Abstract
Since their invention, plastics have driven a revolution in behavior in all aspects of our lives, including agriculture. In-use and as a waste material, plastics degrade and accumulate in agricultural systems. Accumulation of plastic pollution in agricultural systems has negative impacts on human health and agricultural productivity but little is known about concentrations of microplastics in soils. Here we used a historical time series to examine changes to microplastic concentrations in agricultural soils over time. Microplastics were stained with Nile Red and quantified using fluorescence microscopy. We demonstrate that microplastic concentrations increased at higher rates in soils that are amended with either organic or inorganic fertiliser between 1966 and 2022, suggesting that agricultural fertilisers are an important contributor to microplastic concentrations in agricultural soils over time. This study provides evidence that agricultural soils are receptors and reservoirs of microplastic pollution, a legacy which is growing over time.
Similar content being viewed by others
Introduction
Plastics have revolutionised the world we live in today. Much like the industrial revolution, the introduction of plastics into everyday life is responsible for substantial changes to economic and social organisation. Plastics have facilitated advancements in packaging, construction, transportation, agriculture, medicine and electronics. Approximately, 450 million tonnes of plastic are produced annually, of which 12.5 million tonnes are used in agriculture1,2. Of all plastics created, 76% has become waste, 79% of which has accumulated in landfill and the natural environment3.
In-use and as a waste material, plastic breaks down via a series of degradation pathways4,5,6. Plastic degradation is multifaceted, involving chemical weathering, physical fragmentation and bio-, photo- and oxidative degradation2,4,5. Degradation rates are variable, dependent on polymer characteristics and the environmental conditions to which the plastic is exposed. Even though biodegradable plastics are intended to biodegrade into CO2 and biomass via microbial metabolic utilization, complete degradation does not always occur2. Microplastic pollution is considered poorly reversible and negatively impacts planetary and human health2,4. The properties and characteristics that make plastics so versatile are some of the reasons why the accumulation of the material is of growing environmental concern; they are persistent, durable and capable of being transported globally through a range of environmental media4.
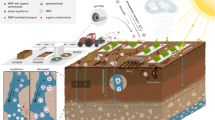
The long-term and widespread use of plastics, combined with substandard management strategies has led to the accumulation of plastic residues (macro-, micro- and nanoplastics) in agricultural soils worldwide7,8,9. Generally, macroplastics are defined as any polymer > 5 mm, microplastics between 1 μm and 5 mm, and nanoplastics < 1 μm, although these ranges are still debated5. For the purpose of this study, we defined microplastics as any polymer between 10 μm and 5 mm, the range between which we can confidently identify a microplastic particle using the techniques described below. Direct agricultural sources of plastic residues to agricultural soils include plastic mulch films, crop covers, crop housing, polymer-coated agrochemicals, silage films and containers, sacks and trays7,8. Indirect sources include biosolids, wastewater irrigation and equipment, wear of farm machinery, organic manure and other field amendments, as well as atmospheric deposition2,5,9,10. Non-biodegradable polymers intended for use in slow- and controlled release inorganic fertilisers are direct contributors of microplastic particles, whereas both inorganic fertilisers and farmyard manure can be indirect contributors of microplastics, with plastics originating from animal feces, feeds, plastic tools, equipment and packaging materials11. The atmospheric transport and deposition of microplastics is borderless, exposing agricultural soils to microplastic pollution from industrial emissions, textile fibers, the deterioration of polymer-coated surfaces and domestic effluents, largely of urban origin3,4,9,10. Consequently, microplastic shape in agricultural soils are unevenly distributed between films, fragment, fiber, foam and pellet, in order of abundance12, although this will differ depending on the cultivation methods and farming practices at each site.
