Abstract
Rice paddies account for ~9% or the world’s cropland area and are characterized by environmental conditions promoting soil organic carbon storage, methane emissions and to a lesser extent nitrous oxide emissions. Here, we synthesize data from 612 sites across 51 countries to estimate global carbon stocks in paddy soils and determine the main factors affecting paddy soil carbon storage. Paddy soils (0–100 cm) contain 18 Pg carbon worldwide. Paddy soil carbon stocks decrease with increasing mean annual temperature and soil pH, whereas mean annual precipitation and clay content had minor impacts. Meta-analysis shows that paddy soil carbon stocks can be increased through several management practices. However, greenhouse gas mitigation through paddy soil carbon storage is generally outweighed by increases in methane and nitrous oxide emissions. Our results emphasize the key role of paddies in the global carbon cycle, and the importance of paddy management in minimizing anthropogenic greenhouse gas emissions.
Similar content being viewed by others
Introduction
Soils contain the largest reservoir of terrestrial organic carbon (C) and they are a main natural source of atmospheric carbon dioxide (CO2)1. Soil organic carbon (SOC) is widely recognized as a key element of soil fertility, and croplands with high SOC contents have better structure and lower risks of erosion2. Insights into the global distribution of SOC stocks and the effects of environmental variables will thus improve estimates of C-climate feedbacks, and may contribute to agricultural policies designed to improve soil quality3,4,5. Over the last decade, SOC stocks have been increasingly estimated at global and regional scales for numerous ecosystems, including croplands6, grasslands7, wetlands8,9, and forests10,11. However, even though rice paddies cover ~9% of the global cropland area and provide staple food for roughly half the world’s population12, a global assessment of SOC stocks in rice paddies is still lacking.
Paddy soils are anthropogenic soils (Anthrosols) for cultivation of rice, which are intentionally flooded and puddled, i.e., tilled under water saturated conditions. Paddy soils are widely distributed from temperate to tropical climates on all continents, but mainly in Asia. Rice paddies can be established on various natural and previously agriculturally used soil types, and on various parent materials, but are highly modified by management practices during rice-paddy cultivation13. Because rice paddies are frequently flooded and puddled, their properties differ substantially from those of all other arable upland soil. Anaerobic conditions induced by flooding slow down organic matter decomposition, and thus beneficial to SOC accumulation14. At the same time, these anaerobic conditions promote CH4 production by methanogens, making rice paddies a main source of anthropogenic CH4 emissions15.
The development of efficient irrigation techniques led to expansion of the global paddy area by >30% since the 1960s16. During this period, rising levels of mineral fertilizer application and subsequent increased straw return to soil stimulated SOC storage in paddy soils around the world17,18. For example, the topsoil layer (0–30 cm) of rice paddies in China store ~30% more SOC (45 Mg ha−1) than corresponding upland soils (35 Mg ha−1)19. Therefore, changes in the C pool size of paddies could strongly affect atmospheric CO2 concentrations. However, the size of global rice-paddy SOC pool is still unclear. On a global scale, SOC stocks in upland soils increase with precipitation and clay content and decrease with temperature20, but the main environmental and management factors affecting paddy SOC stocks at different climates have not yet been determined. This information could help to optimize agronomical management designed to enhance SOC sequestration, inform agricultural policy measures designed to improve soil quality, and predict the potential impacts of climate changes on SOC stocks.
Several recent studies have reported paddy SOC stocks in regions that were previously underrepresented in rice-paddy research, such as South America and Africa (e.g., ref. 21). With SOC inventories now being available for most of the world’s rice-growing areas, a data synthesis may reduce the uncertainty regarding paddy soil C stocks and identify practices and areas with high potential for soil C storage. We thus conducted a global synthesis of SOC stocks in the topsoil (0–30 cm) and subsoil (30–100 cm) of rice paddies, including data from 612 sites around the world (Fig. 1a; see Methods and Supplementary Data 1). Our objectives were (1) to determine climatic factors, soil properties, and management practices that affect SOC stocks of paddy topsoils on a global scale; (2) to compare paddy SOC storage between the main rice-producing countries and their contribution to the global paddy SOC pool; and (3) to determine the contribution of SOC storage in paddy soils to the global terrestrial and agricultural SOC pool.
We found that paddy soils (0–100 cm) contain 18 Pg SOC worldwide, ~1.2% of the global SOC pool, corresponding to 14% of the total SOC pool in croplands. Paddy SOC stocks decrease with increasing mean annual temperature and soil pH, but mean annual precipitation and clay content had minor impacts. Meta-analysis further indicates that paddy SOC stocks (0–30 cm) increase with fertilization (9–32%), straw return (13%), and conservation tillage (8–10%). However, climate benefits of SOC storage in paddies are generally negated by increases in CH4 and N2O emissions.
Results
Our database included information about rice paddies between 48°N and 38°S and between 147°E and 90°W. The distribution of sites was skewed towards low elevations, with most sites located below 200 m a.s.l. (Fig. 1b). The SOC content in the topsoil of most sites (>70%) ranged from 7 to 16 g kg−1, with a mean of 13.8 g kg−1. The bulk density (BD) of the topsoil at most sites (>70%) ranged between 1.2 and 1.6 g cm−3, with a mean of 1.3 g cm−3 (Supplementary Fig. 1).
The estimated global average SOC stock of rice paddies is 108 Mg ha−1 for the 0–100 cm layer, ~10% higher than the global average for all soils (Table 1). Average SOC stocks in rice paddies are lower than for mangroves, forests, and wetlands, but substantially higher than for grasslands and croplands (Table 1). Totaled across the globe, the upper 1 m of paddy soils contains 18 Pg (95% CI: 17.2–18.9) organic C. This amounts to ~1.2% of the global SOC pool, or ~14.2% of the total SOC pool in croplands worldwide (Table 2).
