Abstract
Peatlands have persisted as massive carbon sinks over millennia, even during past periods of climate change. The commonly accepted theory of abiotic controls (mainly anoxia and low temperature) over carbon decomposition cannot fully explain how vast low-latitude shrub/tree dominated (wooded) peatlands consistently accrete peat under warm and seasonally unsaturated conditions. Here we show, by comparing the composition and ecological traits of microbes between Sphagnum- and shrub-dominated peatlands, that slow-growing microbes decisively dominate the studied shrub-dominated peatlands, concomitant with plant-induced increases in highly recalcitrant carbon and phenolics. The slow-growing microbes metabolize organic matter thirty times slower than the fast-growing microbes that dominate our Sphagnum-dominated site. We suggest that the high-phenolic shrub/tree induced shifts in microbial composition may compensate for positive effects of temperature and/or drought on metabolism over time in peatlands. This biotic self-sustaining process that modulates abiotic controls on carbon cycling may improve projections of long-term, climate-carbon feedbacks in peatlands.
Similar content being viewed by others
Introduction
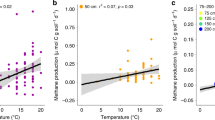
Peatlands cover only 3% of land surface but currently maintain 600–700 Gt of carbon (1 Gt = 1015 g), which exceeds global vegetation carbon stores and is close to the pool of atmospheric CO21,2,3,4. Hence, both the fate of the massive carbon stores in peat and the way peatlands, particularly their carbon-sequestration/release processes, respond to climate change are highly important to future climates. Generally, rates of carbon decomposition via soil microbial respiration increase exponentially with rising temperature in the short term5. Many experiments show that climate warming and drought may not only increase peat loss by accelerating decomposition6 but also could cause substantial losses of the keystone mosses like Sphagnum in the vast boreal peatlands7,8,9, followed by shrub/tree expansion and its uncertain effects10,11,12,13. Such cascading events could provoke a substantial positive feedback to global warming6,12,14. However, long-term warming experiments in grasslands15,16 and studies spanning a wide range of mean annual temperature (MAT) globally17,18 show declining microbial metabolism over time under experimental warming or in warmer regions. To date, most experimental studies in peatlands have lasted only for months to decades, and such time scales are deemed too short to detect long-term (>100 years) effects of climate change on millennial peatlands that may have complex evolutions/successions during past climatic fluctuations19. High-resolution stratigraphic analyses on peat profiles across boreal areas have documented that vegetation composition and net primary productivity played key roles in carbon accumulation during the last millennium19,20. Moreover, a recent study shows that plant taxonomic and functional turnover are decoupled across European peat bogs, which make these ecosystems much more resilient to climate change21. We compiled soil respiration data from >200 peatland sites across latitudes between 2°S and 75°N (Supplementary Data 1) to further test whether the dependence of decomposition on temperature applies to a wide range of MAT in peatlands. As both heterotrophic and autotrophic respirations were included here and plants with higher biomass in the tropical regions beget higher autotrophic respiration22, we expected to see an apparent exponential rise in soil respiration along with increasing MAT. However, we found the relationship did not exist (Fig. 1). The paleontological evidence19,20, apparent decoupling of plant taxonomic and functional turnover21, and our large-scale soil respiration analysis (Fig. 1) together challenge the current abiotic-factor-dependent peat decay models (mainly temperature and water level) that is embedded into the Earth System Models to project climate-carbon feedback14,23. This discrepancy, we assume, could mainly result from the latent role of changing plant communities and their associated ecological (mainly microbial) and biogeochemical processes—a commonly occurring state shift in peatland communities induced by persistent climate change19. Changes in dominant plant communities among mosses, sedges, shrubs, and trees may bring forth substantial top-down and bottom-up regulations24 on the peatland ecosystem through alteration of plant–microbe traits, specifically plant/soil chemistry25 and microbial composition/function10,26,27,28 while maintaining similar functions29,30. Although temperature as an abiotic factor dominantly controls microbial metabolism of soil carbon in monocultures or a constant environment, some evolutional acclimations and interactions in plant/microbial physiology and community composition, as biotic factors in response to long-term climate change in peatlands16,26,27,28,31,32,33 are still unclear. We therefore hypothesized that the unknown biotic controls and interactions (vegetation and microbes) developed over time might be one of the major uncertainties and challenges in projecting the long-term carbon-climate feedbacks in peatlands in the Earth System Models34, thus recognition of which could be central to the development of a meaningful framework for unraveling the future of peatlands26,28.