The release of plastic residues into the agricultural environment causes physical, chemical and biological harm, dictated by polymer type, size, shape, aging, time of exposure and associated additives5,7,9. Microplastics are an emerging concern in agricultural systems and impact agricultural productivity directly or as a result of the additives incorporated into the polymer during production or adsorbed from the surrounding environment7,10,13. Additives include, but are not exclusive to, stabilizers, plasticizers, flame retardants and coloring agents, many of which are known endocrine disruptors which can impair the health of living organisms7,9,10,13.
In agricultural soils, microplastics have been shown to negatively impact crop quality, yield and the physical, chemical and biological properties of soil7,10,13,14. Most of these effects are thought to worsen at greater loads of microplastic residues, although many studies have been conducted at artificially large microplastic concentrations, with the potential to compromise agroecosystem functioning and productivity in the long-term5,13,14. The extent, effects and severity of microplastic pollution in agricultural soils, in the short- and long-term, are largely unknown.
The use of much plastic in agriculture is driven by the almost immediate benefits of the practice which translate to an increase in crop yield and quality, early- and late-season production, as well as resource-use efficiency, particularly in yield-limiting conditions2,6,8. Plastic use is crucial to maintaining the food security and productivity of many regions. The use of plastics is deeply embedded in global agricultural systems and is likely to increase in the future due to population increase, stricter resource and agrochemical regulations, as well as the observed and expected impacts of climate change8. Removing plastics from agriculture may have severe repercussions for agroecosystems, global food security and greenhouse gas emissions, compromising human and planetary health2,6. Although temporal records of microplastics in urban environments have demonstrated a proliferation of microplastics from the 1950’s to the present15 changing concentrations have never been shown in agricultural soils. In this study, we used samples from the Rothamsted Sample Archive16 to determine a temporal record of microplastics in a typical agricultural soil. Here, we provide evidence that agricultural soils are receptors and reservoirs of microplastics, and that the appearance of microplastics in agricultural soils predates modern plasticulture.
Results and Discussion
Soil samples were collected (0–23 cm depth) from the Broadbalk winter wheat experiment sample archive at Rothamsted Research, UK at 18 different time points between 1846 and 2022. A significant increase in microplastic concentrations was observed between 1966 − 2022 across all three treatments selected, farmyard manure (FYM) inorganic fertiliser (N3(P)KMg) and no soil amendments (Nil) (R2 = 0.546, F(1, 28) = 33.607, p = < 0.001), consistent with other sediment and archive analysis5,15 (Fig. 1). From 1846 − 1914, no microplastics were detected in the samples, consistent with the creation of ‘modern plastics’; wholly synthetic materials, 100 years ago17. Particles < 10 μm of unknown composition were present in all samples, attributed to unavoidable contamination during milling, storage, collection and analysis, given that lab environments are sources of airborne microplastics, particularly synthetic fibers18. From 1914 – 1966, it is likely that any microplastics detected are not from agricultural sources but are instead a reflection of increased plastic use in wider society; infrastructure, WWII, fashion and domestic use3,17. From 1966 − 2022, microplastic concentrations in the FYM and N3(P)KMg treatments were significantly different from the Nil treatment (R2 = 0.8, F(3, 26) = 4.6, p = < 0.001 and p = < 0.001, respectively) but not from one another (p = 0.441) indicating that the application of organic and inorganic fertiliser is directly contributing to the microplastic load in the soil beyond baseline concentrations.
Microplastic concentrations (particles 1.5 g−1 soil) in agricultural soil samples collected from the 0–23 cm depth of the Broadbalk winter wheat experiment (and stored in the Rothamsted Sample Archive) between 1846 − 2022. Microplastic concentrations were recorded across three treatments a) FYM (Rothamsted plot number: 2.21); farmyard manure at 35 tonnes ha−1, b) Nil (Rothamsted plot number: 031); no soil amendments, c) N3(P)KMg (Rothamsted plot number: 081); 144 kg N ha−1, 35 kg P ha−1 triple superphosphate until 2000, 90 kg K ha−1 potassium sulphate, 12 kg Mg ha−1 Kieserite (35 kg Mg every third year between 1974 −2000).