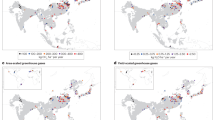
Topsoil paddy SOC stocks ranged between 7 and 330 Mg ha−1 (Fig. 2a). Mean SOC stocks increased with latitude (p < 0.01), from 50 Mg ha−1 in the tropics to 62 Mg ha−1 in temperate regions (Supplementary Fig. 2). Topsoil SOC stocks in rice paddies differed more than three fold between main rice-producing countries (Fig. 2b): paddies in Indonesia and Vietnam had the highest SOC stocks (~78 Mg ha−1), whereas paddies in Pakistan, Cambodia, Africa, and Central and South America contained less than 30 Mg C ha−1. Paddies in China, India, and Indonesia together accounted for ~56% of the global paddy SOC pool (Fig. 2c).
a Spatial distribution of SOC stocks in paddy topsoil (0–30 cm). b Boxplots of SOC stocks in the topsoil (0–30 cm) of the 13 countries. Upper and lower bars: 95th and 5th percentiles of all observations, respectively; top and bottom of boxes: third and first quartiles; black horizontal solid lines in boxes: median values; red dashed lines: mean values. Blue dashed lines indicate global average SOC stock (51 Mg ha−1). The embedded pie chart shows the percentage contribution of each country to global rice production. Countries are arranged by decreasing mean SOC stocks per ha: INA Indonesia, VIE Vietnam, CHN China, JPN Japan, MYA Myanmar, NGR Nigeria, THA Thailand, PHI Philippines, BRA Brazil, BNA Bangladesh, IND India, PAK Pakistan, CAM Cambodia. The numbers below the x-axis indicate the number of datapoints for each country. Three datapoints for Indonesia fell outside the y-axis; these data were included in SOC stock calculations. c Total paddy SOC stocks in the topsoil (0–30 cm) and the 100 cm profile in the 13 countries. The contributions of paddy SOC stocks (0–100 cm) to the global paddy SOC stock for each country are shown as percentages. Error bars indicate standard errors (±SE).
Correlation analyses indicated that paddy SOC stocks are mainly determined by soil pH and mean annual temperature (MAT), and to a much smaller extent by mean annual precipitation (MAP) and clay content (Table 3). SOC stocks decreased with increases in pH and MAT, and slightly increased with increasing MAP and clay content.
Our meta-analysis indicates that N fertilization increased SOC stocks by 9% on average, whereas combined NPK application doubled the increase in SOC stocks compared to sole N fertilization application (Fig. 3). Organic fertilizer application alone and combined with NPK increased SOC stocks by 19% and 32%, respectively. Returning straw to the soil increased C stocks by 13%. Compared to conventional tillage practices, no-till and reduced tillage increased SOC stocks by 10% and 8%, respectively.
Error bars indicate 95% confidence intervals. The number of observations included in the meta-analysis is shown next to the corresponding data point. OF organic fertilizer. Dashed vertical line shows the average of all agronomical practices on the SOC increase. The effects of all presented management practices are significant (p < 0.05).
Discussion
Our data synthesis and meta-analysis reveal the importance of rice paddies for the global C cycle. Per unit area, paddy soils contain more SOC than upland agricultural soils14,22. Whereas rice paddies occupy less than 9% of the global cropland area, they harbor more than 14% of its SOC stocks (Table 2). These large SOC stores can be explained by anaerobic conditions of rice paddies after flooding, slowing down decomposition rates and thus, increasing soil C accumulation compared to other cropland types13,23,24,25.
SOC stocks were best predicted by soil pH (Table 3). This supports previous reports that soil acidity strongly affects ecosystem C balances (e.g., ref. 26). pH regulates several soil properties and processes that play key roles in determining C stocks. For example, the solubility of organic matter decreases under low pH by formation of organic matter complex with polyvalent metal ions such as iron and aluminum26,27. Consequently, leaching of dissolved organic matter will be reduced. Furthermore, low soil pH values slow down litter decomposition by reducing enzyme activity28,29 and by changing the composition of microbial communities30,31.
The MAP was less important than MAT in determining C stocks because of the regular flooding of paddy fields. High temperatures and rainfall in the tropics typically stimulate plant productivity32, but accelerated SOM decomposition negate or even override the effects of increased C inputs from plant production on C stocks33. Similarly, the slowing down of SOC decomposition rates with decreasing temperature34 explains the increase in C stocks with latitude.
Since paddy soils can develop from different parent materials, their initial mineralogy, texture, and fertility can vary considerably13. However, prolonged rice cultivation masks initial soil characteristics and minimizes the influence of parent material on pedogenic features (e.g., ref. 35). This likely explains why clay content explained less of the variation in SOC stock than did other environmental factors (e.g., pH and MAT, Table 3). Upland soils with high clay contents generally store more C than sandy soils36, because clay minerals provide binding surfaces for organic matter and creates anoxic microsites within aggregates. Soil aggregation has minor impact on C dynamics by regular puddling35, and strongly variable redox conditions may reduce the formation and stability of organic matter-clay complexes37. Other recent SOC inventories also suggested that clay contents accounted for a small amount of variation in SOC stocks in rice paddies38. Rather, SOC stabilization in paddies is largely regulated by thermodynamic constraints of organic matter decomposition under anaerobic conditions39.
The impact of environmental factors on paddy soil C stocks can explain some of the main differences between countries. For instance, average paddy SOC stocks were 1.8-fold larger for China (65 Mg ha−1) than India (36 Mg ha−1). The difference between countries partly reflects the specifics of climate; Chinese rice-growing regions predominantly have subtropical climates, whereas Indian rice-growing regions have predominantly hot tropical climates40. Compared to other climate zones, the rate of paddy SOC decomposition in tropical climates is fast40.