Data were compiled from 36 published studies that contain annual mean soil respirations observed in >200 sites globally (Supplementary Data 1).
Here, we set up a series of field and lab experiments in a logically progressive way and suggest that climate-change-induced shifts in dominant microbes to vegetation change, as biotic control, may sustain soil organic matter over time in peatlands facing long-term climate change. We first compared a boreal Sphagnum-dominated peatland with a shrub-dominated subtropical peatland in terms of the composition and functional traits of fungi—the predominant peat decomposers—and their relationships with soil physicochemical parameters. To further verify whether such functional traits exist in other wooded (shrub/tree-dominated) peatlands, we reanalyzed and compared fungal data from a subtropical peatland in China and a coastal wooded peatland in Canada. We showed that slow-growing fungi dominate many wooded peatlands globally. Finally, we verified the proposed consequence of the microbial shifts on carbon loss in peatlands through a reciprocal inoculation experiment.
Results and discussion
Slow-growing fungi dominate shrub-dominated peatlands
An ombrotrophic boreal Sphagnum-dominated peatland and a subtropical shrub-dominated peatland in the USA were first selected to study shifts of ecological processes in peatlands over a long term. The Sphagnum-dominated site is located in the Marcell Experimental Forest, MN, USA, and the shrub-dominated site is found in Pocosin Lakes National Wildlife Refuge (Pocosins), NC, USA (Supplementary Tables 1 and 2). The Sphagnum-dominated site is dominated by Sphagnum mosses (coverage >90%) with black spruce (Picea mariana) and scattered shrubs, while the shrub-dominated site has responded to climate change over the past 12,000 years through a transition of plant communities from boreal Sphagnum/spruce during late-glacial to the modern ericaceous shrubs (coverage >90%) found today13,35. The shrub species in Pocosins are similar in physiognomy to the expanding ericaceous shrubs found in many drained Sphagnum-dominated peatlands in boreal areas10,11,36. Peat is dominantly formed by Sphagnum moss in the Sphagnum-dominated sites, and shrubs mainly build peat in the shrub-dominated sites.
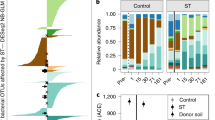
To determine the underlying microbial communities and their ecological traits, we collected triplicate soil cores from the hollows and hummocks in the boreal Sphagnum-dominated peatland and from three sites with different plants and water levels in the subtropical shrub-dominated peatland (Supplementary Table 1), and measured the composition and abundance of fungi, the relative contributions of fungi and bacteria to peat decomposition, and the associated physicochemical peat parameters. Compared to the Sphagnum-formed peat in the boreal site, in the shrub-formed peat in the subtropical site, the dissolved phenolics were 6–8 times higher, while soil pH, concentration of inorganic nitrogen and soil moisture were lower (Supplementary Table 1), which indicate the shrub-dominated peatland was more oligotrophic. Consistent with many previous studies37,38, fungi were the predominant peat decomposers in the unsaturated upper layers with contributions of 93.4% and 95.2% in the Sphagnum- and shrub-dominated sites, respectively (Supplementary Fig. 1, also see Supplementary Note 1). The Sphagnum- and shrub-dominated peatlands distinctively differed in their fungal community composition (Fig. 2). Fungal communities from the Sphagnum-dominated sites were dominated by Pseudeurotium, Saccharomycetales, and Mortierella, whereas the shrub-dominated sites were dominated by Archaeorhizomyces and Helotiales characterized by slower growing rates and also forming symbiotic associations with plant roots (Fig. 2 and Supplementary Data 2). As microbial growth rates significantly affect carbon turnover in soil29,30, we classified the dominant fungi as either fast- or slow-growing groups based on known growth traits of culturable species from each taxonomic group (Supplementary Table 3). Given current limitations on detecting growth rates of unculturable fungi, this gives us a reasonable first approximation although some inevitable biases exist in this classification. Notably, nearly 85% of dominant fungi (relative abundance of each operational taxonomic unit (OTU) > 1%) were fast-growing at the Sphagnum-dominated site, but at the shrub-dominated site a mere 2% were categorized as fast-growing and about 75% as slow-growing based on their ecological traits (Fig. 2 and Supplementary Table 3). Consistent with the majority of fungi found in many boreal peatlands37, the dominant fungi at the Sphagnum-dominated site are members of pioneer saprobe communities that use simple carbon compounds and possibly possess r-selected strategy with fast growth rates29,30,37. Unexpectedly, 11–97% of total fungal sequences at the shrub-dominated site were assigned to Archaeorhizomyces, but only 0.4% on average at the Sphagnum-dominated site. Archaeorhizomyces, which represents one of the ubiquitous lineages of soil fungi, is characterized by markedly slow growth39. Another dominant fungal group (~5%) in the shrub-dominated site is the Helotiales, which includes certain fungi that form ericoid mycorrhizal fungi with resistant melanized cell walls (so-called dark-septate endophytes) which are also characterized by slow growth rates40.