From 1966 – 2010, microplastic load in the FYM treatment increased at a gradual rate. This likely reflects the ubiquity of plastics materials on farms with the application, breakdown and distribution of plastic materials, both intentional and unintentional, being included in farmyard manure19. Microplastic concentrations detected in farmyard manure and other comparable soil amendments are very variable, for example Wu et al. 20 found 0 – 3780 microplastic particles kg−1 soil, partly due to the heterogeneity of farming practices, livestock feed and plastic use on farm. From 2010, microplastic concentrations in the FYM treatment appear to plateau, irrespective of the 180 – 183% increase in the Nil and N3(P)KMg treatments, respectively. A plateau or decrease of microplastic concentrations in the plough layer could be a reflection of the vertical transport of microplastics to greater depths exceeding inputs of microplastics to the plough layer. The rate at which microplastics are vertical transported in agricultural soils is governed by farming practices such as tilling, drilling and harvesting, as well as physical, chemical and biological soil processes; the development of hydraulic conduits, shrink-swelling of soil and earthworm ingestion and excretion5,20,21. Given the observed and predicted change in climate variability, particularly the increased likelihood of intense rainfall, microplastics in the plough layer are likely to be removed and discharged into the riverine and marine environment.
Microplastic concentrations were observed to increase steeply between 1997 – 2005 ( + 350%) and 2010 – 2022 ( + 183%) in the N3(P)KMg treatment., reflecting an overall increase from 1966 – 2022. Between 1997 – 2005, the increase is independent of changes in the Nil treatment. Annually, 22,500 tonnes of microplastics are thought to be released from fertilizers and additives7. Degradation of the coatings is highly variable and is therefore expected to influence inter-annual microplastic load. Once the FYM and N3(P)KMg decouple from the Nil treatment, it is expected that the microplastic load in the latter is reflective of tyre wear from farm machinery during field management and non-agricultural sources; wind-blown redistribution, runoff and atmospheric deposition all of which are substantial sources of microplastics in agricultural soils5,7,9,19. The proliferation in microplastic load across all treatments over time is likely reflective of treatment type and an increase in global plastic use, particularly the last decade.
We demonstrate that microplastic concentrations in agricultural soils increase over time and that the application of organic and inorganic fertilisers are significant contributors of microplastics to soil beyond baseline concentrations. The impacts of microplastic pollution on agricultural productivity are largely unquantified and unreliable, due to the lack of longer-term field trials and the use of unrepresentative concentrations in many studies13. Given that microplastic concentrations will likely continue to accumulate in agricultural soils from agricultural and non-agricultural sources, the effects of microplastics in agricultural systems must be better understood. The legacy of plastic use in agricultural soils is poorly reversible. Considering that most reported interactions between microplastics and soil fauna, vegetation, microbiota and crop production are negative22, the accumulation of microplastics in agricultural soils over time poses a direct threat to agricultural productivity and food security. With no immediate solution to remediate microplastic pollution on the horizon, it is critical to reassess our relationship with plastic use in agriculture and beyond.
Methods
Sample preparation
Soil samples were taken from the Broadbalk winter wheat experiment sample archive at 18 different time points between 1846 and 2022. Rothamsted Research (Harpenden, Herts, UK, AL5 2JQ; 51°48′N, 0°22′W). Broadbalk is one of the oldest continuous agronomic experiments in the world and is managed using conventional tillage, pesticide applications when necessary and no irrigation. The plough layer is limed to maintain soil pH between 7.0 – 7.5. The soil type is classified as a Chromic luvisol and has a clay loam to silty clay loam texture15. Samples were collected from three different treatments across 18 different time points, from 1846 – 2022. Samples were collected from the plough layer (0 – 23 cm), milled to 2 mm and stored in sealed glass bottles or card boxes. For each treatment, at each time point, a 1.5 g sample was used for analysis. Three procedural blanks were run to quantify any microplastic contamination during analysis.