Management practices also strongly affect paddy SOC stocks, which probably explains the relatively low amount of variation explained by environmental factors (Table 3) compared to nonagricultural upland soils (e.g., ref. 19). Our global meta-analysis corroborates previous national syntheses of paddy SOC dynamics under various management practices41,42. C gains in fertilized soils are explained by N and other nutrients stimulating plant growth and rhizodeposition, thereby increasing soil C input rates43. Fertilizer N addition can also stimulate soil C storage by slowing down the decomposition of plant litter and SOM (e.g., refs. 44,45). Specifically, N additions might reduce so-called “microbial N mining”, whereby nutrient-poor conditions (e.g., low N) stimulate recalcitrant SOC decomposition by N-acquiring microbes46,47,48. Furthermore, organic fertilizers are additional C input into soil. Finally, organic fertilizers stimulate the succession of microbial communities favorable to SOC accumulation49. However, in addition to previous findings (e.g., ref. 42), significant differences in SOC stocks were recorded between organic fertilizers (OF) and mineral plus organic fertilizers (NPK + OF). High N and other nutrient levels increase microbial growth on the available C pools, and so more necromass will be produced, which is a main component of SOM48.
Our estimates of no-till effects on paddy SOC stocks are quantitatively similar to no-till effects observed in upland soils50 and indicate potential for SOC storage. Conservation tillage increases soil C storage by reducing aeration and the oxidative decomposition of SOC at periods of paddy soil drainage40 and by increasing the physical and chemical protection of C from microbial attacks through organo-mineral associations51. Furthermore, conservation tillage reduces C losses associated with erosion40. Many paddies, especially in Asia, have been continuously tilled for hundreds to thousands of years. These practices likely depleted soil C stocks27, suggesting further potential for C storage under no-till. However, due to data paucity, our meta-analysis only considered C stocks in the top 30 cm. SOC gains in upper soil layers under no-till can be partly offset by losses at lower depths52, thereby reducing the SOC storage potential. Thus, to improve estimates of paddy SOC storage potential under no-till, whole profile analyses are still needed.
Our meta-analysis also explains some of the differences in SOC stocks between countries. For instance, rice paddies in eastern Asia (China, South Korea, and Japan), western Indonesian islands and Madagascar contained more SOC per area unit than paddies in western Africa, southern Asia, and South and Central America (Fig. 2a). These differences can be partly explained by management practices: farmers in southern/southeastern Asia and Africa often cannot afford sufficient mineral fertilizers to improve crop yield and support soil C storage53. Rice straw is also often removed for fodder and other uses in these regions54, thereby reducing soil C input rates and C stocks even further (Fig. 3). In contrast, high fertilizer application rates and high levels of crop residue return in China55,56,57 contribute to high soil C stocks in Chinese rice paddies.
Another factor that might explain low soil C stocks in Africa might be the age of rice paddies. To feed a growing world population and to accommodate changing diets, global croplands have expanded by an average of 4 million hectares per year in recent decades58. Paddy expansion rates during this time differed strongly between continents, with half of the new global paddy area being located in Africa16. African rice paddies contain relatively low amounts of initial SOC compared to other continents8, suggesting a high potential of C sequestration. Our results also indicate considerable potential for paddy soil C sequestration in southern Asia, the central Indochina Peninsula and eastern South America (Fig. 2a). Realizing this potential requires adoption of recommended management practices such as crop residue incorporation, conservation tillage associated with seldom (once per 10–15 years) deep tillage, crop rotations with inclusion of grasses, legumes and deep rooting crops, and integrated nutrient management with pH adjustment through liming2,59. Promoting these practices will require new environmental and economic policies. For instance, aggregating small cropland patches can facilitate efficient fertilizer application, whereas farmer subsidies could provide an incentive for straw incorporation and rotations with deep rooting crops56.
Even though rice paddies store more SOC than the global average, this does not necessarily mean that the recent expansion in paddy area equates to a net climate benefit. First, new rice paddies are often established in ecosystems with relatively high soil C stocks, such as wetlands60,61. Second, rice paddies require a considerable amount of global irrigation water, accounting for 20% of total freshwater withdrawals by crops62. Pumping this water requires energy, which in turn causes ancillary CO2 emissions. In gravity-fed irrigation systems or when pumping water from shallow aquifers this energy requirement can be minimal, but it can be high with diesel-based groundwater extraction systems or when using electricity not generated by hydropower63. Finally, and most importantly, CH4 emissions from rice paddies are substantially higher than for other staple crops62,64, and rice paddies also produce considerable amounts of N2O (e.g., ref. 15). Thus, any benefits in terms of soil C sequestration with rice-paddy establishment need to be considered against a backdrop of increased greenhouse gas (GHG) emissions.
Soil C storage and GHG emissions can be compared directly by expressing them in CO2 equivalents, using the global warming potential (GWP) values over a 100 year time horizon relative to CO2, i.e., 34 for CH4 and 298 for N2O65. Global average GHG emissions from rice paddies have previously been estimated as 6300 kg CO2-eq ha−1 yr–1 for CH416 and 280 kg CO2-eq ha−1 yr−1 for N2O15 (Supplementary Table 1). Average annual soil C storage in rice paddies can be estimated from average rice yields16 and previously reported conversion factors66,67, and amounts to roughly 314 kg CO2-eq ha−1 yr−1, i.e., an order of magnitude less than the combined emissions of CH4 and N2O (Supplementary Table 1). Our estimates are corroborated by field studies showing that even after accounting for soil C storage, rice paddies remain a large net source of GHGs (e.g., refs. 68,69,70. Supplementary Table 2).
