. Nat. Methods 11, 699–700 (2014).
Krzywinski, M. & Altman, N. Points of significance: nonparametric tests. Nat. Methods 11, 467–468 (2014).
Hong, G. et al. Near-infrared II fluorescence for imaging hindlimb vessel regeneration with dynamic tissue perfusion measurement. Circ. Cardiovasc. Imaging 7, 517–525 (2014).
Cosco, E. D. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020).
Robinson, J. T. et al. In vivo fluorescence imaging in the second near-infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake. J. Am. Chem. Soc. 134, 10664–10669 (2012).
Ghosh, D. et al. Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. Proc. Natl Acad. Sci. USA 111, 13948–13953 (2014).
Yang, F. et al. A biomineral-inspired approach of synthesizing colloidal persistent phosphors as a multicolor, intravital light source. Sci. Adv. 8, eabo6743 (2022).
Fang, Z. et al. Oxyhaemoglobin saturation NIR-IIb imaging for assessing cancer metabolism and predicting the response to immunotherapy. Nat. Nanotechnol. 19, 124–130 (2023).
Choi, H. S. et al. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 5, 42–47 (2010).
Rosenthal, E. L. et al. Sensitivity and specificity of cetuximab-IRDye800CW to identify regional metastatic disease in head and neck cancer. Clin. Cancer Res. 23, 4744–4752 (2017).
Ibrahim, N. E.-S. Shortwave-infrared imaging of cancer vaccine uncovers immune response. Nat. Rev. Bioeng. 1, 694–694 (2023).
Gioux, S., Choi, H. S. & Frangioni, J. V. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol. Imaging 9, 237–255 (2010).
Chen, Y., Wang, S. & Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 1, 60–78 (2023).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Balzarotti, F. et al. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 355, 606–612 (2017).
Semonin, O. E. et al. Absolute photoluminescence quantum yields of IR-26 Dye, PbS, and PbSe quantum dots. J. Phys. Chem. Lett. 1, 2445–2450 (2010).
Murphy, J. E. et al. PbTe colloidal nanocrystals: synthesis, characterization, and multiple exciton generation. J. Am. Chem. Soc. 128, 3241–3247 (2006).
Su, Y. et al. An optimized bioluminescent substrate for non-invasive imaging in the brain. Nat. Chem. Biol. 19, 731–739 (2023).
Williams, E. et al. The Image Data Resource: a bioimage data integration and publication platform. Nat. Methods 14, 775–781 (2017).
Li, C. & Wang, Q. Challenges and opportunities for intravital near-infrared fluorescence imaging technology in the second transparency window. ACS Nano 12, 9654–9659 (2018).
Kamath, A., Schaller, R. D. & Guyot-Sionnest, P. Bright fluorophores in the second near-infrared window: HgSe/CdSe quantum dots. J. Am. Chem. Soc. 145, 10809–10816 (2023).
Zhong, Y. et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 8, 737 (2017).
Ludvikova, L. et al. Near-infrared co-illumination of fluorescent proteins reduces photobleaching and phototoxicity. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01893-7 (2023).
Villa, I. et al. 1.3 μm emitting SrF2:Nd3+ nanoparticles for high contrast in vivo imaging in the second biological window. Nano Res. 8, 649–665 (2015).
Del Rosal, B., Villa, I., Jaque, D. & Sanz-Rodríguez, F. In vivo autofluorescence in the biological windows: the role of pigmentation. J. Biophotonics 9, 1059–1067 (2016).
Tanzid, M. et al. Absorption-induced image resolution enhancement in scattering media. ACS Photonics 3, 1787–1793 (2016).
Carr, J. A. et al. Absorption by water increases fluorescence image contrast of biological tissue in the shortwave infrared. Proc. Natl Acad. Sci. USA 115, 9080–9085 (2018).
Feng, Z. et al. Perfecting and extending the near-infrared imaging window. Light. Sci. Appl. 10, 197 (2021).
Chang, Y. et al. Bright Tm3+-based downshifting luminescence nanoprobe operating around 1800 nm for NIR-IIb and c bioimaging. Nat. Commun. 14, 1079 (2023).
Zhang, P. et al. Deep learning driven adaptive optics for single molecule localization microscopy. Nat. Methods 20, 1748–1758 (2023).
Prakash, M. Frugal science: a physicist view on tackling global health and education challenges. APS Meet. Abstr. 2018, P61.004 (2018).
Lai, Y. et al. Design of an activatable NIR-II nanoprobe for the in vivo elucidation of Alzheimer’s disease-related variations in methylglyoxal concentrations. Chem. Sci. 13, 12511–12518 (2022).
Hou, S. S. et al. Near-infrared fluorescence lifetime imaging of amyloid-β aggregates and tau fibrils through the intact skull of mice. Nat. Biomed. Eng. 7, 270–280 (2023).
Wu, X., Yang, F., Cai, S., Pu, K. & Hong, G. Nanotransducer-enabled deep-brain neuromodulation with NIR-II light. ACS Nano 17, 7941–7952 (2023).