Relative abundance (mean ± S.E.) of the dominant fungi (OTU relative abundance >1%) at the research sites in the shrub-dominated subtropical peatland in Pocosin Lakes National Wildlife Refuge (PLNWR), the Sphagnum-dominated boreal peatland in Marcell Experimental Forest (MEF), and the subtropical peatland in Dajiuhu (DJH). Ecological traits of slow-growing and fast-growing fungi were listed in Supplementary Table 3.
The fungal composition and their growth-rate traits in the Sphagnum-dominated site are in line with the observations found in many boreal peatlands37, while such studies in wooded peatlands, particularly in low-latitude areas, are still rare. Hence, we further examined the fungal communities in a subtropical peatland with dense shrubs (coverage: 41%) and Sphagnum layer underneath in Dajiuhu peatlands in Shennongjia, China (31°29′N, 109°59′E) and reanalyzed the fungal composition in a bog forest and a Sphagnum-shrub mixed peat bog (shrub coverage: 52%) in the Pacific coastal temperate rainforest in Canada41 (Supplementary Tables 1 and 2). The majority of fungal taxa in both shrub/tree-dominated sites were also found to be slow-growing, e.g., Archaeorhizomyces spp. (26.5%) and Cryptococcus sp. (34.0%) in Dajiuhu Peatlands (Fig. 2, Supplementary Fig. 2, and Supplementary Table 3). Importantly there was a significant negative relationship between soil respiration and richness of slow-growing fungi in the Pacific coastal rainforest (Fig. 3), which indicates that the slow-growing fungi likely regulate the carbon turnover rates in these peatlands.
The richness of fungi was recalculated from raw amplicon reads in the European Nucleotide Arch Archive ITS(ERS1798771-ERS1799064)41, CO2 emission data were collected from the Hakai Institute data repository at https://doi.org/10.21966/1.71563041. SG = slow-growing fungi.
Further supporting the influence of shrubs on fostering slow-growing fungi, a recent study showed a boreal peatland in MN, USA, where ericaceous shrubs dominated the wooded cover, was also dominated by slow-growing fungi (Helotiales and Archaeorhizomyces, >80%)42. By comparison on an upland plateau peatland in Czech Republic, the relative abundances of Archaeorhyzomycetes were 1.4 ± 3.3%, 0.5 ± 0.6%, and 42.7 ± 28.8% in mosses-, graminoids-, and ericoid shrub-dominated sites, respectively43. Collectively, these studies indicate that slow-growing fungi are dominant in many wooded peatlands in North America (this study and ref. 42), Asia (this study), and Europe43.
Phenolics primarily control microbial communities
Mantel test and redundancy analysis (RDA) were performed to determine what soil physicochemical variables (including dissolved organic carbon (DOC), dissolved phenolics, soil pH, soil moisture, NO3−–N, and NH4+–N) might control the fungal composition in the sites in NC and MN, USA. Both analyses showed that soluble phenolics and pH were the most important drivers (Supplementary Fig. 3 and Supplementary Table 4). Analyses of stepwise regression and correlation further show that the relative richness of slow-growing and fast-growing fungi were primarily controlled by dissolved phenolics (Fig. 4a, b) and pH (Fig. 4c, d), respectively. Because stepwise regression showed phenolics was the dominant factor controlling soil pH (r2 = 0.455, P < 0.0001) in these sites, we speculated that phenolic content in soil primarily driven by plant communities13 likely acted as the overarching regulator, not only directly limiting microbial activities13,44 but also allowing slow-growing fungi to thrive while impeding fast-growing fungi. The dissolved phenolics in these peatlands might be mainly phenolic/humic acids that increase soil acidity and reduce nitrogen availability by complexing with proteins45, thus further exacerbating the extreme oligotrophic conditions that benefit mainly the slow-growing fungi29 while simultaneously inhibiting bacterial growth and shifting bacterial communities as well. This is further demonstrated by the relatively small contribution of bacteria to the peat decomposition at both sites (Supplementary Fig. 1). Recent measurements in bacteria composition46,47 at the same sites in North Carolina and Minnesota showed that oligotrophic slow-growing Acidobacteria30 dominated both peatlands. The relative abundance of Acidobacteria in the shrub-dominated sites (57%) was substantially higher than that in the Sphagnum-dominated sites (36%). Moreover, the fast-growing bacteria—including Betaproteobacteria and Bacteroidetes as copiotrophs30 —were nearly absent from the shrub-dominated site46 but contributed about 9% and 4%, respectively, in the Sphagnum-dominated site47.