Management practices that increase SOC sequestration in rice paddies need to account for increased CH4 emissions as well. Even though rice straw incorporation stimulates soil C storage, it more than doubles CH4 emissions from rice paddies on average71. Previous syntheses suggest that the net effect of these two responses is negative, i.e., straw incorporation constitutes a net source of GHG emissions72. Whereas reduced till and no-till practices generally increase paddy soil C stocks, their effect on CH4 emissions remains uncertain, with recent syntheses suggesting either increases73 or decreases74 in CH4 emissions with no-till. Higher surface SOC with no-till may stimulate CH4 production by increasing the availability of organic substrates75. On the other hand, increased soil macroporosity and soil pore continuity with no-till may accelerate gas diffusion and increase CH4 oxidation76. The net effect of these opposing mechanisms is still unclear, and further research is needed to determine which of these mechanisms dominates under which conditions. Fertilizer N addition not only stimulates paddy SOC storage; it also stimulates N2O emissions from rice paddies77. The effect of fertilizer N on CH4 emissions depends on application rates, with positive effects at low and medium rates, but negative effects at very high rates77. The increase in GHG emissions with fertilizer addition generally outweighs the climate benefit of soil C storage68,69. Moreover, the manufacturing and distribution of fertilizer requires energy and thus produces ancillary CO2 emissions, possibly negating climate benefits78. In addition, excessive fertilizer N application in rice paddies causes a range of other environmental problems79.
Although rice agriculture represents a large net source of GHGs compared to other staple crops, it also shows large potential for GHG mitigation through management64. For instance, mid-season drainage and intermittent irrigation can prevent the development of strong anaerobic conditions, thereby reducing CH4 emissions by 53%80. While these practices stimulate N2O emissions, their net effect on GHG emissions is still negative80. Combining intermittent irrigation with several other management practices, the System of Rice Intensification may reduce both GHG emissions and the use of irrigation water (e.g., refs. 63,81). Applying rice straw off season rather than in season may reduce global CH4 emissions by 4.1 Tg year–182. Moreover, selecting high-yielding cultivars can simultaneously reduce CH4 emissions and increase crop yields83, and so, the C input into soil. A full accounting of the mitigation potential of these measures calls for long-term experiments under real-world conditions that account for changes in soil C stocks, interactions between management practices, as well as direct and ancillary GHG emissions. In addition, models such as DNDC-rice (e.g., ref. 84) may be used to evaluate trade-offs between soil C sequestration and CH4 and N2O emissions under a range of management practices85.
In summary, we present the first global assessment of paddy soil C stocks. Our results identify paddy soils as an important C pool, containing ~20% more SOC per hectare than croplands on average. Our analysis underlines the role of both natural factors and agronomical management in determining paddy SOC stocks; fertilization, straw incorporation, and no-till practices all increased paddy SOC storage, whereas SOC levels decreased with MAT and soil pH. However, the climate benefit of SOC storage in rice paddies is generally outweighed by increases in GHG emissions. These data underline the importance of paddies in the terrestrial C cycle, and should be used to improve global C inventories and to inform policy advice related to land use.
Methods
Data collection
We used Web of Science, Google Scholar, and China National Knowledge Infrastructure to search for studies published between 1999 and 2019, applying the search terms “paddy AND soil organic carbon” and “rice AND soil organic carbon”. We only considered studies reporting contents for soil organic carbon (SOC) or soil organic matter (SOM) and sampling depth for the quantitative determination of paddy SOC storage (excluding upland rice). For each study we tabulated SOC contents and sampling depth; we also tabulated soil bulk density (BD) data when these were reported. We selected a total of 239 publications reporting 2234 sets of raw data from rice paddies around the world for analyses (see Supplementary Data 1). To avoid data duplication, we checked the latitude and longitude of all the sites included in our dataset, and we eliminated duplicated sites. Finally, our dataset included 612 sampling locations in 50 countries (out of 118 rice-producing countries, Fig. 1a) encompassing 95% of the global paddy area and 98% of the global rice production.
We also tabulated the following information for each study: (1) geographical location of sites (latitude, longitude, elevation above sea level, and country), (2) climatic conditions (mean annual temperature, MAT; and mean annual precipitation, MAP), and (3) properties of the paddy soil (pH and clay content). Missing data for latitude, longitude, and elevation were estimated using Google Maps (https://maps.google.com/). Missing data for MAT and MAP were obtained from https://en.climate-data.org/.
Data processing
Estimating missing data for SOC content and BD
Paddy SOC stocks were calculated separately for the topsoil (0–30 cm) and subsoil (30–100 cm) to facilitate a comparison between our results and global SOC storage data in the Harmonized World Soil Database86. Original data reported as SOM content were converted to SOC content using the conventional “van Bemmelen factor” of 1.72487.
Most SOC data in our database were for soil layers to a maximum depth of 30 cm or less. To extrapolate these data to lower depths, we used a subset of 42 studies that reported SOC contents for 409 profiles ≥50 cm. We assume that soil compaction was similar across all the profiles and could be extrapolated to other SOC data in the deep soil. The relative ratio of SOC content (RRSOC, see ref. 88) was first calculated as:
where SOCsurface is the SOC content (g kg−1) of surface soil and SOCbelow is the SOC content below the surface soil for various depths in the profile. Combing the data for all studies in the subset, the relationship between RRSOC and soil depth could be described by a logarithmic curve (Supplementary Fig. 3, R2 = 0.63, n = 1227, see ref. 89). SOC content at depth i (cm) was then estimated as:
The depth gradient for BD was less pronounced than for SOC content (Supplementary Fig. 4a). The relationship between BD and SOC content of the topsoil in our dataset could be described by a negative power function (Fig. S5; R2 = 0.49, n = 1370):
The availability of subsoil BD data was insufficient to perform a regression analysis with SOC content. Because the ratio of subsoil over topsoil bulk density averaged 1.18 across our dataset (Supplementary Fig. 4b; standard error = 0.01, n = 376), we estimated missing subsoil BDs by multiplying the topsoil BDs by 1.18.