Jiang, S., Wu, X., Rommelfanger, N. J., Ou, Z. & Hong, G. Shedding light on neurons: optical approaches for neuromodulation. Natl. Sci. Rev. 9, nwac007 (2022).
Cui, H., Zhao, S. & Hong, G. Wireless deep-brain neuromodulation using photovoltaics in the second near-infrared spectrum. Device 1, 100113 (2023).
Wang, F., Dukovic, G., Brus, L. E. & Heinz, T. F. Time-resolved fluorescence of carbon nanotubes and its implication for radiative lifetimes. Phys. Rev. Lett. 92, 177401 (2004).
Zhang, Y. et al. Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano 6, 3695–3702 (2012).
Zhang, Y., Liu, Y., Li, C., Chen, X. & Wang, Q. Controlled synthesis of Ag2S quantum dots and experimental determination of the exciton bohr radius. J. Phys. Chem. C. 118, 4918–4923 (2014).
Wu, Q. et al. Synthesis of water-soluble Ag2S quantum dots with fluorescence in the second near-infrared window for turn-on detection of Zn(II) and Cd(II). Anal. Chem. 89, 6616–6623 (2017).
Li, C. et al. An activatable NIR-II nanoprobe for in vivo early real-time diagnosis of traumatic brain injury. Angew. Chem. Int. Ed. 59, 247–252 (2020).
Ren, F., Zhao, H., Vetrone, F. & Ma, D. Microwave-assisted cation exchange toward synthesis of near-infrared emitting PbS/CdS core/shell quantum dots with significantly improved quantum yields through a uniform growth path. Nanoscale 5, 7800–7804 (2013).
Franke, D. et al. Continuous injection synthesis of indium arsenide quantum dots emissive in the short-wavelength infrared. Nat. Commun. 7, 12749 (2016).
Wang, P. et al. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 9, 2898 (2018).
Liu, J. et al. Luminescent gold nanoparticles with size-independent emission. Angew. Chem. Int. Ed. 55, 8894–8898 (2016).
Kopainsky, B., Qiu, P., Kaiser, W., Sens, B. & Drexhage, K. H. Lifetime, photostability, and chemical structure of IR heptamethine cyanine dyes absorbing beyond 1 μm. Appl. Phys. B 29, 15–18 (1982).
Gerega, A. et al. Wavelength-resolved measurements of fluorescence lifetime of indocyanine green. J. Biomed. Opt. 16, 067010 (2011).
Smith, J. T. et al. In vitro and in vivo NIR fluorescence lifetime imaging with a time-gated SPAD camera. Optica 9, 532–544 (2022).
Wang, F., Zhong, Y., Bruns, O., Liang, Y. & Dai, H. In vivo NIR-II fluorescence imaging for biology and medicine. Nat. Photon. https://doi.org/10.1038/s41566-024-01391-5 (2024).
Acknowledgements
G.H. acknowledges a National Science Foundation (NSF) EAGER Award (2217582) and a Beckman Technology Development Grant. E.L.S. acknowledges the support of a fellowship from the Bio-X Initiative of Stanford University. Z.O. is supported by the Wu Tsai Neurosciences Institute. H.C. acknowledges support from a Stanford Interdisciplinary Graduate Fellowship. C.H.C.K. acknowledges support from the NSF Graduate Research Fellowships Program and from the NeuroTech training programme from the Wu Tsai Neuroscience Institute. D.J. and E.X. acknowledge Grant PID2019-106211RB-I00 (NANONERV) funded by MCIN/AEI/10.13039/501100011033, and the S2022/BMD7403 REMIN-CM project supported by Comunidad Autónoma de Madrid. E.X. is grateful for a Juan de la Cierva Incorporación scholarship (IJC2020-045229-I). The initial draft of Figs. 1, 2, 4 and 7 were prepared using BioRender.
Author information
Authors and Affiliations
Contributions
Introduction (E.L.S., Z.O., H.C., C.H.C.K. and G.H.); Experimentation (E.X., H.C., D.J. and G.H.); Results (E.L.S., Z.O., H.C. and G.H.); Applications (E.X., H.C., D.J. and G.H.); Reproducibility and data deposition (E.L.S., Z.O., H.C., C.H.C.K. and G.H.); Limitations and optimizations (E.L.S., Z.O., H.C., C.H.C.K. and G.H.); Outlook (E.L.S., Z.O. and G.H.); overview of the Primer (all authors).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Fan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Brain Image Library: https://www.brainimagelibrary.org/
Distributed Archives for Neurophysiology Data Integration: https://www.dandiarchive.org/
Image Data Resource: http://idr.openmicroscopy.org/about/
The Cancer Imaging Archive: https://www.cancerimagingarchive.net/
Supplementary information
Glossary
- Adjuvant
-
A substance added to vaccines to enhance the body’s immune response to the vaccine’s antigen.
- Affibody
-
Small protein scaffolds derived from the Z domain of staphylococcal protein A, engineered to bind specific target proteins with high specificity and affinity.