We postulate that these slow-growing microbes including both fungi and bacteria have adapted to high-phenolic acidic conditions and become the dominant microbes with inherent slow metabolic processes, a major underlying feature of high-phenolic wooded peatlands developed under warm and relatively dry conditions. Although higher microbial biomass carbon (MBC) was present in the shrub-formed peat (3.8 ± 0.2 mg C g−1 dry soil) in North Carolina relative to the Sphagnum-formed peat (2.6 ± 0.3 mg C g−1 dry soil) in Minnesota, the decomposition rate of the shrub-formed peat was much slower at the same temperature and displayed lower temperature sensitivity than the Sphagnum-formed peat (Supplementary Fig. 4).
Slow-growing microbes lead to slow peat decay
It is still impossible to measure the relative growth rates of the dominant microbes in this study, because so many of the fungi are unculturable. To further test and verify the consequences of these likely fast-growing versus slow-growing microbes including both fungi and bacteria, we compared soil respiration rates per gram MBC17,31 in the Sphagnum- and the shrub-formed peats through a reciprocal inoculation experiment using inocula and peat materials from both sites. We also added each inoculum to labile carbon-enriched mineral soil to test how the microbes decompose labile carbon under a condition without phenolic inhibition. All soil media and incubators were sterilized by an autoclave before inoculation. Consistent with the microbial growth traits found in the literature (Supplementary Table 3), the microbes from the shrub-dominated peatland decomposed both labile glucose and complex peats at much slower rates than the microbes from the Sphagnum-dominated peatland (Fig. 5). Although the shrub-formed peat is more highly recalcitrant than the Sphagnum-formed peat25, the fast-growing microbes from the Sphagnum inoculum decomposed the soil carbon in the shrub-formed peat 4 times faster than the microbes from the shrub-formed peat did, which indicates the reproductive strategies of decomposers (r- or K-selective) significantly impact the decomposition rate. Moreover, the soil respiration rates from Sphagnum inoculum were dependent on the source of carbon, with rates at 320, 838, and 2315 mg C h−1 g−1 MBC in the shrub-formed peat, the Sphagnum-formed peat, and the labile carbon-enriched mineral soil, respectively. Hence, the dominant decomposers in the Sphagnum-dominated peatlands are likely fast-growing copiotrophs that generally adapt to using available resource rapidly29. In contrast, soil media—including labile carbon—did not significantly impact the microbial activities from the shrub-formed peat inoculum (71–92 mg C h−1 g−1 MBC) in the short term. These results further support our hypothesis that microbial metabolism in high-phenolic shrub-dominated peatlands is slower and that growth traits of a specific microbial community might be inherent29.
All media were inoculated by inoculum made from the Sphagnum-formed or shrub-formed peat. Standardized CO2 emission (mean ± S.E.) was calculated based on the amount of MBC added to the soil media. Different letters indicate significant differences among treatments. MBC = microbial biomass carbon. Sh. = shrub-formed peat, Sp. = Sphagnum-formed peat, Min. = labile carbon–enriched mineral soil, Sh. = inoculum made from the shrub-formed peat, Sp. = inoculum made from the Sphagnum-formed peat.
We postulate that the slow-growing microbes which dominate the high-phenolic shrub-dominated site behave like K-selected taxa outcompeting fast-growing r-selected taxa under steadily warmer and dryer conditions. The established slow-growing fungi, as well as bacteria46,47 lead to a lower carbon turnover in soil13,30. The dominance of the slow-growing microbes may explain why plant necromass does not completely decompose, but continues to accumulate as peat in low-latitude wooded peatlands, despite constant warming and frequent drought over millennia13. This also explains the observed slow decomposition under drought in the subtropical shrub-dominated peatlands13, which was likely caused by the anti-microbe role of increased phenolics13,48 and also the magnified slow-growing decomposers induced by higher phenolics. Collectively, our field and lab experiments demonstrate that a phenolics-linked plant–microbe interaction may act as a natural curb on carbon loss in low-latitude wooded peatlands and would likely function in the same way in forthcoming boreal peatlands with climate-induced shrub expansion. This biotic self-sustaining process driven by consistent increases in temperature and drought over time appears to override direct abiotic controls in regulating long-term carbon-climate feedbacks in peatlands, which is critical for understanding and modeling how ongoing climate change affects peatlands across the globe.