Estimating paddy SOC stocks in the topsoil and subsoil
For each soil layer at each sampling location in our dataset, total SOC stock (SOCT, Mg ha−1) was calculated according to ref. 21
where SOC and BD are SOC content (g kg−1) and bulk density (g cm−3), respectively, H is soil thickness (cm) and δ2mm is the fraction (%) of fragments >2 mm in the soil. Since the paddy soils were mostly derived from deposits in flat areas, the >2 mm fraction of the total mass is usually negligible21.
When SOC content and BD data were available for the entire 0–100 cm profile, we calculated SOC stock (Mg ha−1) in the 0–30 cm topsoil layer (SOCT30) and the entire 0–100 cm profile (SOCT100) by adding SOCT of all soil layers within the 0–30 cm and 0–100 cm range, respectively.
When SOC content or BD were not available for some of the 0–30 cm or 0–100 cm profile, we used the following formulas instead:
where SOCa and SOCTa are the SOC content (g kg−1) and SOC stock in the topsoil, respectively, BD is bulk density (g cm−3) in the topsoil. Missing BDs were estimated using the formula in Supplementary Fig. 5.
Estimating national and global paddy SOC stocks
National paddy SOC stocks (SOCTN, Pg) for any country in our dataset were estimated as:
where SOCTmean (Mg ha−1) is the mean SOC stock across all sampling locations in that country and HA is the rice harvest area (ha) in that country. HA data for all countries in our analysis were derived from FAO15.
The global SOC stock (SOCTG, Pg) was estimated as:
where SOCTNa is paddy SOC stock (Pg) in country a, and SOCTNn is paddy SOC stock (Pg) in country n. No SOC data were available for some countries where HA was small (e.g., Congo, Mali, and Peru). For these countries, we estimated SOCTN using the SOCN data from neighboring countries with the closest climate based on Köppen-Geiger climate classification. These estimates did not substantially affect the estimates of the global SOC stocks because of the small areas of these countries (~5% of total HA). Global mean SOC stocks per unit area was calculated based on global SOC stocks (SOCTG) divided by global rice-paddy area.
Data analysis
The importance of the environmental variables was estimated using Pearson’s and partial correlation coefficients, which are commonly used to measure the association between variables, implemented in SPSS 20.0 (SPSS, Chicago, USA). In these correlations, the p value defines whether two variables are statistically correlated. p values below 0.05 were accepted as significant correlations. Optimized model for SOC with environmental variables was determined by stepwise regression using forward selection criteria (p of 0.05 for entering and 0.1 for removal). ArcGIS 10.3 (Esri, Redlands, USA) was used to analyze and visualize the spatial distribution of SOC stocks.
The 95% confidence interval (CI) of SOC stocks (SOCTN) in each country was calculated by bootstrapping, using 4999 iterations90. The uncertainty (U) of the total SOC stock in each country was then calculated as:
where x is the SOC stock in a country, and CI is the 95% confidence interval of x. The total uncertainty (Utotal) at the global scale was calculated as:
where Ua and Un are the uncertainties associated with xa and xn in country a and country n. Because some countries were represented by only one site, the CI of that country could not be calculated. In these cases, the coefficient of variation was conservatively set to 50%91. Global SOC stocks were then estimated as described by Eq. 8, and the 95% confidence CI of this estimate was calculated using Eq. 9.
Meta-analysis
We assessed the effects of management practices (fertilization, return of straw, and tillage) on paddy SOC stocks by creating subsets of experiments that included side-by-side comparisons between management practices. Studies had to meet specific criteria to be included in the dataset. First, growing conditions in the control and treatment plots had to be identical (except for the management practice being studied). Second, mean SOC stock and the number of field replicates had to be reported for both control and treatment plots. Studies were incorporated into seven datasets, based on seven types of management practices: (1) addition of mineral nitrogen (N) fertilizer, (2) addition of mineral nitrogen–phosphorus–potassium (NPK) fertilizer, (3) addition of organic fertilizer (e.g., green/farmyard manure, compost), (4) addition of mineral NPK and organic fertilizer, (5) no tillage, (6) reduced tillage, and (7) the return of straw.
The effects of these management practices on SOC stocks were quantified as the natural log of the response ratio (lnRR), a metric commonly used in meta-analyses:92
where SOCTt and SOCTc represent the mean SOC stock in the 0–30 cm layer of the treatment and control groups, respectively. SOC stocks for the 0–30 cm layer were calculated as described above. Treatments included (i) the application of mineral fertilizers (e.g., N or NPK), organic fertilizer (OF), and mineral plus organic fertilizers (NPK + OF) versus no application, (ii) straw return versus no return, and (iii) no or reduced tillage versus conventional tillage.
Most studies in our analysis did not report the standard deviations of the means. We therefore adopted a replication‐based weighting method:93,94
where nt and nc are the numbers of replicates of the treatment and control, respectively.
Mean effect sizes and 95% confidence intervals (CIs) were generated by bootstrapping with 4999 iterations using MetaWin 2.190. Effects of paddy management were considered significant if the 95% CIs did not overlap with zero. To ease interpretation, results were back-transformed to percent change ((RR − 1) × 100) in SOC stocks. Positive and negative changes indicate increases and decreases due to the management practices, respectively.
Data availability
The datasets generated during the current study are available at https://doi.org/10.5281/zenodo.5102775
References
Batjes, N. H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 65, 10–21 (1996).
Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623–1627 (2004).