Sample treatment
H2O2 was added to each soil sample and heated to 60 °C to remove organic matter8,23. Once effervescence subsided and all visible organic matter was removed, samples were cooled to 40 °C and 5 ml of 0.05 M Fe(II)SO4 was added. Samples were reheated to 60 °C and covered for 24 h to allow Fe(II)SO4 to decompose remaining H2O2 and flocculate clay particles.
Density separation was performed to separate microplastics from the inorganic fraction of the sample. 600 ml 26% w/v NaCl solutions were added, mixed and left to settle for 24 h. The supernatant was filtered through a 0.45 µm glass fibre filter. Beakers were rinsed with HPLC water to capture residual microplastics and the washings filtered. 3 ml of a 0.5% Nile Red solution in n-hexane was applied to the filter paper to stain any microplastics. 3 ml of n-hexane was added to the filter paper. Once dried, filter was transferred to a microscope slide, covered with glass slips and wrapped in foil.
Samples were sealed in sterilised aluminum trays throughout to minimise contamination. Preparation and analysis of samples took place in a sterilised fume cupboard. All glassware, instruments and applicable chemicals were leached with acetone and baked at 400 °C for 4 h. Cotton lab coats and non-synthetic clothing were worn during preparation and analysis. Samples were analysed in triplicate along with blanks. Recovery rates of microplastics were measured and deemed satisfactory.
Sample analysis
A fluorescence microscope (Leica MZFLIII Stereo Fluorescence Microscope) equipped with an integrated digital camera using GXCapture software was used to analyse the samples. Microplastics were examined at three combinations of excitation:emission (Ex:Em) wavelength: 425:480 nm, 475:535 nm, and 510:560 nm; Nile Red-stained plastics have previously been found to fluoresce well at Ex: 450–490 nm; Em: 515–565 nm24. Nile Red approaches to detecting microplastics are rapid, cost-effective and require little technical expertise, but require appropriate quality control23,24,25. Combining H2O2 digestion and multiple density separations is effective to improve the recovery rate of microplastics in complex environmental matrices23. The use of a selection criteria incorporating shape, edge-definition, fluorescence intensity and surface properties is important to reduce misidentification of inorganic matter and any remaining organic matter25. A particle was identified as a microplastic if the following selection criteria were met: (1) the outline of the particle is clearly visible and has well-defined edges; (2) the particle has a 3-dimensional shape resembling a synthetic material; (3) the particle size was greater than 10 µm; (4) there are no internal organic structures; (5) the particle clearly fluoresces in green-yellow; (6) the particle is visible and physically present in all Ex:Em combinations used. Microplastic analysis, identification and quantification was performed by one individual to reduce the risk of overestimation or underestimation of microplastic particles.
Statistical analysis
To measure the relationship between microplastic concentrations, treatment type and time, we performed simple linear regressions with groups analysis, using dummy coding of multi-categorical predictors. Each regression was fitted to the data from 1966, when microplastics were detected in all three plots. Relationships were considered significant at the P < 0.05 level. Analyses were performed using SPSS (IBM Statistics for Windows, Version 28.0).
Data availability
The data that support the findings of this study are published on figshare (https://doi.org/10.6084/m9.figshare.24759615).
References
Bergmann, M. et al. A global plastic treaty must cap production. Science 376, 469–470 (2022).
Hofmann, T. et al. Plastics can be used more sustainably in agriculture. Commun. Earth Environ. 4, 332 (2023).
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
MacLeod, M., Arp, H. P. H., Tekman, M. B. & Jahnke, A. The global threat from plastic pollution. Science 373, 61–65 (2021).
Allen, S. et al. Micro(nano)plastics sources, fate, and effects: What we know after ten years of research. J. Hazardous Mater. Adv. 6, 100057 (2022).
Steinmetz, Z. et al. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 550, 690–705 (2016).