Finally, our findings suggest that enduring peatlands that are highly resistant to increased temperature and natural drought may gradually shift to a new equilibrium state26 with different microbes and plants that have adapted to the changed climate over time through their self-sustaining plant–microbe interactions (likely connected by plant-induced phenolics). As biotic regulators, the co-shifting microbe and plant communities that were initially triggered by climate change appear to exert very important controls on ecosystem C cycling and soil C sequestration over time, thus ensuring for continuing peat accretion in a new steady state. Though beyond the scope of this study, our findings may have more immediate applications in carbon-climate feedback models and geoengineering strategies48,49. Embedding dynamic biotic factors into current abiotic-factor-dependent decay models could greatly advance the accuracy of the Earth System Models in projecting the fate of boreal peatlands with shifting plant/microbe communities10,11,42 under climate change. This mechanism added to the framework would allow models to predict how the biotic processes of a peatland could modulate abiotic controls on the carbon cycle over time. Moreover, this mechanism further indicates that peatland geoengineering49 adding high-phenolics natural materials like woody litter48 could be an enhanced nature-based solution, similar to a natural state shift, preserving degraded peatlands not only in the short term through increasing phenolic contents48 but also in the long term by encouraging phenolics-magnified, slow-growing microbes.
Materials and methods
Study sites and soil sampling
Our major study sites were located in a shrub-dominated bog13 in the Pocosin Lakes National Wildlife Refuge, NC, USA and a Sphagnum-dominated bog47 in the Marcell Experimental Forest, MN, USA (Supplementary Tables 1 and 2). Three sites (>1 km apart) around Pungo Lake including Pungo West, Pungo Southwest, and Pungo East were selected at the shrub bogs in North Carolina. Ilex glabra and Lyonia lucida cover about 85% and 10%, respectively at Pungo West. Ilex glabra and Lyonia lucida also dominate Pungo Southwest but distribute evenly, also there are many Woodwardia virginica ferns during the growing season. The water level at Pungo Southwest is always higher than at Pungo West. Both Pungo West and Pungo Southwest have prescribed light fire every 4–5 years. There has been no fire disturbance at the Pungo East site over last 30 years, where more dominant plant species exist, including Lyonia lucida, Ilex glabra, Zenobia pulverulenta, Gaylussacia frondosa, Vaccinium formosum.
One hollow and one hummock were selected at the Sphagnum-dominated bogs in Minnesota. A lot of mature trees including Picea mariana, Pinus resinosa, Larix laricina with different bryophytes and shrubs grow at both the hollows and the hummocks. S. fallax dominates the bryophyte layer at the hollows, and S. angustifolium and S. magellanicum dominate at the hummocks. The understory has a layer of ericaceous shrubs including Rhododendron groenlandicum, Chamaedaphne calyculata, Vaccinium oxycoccos at the hummocks, however, only scattered shrubs present in the hollows. Other site information is described in Supplementary Tables 1 and 2. We took three soil cores at each sites (with a distance >4 m from each other), and each soil core was sliced to four subsamples (0–5, 5–10, 10–15, and 15–20 cm). Big roots were removed in lab. The hair roots of all plants were included in the soil samples.
Additionally, we took three soil cores at depth 0–10 cm in the shrub-dominated area in Dajiuhu peatlands in Shennongjia, China (31°29′N, 109°59′E) in May 2017. The dominant shrub at Dajiuhu is Betula albosinensis and Spiraea salicifolia with a dense Sphagnum layer (detailed plant information is described in Supplementary Tables 1 and 2). The samples were transported to the laboratory in iceboxes. Half of the samples were frozen at −80 °C for DNA isolation; the other half was stored at 4 °C for chemical analysis.
Soil chemistry analysis
We used the deionized water extraction of fresh soil for DOC and soluble phenolics measurements. DOC was measured as the difference between total C and inorganic C with a total C analyzer (Shimadzu 5000 A, Kyoto, Japan). Soluble phenolics were measured by following the Folin-Ciocalteu procedure50. Inorganic nitrogen (NH4+–N and NO3−–N + NO2−–N) extract with 2 M KCl was determined colorimetrically on a flow-injection analyzer (Lachat QuikChem 8000, Milwaukee, WI, USA). Total carbon and nitrogen in soil were analyzed with combustion CN soil analyzer equipped with a TCD detector (ThermoQuest Flash EA1112, Milan, Italy). A 1:10 soil/water solution was used to measure soil pH.