Buringh, P. in The role of terrestrial vegetation in the global carbon cycle: Measurement by remote sensing, 91–109 (Wiley, 1984).
Hiederer, R. & Köchy, M. Global soil organic carbon estimates and the harmonized world soil database. EUR 79, 25225 (2011).
Smith, P. et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 22, 1008–1028 (2016).
Schlesinger, W. H. The Role of Terrestrial Vegetation in the Global Carbon Cycle: Measurement by Remote Sensing (Wiley, 1984).
Conant, R. T., Cerri, C. E., Osborne, B. B. & Paustian, K. Grassland management impacts on soil carbon stocks: a new synthesis. Ecol. Appl. 27, 662–668 (2017).
Köchy, M., Hiederer, R. & Freibauer, A. Global distribution of soil organic carbon–Part 1: masses and frequency distributions of SOC stocks for the tropics, permafrost regions, wetlands, and the world. Soil 1, 351–365 (2015).
Nahlik, A. M. & Fennessy, M. S. Carbon storage in US wetlands. Nat. Commun. 7, 1–9 (2016).
Dixon, R. K. et al. Carbon pools and flux of global forest ecosystems. Science 263, 185–190 (1994).
Atwood, T. B. et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 7, 523–528 (2017).
Maclean, J. L., Dawe, D. C., Hardy, B. & Hettel, G. P. Rice Almanac: Source book for the most important economic activity on earth, 3rd edn. (CABI Publishing, 2002).
Kögel-Knabner, I. et al. Biogeochemistry of paddy soils. Geoderma 157, 1–14 (2010).
Wu, J. Carbon accumulation in paddy ecosystems in subtropical China: evidence from landscape studies. Eur. J. Soil Sci. 62, 29–34 (2011).
Carlson, K. M. et al. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 7, 63–68 (2017).
FAO (Food and Agriculture Organization of the United Nations). FAOSTAT: FAO Statistical Databases. http://faostat.fao.org/default.aspx (2018).
Gattinger, A. et al. Enhanced top soil carbon stocks under organic farming. Proc. Natl Acad. Sci. USA 109, 18226–18231 (2012).
Xie, Z. et al. Soil organic carbon stocks in China and changes from 1980s to 2000s. Glob. Chang. Biol. 13, 1989–2007 (2007).
Qin, Z., Huang, Y. & Zhuang, Q. Soil organic carbon sequestration potential of cropland in China. Glob. Biogeochem. Cycles 27, 711–722 (2013).
Jobbágy, E. G. & Jackson, R. B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 10, 423–436 (2000).
Haefele, S. M., Nelson, A. & Hijmans, R. J. Soil quality and constraints in global rice production. Geoderma 235, 250–259 (2014).
Pan, G., Li, L., Wu, L. & Zhang, X. Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Glob. Chang. Biol. 10, 79–92 (2004).
Wei, L. et al. Comparing carbon and nitrogen stocks in paddy and upland soils: Accumulation, stabilization mechanisms, and environmental drivers. Geoderma 398, 115121 (2021).
Wang, P. et al. Long-term rice cultivation stabilizes soil organic carbon and promotes soil microbial activity in a salt marsh derived soil chronosequence. Sci. Rep. 5, 15704 (2015).
Li, Y. et al. Oxygen availability determines key regulators in soil organic carbon mineralisation in paddy soils. Soil Biol. Biochem. 153, 108106 (2021).
Evans, C. D. et al. Acidity controls on dissolved organic carbon mobility in organic soils. Glob. Chang. Biol. 18, 3317–3331 (2012).
Liu, Y. et al. Impact of prolonged rice cultivation on coupling relationship among C, Fe, and Fe-reducing bacteria over a 1000-year paddy soil chronosequence. Biol. Fertil. Soils 55, 589–602 (2019).
Sinsabaugh, R. L. et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 11, 1252–1264 (2008).
Liu, Y. et al. Microbial activity promoted with organic carbon accumulation in macroaggregates of paddy soils under long-term rice cultivation. Biogeosciences 13, 6565–6586 (2016).
Liu, Y. et al. Methanogenic abundance and changes in community structure along a rice soil chronosequence from east China. Eur. J. Soil Sci. 67, 443–455 (2016).
Malik, A. A. et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 9, 1–10 (2018).
Don, A., Schumacher, J. & Freibauer, A. Impact of tropical land‐use change on soil organic carbon stocks-a meta‐analysis. Glob. Chang. Biol. 17, 1658–1670 (2011).
Piao, S. et al. The carbon balance of terrestrial ecosystems in China. Nature 458, 1009–1013 (2009).
Davidson, E. A. & Janssens, I. A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173 (2006).
Kirk, G. The Biogeochemistry of Submerged Soils (Wiley, 2004).
Kramer, M. G., Sanderman, J., Chadwick, O. A., Chorover, J. & Vitousek, P. M. Long‐term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Chang. Biol. 18, 2594–2605 (2012).
Scharpenseel, H. W., Pfeiffer, E. M. & Becker-Heidmann, P. in Advances in Soil Science (eds. Carter, MR, Stewart, BA) (Lewis Publishers, 1996).
Liao, Q. et al. Increase in soil organic carbon stock over the last two decades in China’s Jiangsu Province. Glob. Chang. Biol. 15, 861–875 (2009).
Keiluweit, M., Wanzek, T., Kleber, M., Nico, P. & Fendorf, S. Anaerobic microsites have an unaccounted role in soil carbon stabilization. Nat. Commun. 8, 1–10 (2017).
Ghimire, R., Lamichhane, S., Acharya, B. S., Bista, P. & Sainju, U. M. Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: a review. J. Integr. Agric. 16, 1–15 (2017).
Maillard, É. & Angers, D. A. Animal manure application and soil organic carbon stocks: a meta‐analysis. Glob. Chang. Biol. 20, 666–679 (2014).