- Aggregation-induced emission
-
A phenomenon where a material, often an organic compound, emits light more efficiently when it is aggregated or clustered together than when it is in an isolated, dissolved state.
- Autofluorescence
-
The natural emission of light upon excitation of biological tissues, largely contributed by endogenous chromophores such as NADH (emission ~460 nm) and flavins (500–600 nm), as well as pigmented cellular structures such as lipofuscin (450–650 nm) and reticulin (470–520 nm).
- Avalanche photodetectors
-
Photodiodes that are specifically designed to use the avalanche effect, which involves the multiplication of charge carriers (electrons and holes) due to high applied voltages, to amplify the electrical signals generated by the absorption of photons.
- Dichroic mirrors
-
Optical filters that reflect light below (for short pass) or above (for long pass) a specific cut-off or cut-on wavelength, respectively, while transmitting the rest.
- Emission filter
-
An optical filter, typically positioned in front of the detector, selectively transmits wavelengths corresponding to the emission of a specific fluorophore while blocking other undesired wavelengths, such as those from the excitation light source.
- Epifluorescence
-
The fluorescence observed in an optical microscope or imaging system when the object is illuminated from the side that is being viewed.
- Excitation filter
-
An optical filter, typically positioned in front of the excitation light source, selectively transmits wavelengths suitable for exciting a specific fluorophore while blocking other undesired wavelengths.
- High endothelial venules
-
Specialized post-capillary venous structures found in lymph nodes and Peyer’s patches that facilitate the entry of lymphocytes from the bloodstream into lymphatic tissues.
- Human embryonic kidney cells
-
A cell line derived from human embryonic kidney tissue, known for robust growth and ease of transfection, commonly used in the production of recombinant proteins, viral vectors and in vitro drug toxicity assays.
- Indium gallium arsenide
-
(InGaAs). A compound semiconductor material that is sensitive to infrared light and commonly used in photodetectors for second near-infrared (NIR-II) fluorescence imaging.
- Infinity-corrected objective
-
An optical lens system designed to produce parallel rays between the objective and the eyepiece or camera, typically used in microscopy for clearer imaging and easier integration of additional optical components.
- Intralipid
-
A sterile fat emulsion commonly used in medical settings as a parenteral nutrition supplement and in research as a scattering medium to simulate biological tissues in optical imaging experiments.
- Optical density
-
A measure of how much a substance or an object attenuates the intensity of light that passes through it. Mathematically, optical density is defined as OD = –log10(I/I0), where I is the intensity of light transmitted through the substance and I0 is the intensity of the incident light.
- Optical diffuser
-
A device that scatters light in various directions to produce a uniform illumination.
- Optomechanics
-
Elements including optical tables, breadboards, construction components such as mounts and mechanically integrated optoelectronic devices.
- Organic colour centres
-
Synthetic defects in semiconducting single-walled carbon nanotubes (CNTs) created by covalently bonding organic molecules to the crystal lattice, resulting in quantum emitters that fluoresce in the second near-infrared (NIR-II) spectrum, emitting pure single photons at room temperature.
- Overtone absorption
-
The absorption of light by a molecule at a frequency (or wavelength) that is a multiple of the fundamental frequency of a vibrational mode of that molecule.
- Particle image velocimetry
-
A visual measurement technique used to obtain instantaneous velocity fields by tracking the movement of small particles seeded in a fluid flow.
- Peripheral node addressin
-
A carbohydrate ligand for l-selectin that plays a crucial role in the homing of white blood cells, specifically directing their migration to peripheral lymph nodes during the immune response.
- Photodiodes
-
Semiconductor devices that convert light into an electrical current, the amplitude of which is directly proportional to the light intensity shining on the diode.
- Photomultiplier tubes
-
Electronic devices that detect and greatly amplify weak light signals by converting photons generated by a photocathode into an intensified electrical signal through a series of secondary electron multipliers.
- Reactive oxygen species
-
Chemically reactive molecules that contain oxygen, such as hydrogen peroxide (H2O2), superoxide anion (O2−), hydroxyl radical (•OH) and singlet oxygen (1O2).
- Scattering
-
The deviation of light rays from their original path, a phenomenon exacerbated in animal tissue by the inhomogeneity of refractive indices among components such as water, lipid membranes and subcellular organelles.
- Semiconductor diode lasers
-
Lasers with a semiconductor active medium, akin to a light-emitting diode, but that produce coherent light through stimulated emission from the recombination of electrons and holes.
- Signal-to-noise ratio
-
(SNR). The ratio of fluorescence signal to the background noise, the latter of which comprises the shot noise and dark noise of the photodetector, the read-out noise from the camera electronics as well as autofluorescence and scattering from biological tissues.
- Superconducting nanowire single-photon detectors
-
Ultra-sensitive devices that detect individual photons by measuring the disruption in the bias current, which arises when single photons absorbed by the superconducting nanowire break Cooper pairs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schmidt, E.L., Ou, Z., Ximendes, E. et al. Near-infrared II fluorescence imaging. Nat Rev Methods Primers 4, 23 (2024). https://doi.org/10.1038/s43586-024-00301-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-024-00301-x