DNA extraction, PCR, and sequencing
Genomic DNA was extracted from 0.25 g (fresh weight) of each homogenized soil sample using the PowerSoil DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA, USA). DNA of each replicate was extracted 3 times and homogenized together as one DNA template. For Pocosin and Minnesota samples, a set of fungus-specific primers, ITS1F (3′-CTTGGTCATTTAGAGGAAGTAA-5′) and ITS4 (3′-TCCTCCGCTTATTGATATGC-5′), were used to amplify the internal transcribed spacer (ITS) region using barcoded ITS1F primers. For Dajiuhu samples, ITS1F and ITS2 (3′-GCTGCGTTCTTCATCGATGC-5′) were used. All PCR reactions were repeated in triplicate, together with the negative controls in which the template DNA was replaced with deionized H2O. The amplicon concentration of each sample was determined after purification using Qubit® 2.0 Fluorometer (Invitrogen, Grand Island, NY, USA), samples pooled at equimolar concentrations, purified using AMPure Bead cleanup. The amplicons from Pocosin, Minnesota and Dajiuhu samples were submitted to the core facility at Duke University (Durham, NC, USA) and Allwegene Tech Beijing (Beijing, China) for sequencing using Illumina MiSeq (Illumina, San Diego, CA, USA), respectively.
Bioinformatics processing
Sequence data of Pocosins and Minnesota samples were obtained from both ITS1 and ITS2 gene regions. ITS sequences were quality filtered and processed using the standard QIIME pipeline, with each fungal taxon represented by an OTU at the 97% sequence similarity level. Singleton OTUs were omitted51, and OTUs classified taxonomically using a QIIME-based wrapper of BLAST against the UNITE database52,53 (see Supplementary Methods for further details). The quality and depth of coverage of both primers’ reads were not significantly different, thus libraries from ITS4 reads were used for further analysis of fungal communities. Taxonomic-based alpha diversity was calculated as the total number of phylotypes (richness) and Shannon’s diversity index (H′). A total of 150,967 ITS sequences from ITS2 region passed quality control criteria in the Pocosin and Minnesota sites. These sorted into 590 OTUs. Following the same procedure, a total of 115,936 ITS1 sequences from Dajiuhu samples were assigned into 307 OTUs. Following the processing procedure described by Wilson et al.47, relative abundance of beta-proteobacteria at the controlled site in the boreal Sphagnum site was recalculated from Wilson and others’ sequence data34 available from the National Center for Biotechnology Information at SRP071256. Relative abundance of fungi from a bog forest at the Calvert Island in Canada was recalculated from the raw amplicon reads in the European Nucleotide Archive, ITS (ERS1798771-ERS1799064).
Lab incubations
The decomposing capability of microbes in the Sphagnum- and shrub-dominated peatlands
We tested the decomposing capability of microbes in the Sphagnum- and shrub-dominated peatlands by amending peat inocula from both sites in North Carolina and Minnesota to their peats and labile carbon-enriched mineral soil. Fresh Sphagnum- and shrub-formed peat inocula were prepared by mixing 0.5 kg of each type of fresh peat (10–20 cm) with 2 L of deionized water. After 1 h of stirring and 1-day settlement, the suspension liquid inoculum was filtered through a Buchner funnels (without filter, pore size 0.25–0.5 mm). We added 2 g of glucose to 50 g of nutrient-poor mineral soil (initially 0.05% total nitrogen, 0.64% total soil carbon) to produce a mineral soil medium with high labile carbon content. All incubation media (peat and mineral soil) and jars were sterilized by an autoclave before inoculation. About 30-g fresh Sphagnum-formed peat (2.5–2.8 g in dry weight) or shrub-formed peat (9.1–9.3 g in dry weight), or 50-g mineral soil with 2-g glucose was placed in Mason jars (triplicate, 8-cm diameter, 12-cm height, vacuum seal lid with a stainless-steel fitting with sampling septum), then 20 ml of its own or other’s inoculum was added to the peat media, and 5 ml of inoculum from each site was added to the mineral soil. Finally, all samples were aerobically incubated at a constant temperature of 25 °C. We initially used Parafilm M® Laboratory film, which is air permeable but water resistant, to seal the top for 3-day equilibration, afterward we collected gas samples by syringe from the headspace of each jar at the beginning and end of 1-h sealed incubation and used a GC (Varian 450, CA, USA) to analyze CO2 concentration. As microbial biomass itself is a factor regulating soil respiration rates, standardized CO2 emissions at the microbial biomass were calculated based on the elevated CO2 concentration, time, air volume in the jar, and the amount of added MBC from the inoculum. To prevent microbial acclimation to the assay chemistry18,31, we only incubated the soils for a short time. A chloroform fumigation-extraction method (0.5 M K2SO4 to extract biomass C)54 was used to determine soil MBC by the difference in measured carbon contents between fumigated and control replicates of each sample.