Tian, K. et al. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: a meta-analysis. Agric. Ecosyst. Environ. 204, 40–50 (2015).
Liu, Y. et al. Initial utilization of rhizodeposits with rice growth in paddy soils: rhizosphere and N fertilization effects. Geoderma 338, 30–39 (2019).
Chen, J. et al. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 4, eaaq1689 (2018).
Zhu, Z. et al. Rice rhizodeposits affect organic matter decomposition in paddy soil: the role of N fertilization and rice growth for enzyme activities, CO2 and CH4 emissions. Soil Biol. Biochem. 116, 369–377 (2018).
Moorhead, D. L. & Sinsabaugh, R. L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 76, 151–174 (2006).
Li, X. et al. Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol. Biochem. 112, 47–55 (2017).
Cui, J. et al. Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol. Biochem. 142, 107720 (2020).
Geisseler, D., Linquist, B. A. & Lazicki, P. A. Effect of fertilization on soil microorganisms in paddy rice systems—a meta-analysis. Soil Biol. Biochem. 115, 452–460 (2017).
Sun, W. et al. Climate drives global soil carbon sequestration and crop yield changes under conservation agriculture. Glob. Chang. Biol. 26, 3325–3335 (2020).
Wissing, L. et al. Management-induced organic carbon accumulation in paddy soils: the role of organo-mineral associations. Soil Tillage Res. 126, 60–71 (2013).
Baker, J. M., Ochsner, T. E., Venterea, R. T. & Griffis, T. J. Tillage and soil carbon sequestration—-what do we really know? Agric. Ecosyst. Environ. 118, 1–5 (2007).
Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 60, 158–169 (2009).
Lal, R. Soil carbon sequestration in India. Clim. Change 65, 277–296 (2004).
Liu, Y. et al. Carbon input and allocation by rice into paddy soils: a review. Soil Biol. Biochem. 133, 97–107 (2019).
Zhao, Y. et al. Economics-and policy-driven organic carbon input enhancement dominates soil organic carbon accumulation in Chinese croplands. Proc. Natl Acad. Sci. USA 115, 4045–4050 (2018).
Wei, X., Zhu, Z., Wei, L., Wu, J. & Ge, T. Biogeochemical cycles of key elements in the paddy-rice rhizosphere: microbial mechanisms and coupling processes. Rhizosphere 10, 100145 (2019).
Alexandratos, N. & Bruinsma, J. World agriculture towards 2030/2050: the 2012 revision. https://doi.org/10.22004/ag.econ.288998. (2012).
Rui, W. & Zhang, W. Effect size and duration of recommended management practices on carbon sequestration in paddy field in Yangtze Delta Plain of China: a meta-analysis. Agric. Ecosyst. Environ. 135, 199–205 (2010).
Song, K. et al. Wetland degradation: its driving forces and environmental impacts in the Sanjiang Plain, China. Environ. Manage. 54, 255–271 (2014).
Dong, J. et al. Northward expansion of paddy rice in northeastern Asia during 2000–2014. Geophys. Res. Lett. 43, 3754–3761 (2016).
Chaturvedi, V. et al. Climate mitigation policy implications for global irrigation water demand. Mitig. Adapt. Strat. Glob. Chang. 20, 389–407 (2015).
Gathorne-Hardy, A. A life cycle assessment (LCA) of greenhouse gas emissions from SRI and flooded rice production in SE India. Taiwan Water Conserv. J. 61, 111–125 (2013).
Linquist, B., Van Groenigen, K. J., Adviento‐Borbe, M. A., Pittelkow, C. & Van Kessel, C. An agronomic assessment of greenhouse gas emissions from major cereal crops. Glob. Chang. Biol. 18, 194–209 (2012).
IPCC. in Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change. (eds. Field, C. B. et al) (Cambridge University Press, 2014).
Xie, Z. et al. CO2 mitigation potential in farmland of China by altering current organic matter amendment pattern. Sci. China Earth Sci. 53, 1351–1357 (2010).
Yan, X. et al. Carbon sequestration efficiency in paddy soil and upland soil under long-term fertilization in southern China. Soil Tillage Res. 130, 42–51 (2013).
Shang, Q. et al. Net annual global warming potential and greenhouse gas intensity in Chinese double rice‐cropping systems: a 3‐year field measurement in long‐term fertilizer experiments. Glob. Chang. Biol. 17, 2196–2210 (2011).
Ma, Y. et al. Net global warming potential and greenhouse gas intensity of annual rice–wheat rotations with integrated soil–crop system management. Agric. Ecosyst. Environ. 164, 209–219 (2013).
Xiong, Z. et al. Differences in net global warming potential and greenhouse gas intensity between major rice-based cropping systems in China. Sci. Rep. 5, 1–9 (2015).
Jiang, Y. et al. Acclimation of methane emissions from rice paddy fields to straw addition. Sci. Adv. 5, eaau9038 (2019).
Liu, C., Lu, M., Cui, J., Li, B. & Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: a meta‐analysis. Glob. Chang. Biol. 20, 1366–1381 (2014).
Shakoor, A. et al. A global meta-analysis of greenhouse gases emission and crop yield under no-tillage as compared to conventional tillage. Sci. Total Environ. 750, 142299 (2021).
Zhao, X. et al. Methane and nitrous oxide emissions under no‐till farming in China: a meta‐analysis. Glob. Chang. Biol. 22, 1372–1384 (2016).
Kim, S. Y., Gutierrez, J. & Kim, P. J. Unexpected stimulation of CH4 emissions under continuous no-tillage system in mono-rice paddy soils during cultivation. Geoderma 267, 34–40 (2016).
Ball, B. C., Scott, A. & Parker, J. P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 53, 29–39 (1999).