Food and Agriculture Organization (FAO). Assessment of agricultural plastics and their sustainability: A call for action. FAO. https://www.fao.org/3/cb7856en/cb7856en.pdf (2021).
Cusworth, S., Davies, W. J., McAinsh, M. R. & Stevens, C. J. A nationwide assessment of microplastic abundance in agricultural soils: The influence of plastic crop covers within the United Kingdom. Plants People Planet 1–11; https://doi.org/10.1002/ppp3.10430 (2023).
Tian, L. et al. Microplastics in agricultural soils: sources, effects, and their fate. Curr. Opin. Environ. Sci. Health 25, 100311 (2022).
Nizzetto, L., Futter, M. & Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 50, 10777–10779 (2016).
Prata, J. & Dias-Pereira, D. Microplastics in terrestrial domestic animals and human health: implications for food security and food safety and their role as sentinels. Animals 13, 661 (2023).
Xu, L. et al. Missing relationship between meso-and microplastics in adjacent soils and sediments. J. Hazardous Mater. 424, 127234 (2022).
Qi, R. et al. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 703, 134722 (2020).
de Souza Machado, A. A. et al. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 52, 9656–9665 (2018).
Turner, S., Horton, A. A., Rose, N. L. & Hall, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 61, 449–462 (2019).
Rothamsted Research. The Rothamsted Sample Archive. Rothamsted Research. https://www.rothamsted.ac.uk/sample-archive (2023).
Millet, H. et al. The nature of plastics and their societal usage in Plastics and the Environment (eds. Harrison, R. M. & Hester, R. E.) 1-20 (Royal Society of Chemistry, 2018).
Hale, R. et al. A global perspective on microplastics. J Geophys. Res.: Oceans 125, e2018JC014719 (2020).
Piehl, S. et al. Identification and quantification of macro-and microplastics on an agricultural farmland. Sci. Rep. 8, 17950 (2018).
Wu, R. T. et al. Occurrence of microplastic in livestock and poultry manure in South China. Environ. Pollut. 277, 116790 (2021).
Rillig, M. C., Ingraffia, R. & de Souza Machado, A. A. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 8, 1805 (2017).
Pérez-Reverón, R. et al. Microplastics in agricultural systems: analytical methodologies and effects on soil quality and crop yield. Agriculture 12, 1162 (2022).
Radford, F. et al. Developing a systematic method for extraction of microplastics in soils. Analyt. Methods 13, 1695–1705 (2021).
Erni-Cassola, G., Gibson, M. I., Thompson, R. C. & Christie-Oleza, J. A. Lost, but found with Nile red: a novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environ. Sci. Technol. 51, 13641–13648 (2017).
Shruti, V. C., Pérez-Guevara, F., Roy, P. D. & Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazardous Mater. 423, 127171 (2022).
Acknowledgements
This project was funded by BBSRC (EAA7536XS04) and Waitrose Agronomy Group as part of the Waitrose Collaborative Training Partnership. Waitrose and Partners contributed to the funding and development of this project. The Rothamsted Sample Archive is part of the Rothamsted Long-Term Experiments - National Biosciences Research Infrastructure (RLTE-NBRI), funded by BBSRC under award BBS/E/RH/23NB0007 (2023–2028).
Author information
Authors and Affiliations
Contributions
S.J.C., W.J.D., M.R.M. and C.J.S. made an equal contribution to the concept and design of the research. A.S.G. and J.S. provided the resources for the research and contributed to data analysis. S.J.C. collected and analysed the data and produced a first draft of the manuscript. All authors contributed to later drafts, edited and approved the article to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Thilo Hofmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Aliénor Lavergne. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cusworth, S.J., Davies, W.J., McAinsh, M.R. et al. Agricultural fertilisers contribute substantially to microplastic concentrations in UK soils. Commun Earth Environ 5, 7 (2024). https://doi.org/10.1038/s43247-023-01172-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-023-01172-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.