Temperature sensitivity
To test temperature sensitivity of soil respiration, nine fresh peat samples (30 g) from each site were added to jars and sealed with Parafilm M® Laboratory film. Triplicate samples were incubated at 4, 25, and 44.5 °C. The highest temperature in this incubation does not match the in situ conditions in our sites, but it may happen shortly in tropical wooded peatlands in the future. After 3-day equilibration, we used the same method as above to measure gas emission and calculated soil respiration based on soil dry weight. We conducted regression analyses for soil from each site using R = αeβT, where R is soil respiration, coefficient α is the intercept of soil respiration when temperature is zero, coefficient β represents the temperature sensitivity of soil respiration, and T is soil temperature.
The relative contributions of fungi and bacteria to peat decomposition
We subsampled 20 g each of our archived material from the Sphagnum-dominated bog in Minnesota and the shrub-dominated peatland in North Carolina, then subsamples were well mixed to make two composite bulk samples (one for Sphagnum-formed peat, one for shrub-formed peat) for the following incubations.
A total of nine broad-spectrum antibiotics were tested either alone or in combination for their inhibition on bacteria or/and fungal respiration using a selective inhibition (SI) technique55 without glucose. The antibiotics include 5 fungicides (cycloheximide, benomyl, nystatin, natamycin, amphotericin B) and 4 bactericides (streptomycin, penicillin, oxytetracycline hydrochloride, neomycin). Both fungicide and bactericide were used alone or combined at concentrations of 0, 10, 20, 100, 500, and 1000 µg g−1 soil for the shrub-formed peat or 0, 35, 71, 357, 1785, and 3571 µg g−1 soil for the Sphagnum-formed peat. Each concentration of antibiotics (triplicate) was added to a 3-g fresh peat placed in a 50-ml tube. Mason jars (8-cm diameter, 12-cm height, vacuum seal lid with a stainless-steel fitting with sampling septum) are used to incubate the treated samples. CO2 accumulation rates over 24 h was measured and calculated as same as testing temperature sensitivity of soil respiration above. We found that all bactericides used in this study increased CO2 emission along with their concentrations. The results suggest that: (1) the contribution of bacteria to peat decomposition in general was simply very little, (2) the bacteria that were inhibited by bactericide contribute negligibly to peat decomposition, (3) the non-targeted bacteria were stimulated after the targeted-bacteria were inhibited, although the bactericides are broad-spectrum antibiotics, they did not inhibit the dominant bacteria at all in our sites, and /or (4) both bacteria and fungi in our sites may utilize these bactericides as a carbon source. As to the fungicide, only cycloheximide at a concentration of 357 µg g−1 soil slightly decrease CO2 emission in the Sphagnum-formed peat, but not the shrub-formed peat. Other fungicides did not suppress the CO2 emission regardless of their concentrations, or increased the CO2 emission along with increase in concentrations of fungicide, which suggest that these fungicides did not inhibit the dominant fungi in our sites. Therefore, we found no evidence that SI technique could detect the relative contribution of bacteria and fungi to peat decomposition in our sites. To further examine our fungal dominance hypothesis, we next used filtration by size to assess dominant decomposers.
According to the literature (e.g., refs. 56,57,58), the average size of most bacteria is between 0.2 and 2.0 µm in diameter, with most of them less than 1.5 µm; while most fungi grow as hyphae in soil, which are cylindrical, thread-like structures 1.5–10 µm in diameter and up to several centimeters in length57,59,60. The sizes of most fungal spore are more than 2.0 µm in diameter61,62,63. Theoretically, porous filters could physically separate bacteria from fungi58. Domeignoz-Horta et al.64 used 0.8-μm filter to exclude fungi successfully64. In our test, filters with pore sizes of 0.22, 0.45, 1.2, and 1.5 μm were selected. The filtrates through 1.2- and 1.5-μm filters contain most of the bacteria, in which a small portion of larger bacteria and small fungi may pass through pores due to a lack of rigidity of their cells58.