Linquist, B. A., Adviento-Borbe, M. A., Pittelkow, C. M., van Kessel, C. & van Groenigen, K. J. Fertilizer management practices and greenhouse gas emissions from rice systems: a quantitative review and analysis. Field Crop. Res. 135, 10–21 (2012).
Schlesinger, W. H. Carbon sequestration in soils: some cautions amidst optimism. Agric. Ecosyst. Environ. 82, 121–127 (2000).
Choudhury, A. T. M. A. & Kennedy, I. R. Nitrogen fertilizer losses from rice soils and control of environmental pollution problems. Commun. Soil Sci. Plan. 36, 1625–1639 (2005).
Jiang, Y. et al. Water management to mitigate the global warming potential of rice systems: a global meta-analysis. Field Crop. Res. 234, 47–54 (2019).
Suryavanshi, P., Singh, Y. V., Prasanna, R., Bhatia, A. & Shivay, Y. S. Pattern of methane emission and water productivity under different methods of rice crop establishment. Paddy Water Environ. 11, 321–329 (2013).
Yan, X., Akiyama, H., Yagi, K. & Akimoto, H. Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 Intergovernmental Panel on Climate Change Guidelines. Glob. Biogeochem. Cycles https://doi.org/10.1029/2008GB003299 (2009).
Jiang, Y. et al. Higher yields and lower methane emissions with new rice cultivars. Glob. Chang. Biol. 23, 4728–4738 (2017).
Li, C. et al. Modeling greenhouse gas emissions from rice-based production systems: sensitivity and upscaling. Glob. Biogeochem. Cycles https://doi.org/10.1029/2003GB002045 (2004).
Yin, S. et al. Carbon sequestration and emissions mitigation in paddy fields based on the DNDC model: a review. Artif. Intell. Agric. 4, 140–149 (2020).
FAO, IIASA, ISRIC, ISSCAS, and JRC: Harmonized World Soil Database (version 1.2), Tech. Rep., FAO, Rome, Italy and IIASA, Laxenburg, Austria (2012).
Allison, L. in Organic carbon. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, (ed. A.g. Norman). (American Society of Agronomy, 1965).
Fang, C. & Moncrieff, J. B. The variation of soil microbial respiration with depth in relation to soil carbon composition. Plant Soil 268, 243–253 (2005).
Yan, X., Cai, Z., Wang, S. & Smith, P. Direct measurement of soil organic carbon content change in the croplands of China. Glob. Chang. Biol. 17, 1487–1496 (2011).
Rosenberg, M. S., Adams, D. C. & Gurevitch, J. MetaWin 2.0: statistical software for meta-analysis (Sinauer, 2000).
Yue, Q. et al. Deriving emission factors and estimating direct nitrous oxide emissions for crop cultivation in China. Environ. Sci. Technol. 53, 10246–10257 (2019).
Hedges, L. V., Gurevitch, J. & Curtis, P. S. The meta‐analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999).
Adams, D. C., Gurevitch, J. & Rosenberg, M. S. Resampling tests for meta‐analysis of ecological data. Ecology 78, 1277–1283 (1997).
Van Groenigen, K. J., Osenberg, C. W. & Hungate, B. A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475, 214–216 (2011).
Acknowledgements
This study was supported by the National Key Research and Development program (2017YFD0800104), the National Natural Science Foundation of China (41977088, 41807089; 41977093; 41761134095); the Natural Science Foundation of Hunan Province (2019JJ10003; 2019JJ30028), the Youth Innovation Team Project of Institute of Subtropical Agriculture, Chinese Academy of Sciences (2017QNCXTD_GTD), and the International Postdoctoral Exchange Fellowship Program 2018 (20180017). The research of J.P. and J.S. was funded by the European Research Council Synergy grant ERC-2013-SyG-610028 IMBALANCE-P. The grants or other support to Ge T. from the Alexander von Humboldt Foundation of Germany and K. C. Wong Magna Fund in Ningbo University are also acknowledged with gratitude.
Author information
Authors and Affiliations
Contributions
Y.L.L., T.G., K.J.v.G., G.G., and Y.K. conceived and designed this work; Y.L.L. and P.W. collected and organized data; Y.L.L., T.G., K.J.v.G., Y.Y., K.C., Z.Z., J.K.W., Y.L., G.G. J.S., J.P., J.S.W., and Y.K. took part in data discussion; Y.L.L. analyzed data and wrote the manuscript with contributions from all authors; Y.L.L. and K.J.v.G. revised the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Earth & Environment thanks Andreas Gattinger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joshua Dean, Joe Aslin and Clare Davis.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Ge, T., van Groenigen, K.J. et al. Rice paddy soils are a quantitatively important carbon store according to a global synthesis. Commun Earth Environ 2, 154 (2021). https://doi.org/10.1038/s43247-021-00229-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-021-00229-0
This article is cited by
-
Co-benefits for net carbon emissions and rice yields through improved management of organic nitrogen and water
Nature Food (2024)
-
Temporal variations in carbon stocks and soil fertility in Inceptisols after 12 years of paddy rice cultivation
Plant and Soil (2024)
-
CO2 Fluxes Over Water-Saving Paddy Fields with Different Straw Management Methods on the Basis of the Same Amount of Carbon Input
Journal of Soil Science and Plant Nutrition (2024)
-
Greenhouse gas emissions and mitigation in rice agriculture
Nature Reviews Earth & Environment (2023)
-
Change in Microbial Metabolic Quotient Under Biochar Amendment Was Associated with Soil Organic Carbon Quality, Microbial Community Composition, and Enzyme Activity in Bulk and Rhizosphere Soils in an Acid Rice Paddy
Journal of Soil Science and Plant Nutrition (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.