Sphagnum- and shrub-formed peat inocula were made by mixing 50 g of each type of fresh peat with 250 ml of sterilized deionized water. After 1 h of stirring and 1-day settlement, the suspension liquid inoculum was first filtered through a Buchner funnels (without filter, pore size 0.25–0.5 mm). The filtrate, we assumed, contain all bacteria and fungi while removing large decomposers like insects and worms. The 0.25–0.5 mm filtrate was used to make other inocula containing no-bacteria/fungi, nanobacteria and non-fungi, and most bacteria and non-fungi by filtering through 0.22- (nylon), 0.45- (nylon), 1.2- (glass fiber), and 1.5-μm (glass fiber) filters, separately. In total, 6 treatments including 5 inocula (filtrates through 0.22, 0.45, 1.2, 1.5, and 250–500-μm filters) and control (sterilized deionized water) were established. Either inoculum or sterilized deionized water was added to a 3-g sterilized Sphagnum- or shrub-formed peat (triplicate) and incubated at 25 °C. CO2 emission was measured within 24 h.
Statistical analysis
One-way ANOVA with Duncan’s multiple-range test was used to compare the means of soil physicochemical parameters. Standard error of the mean was calculated for each mean. The significant level of the test was set at a probability of 0.05. The ANOSIM function in the vegan package in R was used to test statistical significance in fungal composition within and among sites in the shrub- and the Sphagnum-dominated peatlands (999 permutations), which shows that fungal communities were significantly different within sites at the shrub-dominated peatlands (Pungo East, Pungo West, and Pungo Southwest) and at the Sphagnum-dominated peatlands (hollows and hummocks) (Supplementary Fig. 5). Mantel test and redundancy analysis (RDA) were employed to explain the relative roles of soil physicochemical factors in fungal community composition using vegan package in R. The correlation of the redundancy axes with the explanatory matrix was determined with the general permutation test (anova.cca function; 999 permutations). Stepwise regression was further run to test what primarily control the slow-growing versus fast-growing fungi and soil acidity.
Data availability
The generated sequence data are available from the National Center for Biotechnology Information at SRP122579 and SRP158553. Data files containing compiled mean soil respirations from boreal to tropical peatlands, soil physicochemical parameters and results of incubation experiments are available from https://doi.org/10.17632/8zx2mczz6d.1.
References
Yu, Z., Loisel, J., Brosseau, D.P., Beilman, D.W. & Hunt, S.J. Global peatland dynamics since the Last Glacial Maximum.Geophys. Res. Lett. 37, L13402 (2010).
Page, S. E., Rieley, J. O. & Banks, C. J. Global and regional importance of the tropical peatland carbon pool. Global Change Biol. 17, 798–818 (2011).
Dargie, G. C. et al. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 542, 86–90 (2017).
Xu, J., Morris, P. J., Liu, J. & Holden, J. PEATMAP: refining estimates of global peatland distribution based on a meta-analysis. CATENA 160, 134–140 (2018).
Kirschbaum, M. U. F. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol. Biochem. 27, 753–760 (1995).
Fenner, N. & Freeman, C. Drought-induced carbon loss in peatlands. Nat. Geosci. 4, 895–900 (2011).
Norby, R. J., Childs, J., Hanson, P. J. & Warren, J. M. Rapid loss of an ecosystem engineer: Sphagnum decline in an experimentally warmed bog. Ecol. Evol. 9, 12571–12585 (2019).
Berg, E. E., Hillman, K. M., Dial, R. & Deruwe, A. Recent woody invasion of wetlands on the Kenai Peninsula Lowlands, south-central Alaska: a major regime shift after 18,000 years of wet Sphagnum–sedge peat recruitment. Can. J. Forest Res. 39, 2033–2046 (2009).
Harris, L. I., Roulet, N. T. & Moore, T. R. Drainage reduces the resilience of a boreal peatland. Environ. Res. Commun. 2, 065001 (2020).
Bragazza, L., Parisod, J., Buttler, A. & Bardgett, R. D. Biogeochemical plant-soil microbe feedback in response to climate warming in peatlands. Nat. Clim. Change 3, 273–277 (2013).
Talbot, J. Drainage as a Model for Long Term Climate Change Effect on Vegetation Dynamics and Carbon Cycling in Boreal Peatlands. PhD thesis, McGill University (2009).
Gavazov, K. et al. Vascular plant-mediated controls on atmospheric carbon assimilation and peat carbon decomposition under climate change. Global Change Biol. 24, 3911–3921 (2018).
Wang, H., Richardson, C. J. & Ho, M. Dual controls on carbon loss during drought in peatlands. Nat. Clim. Change 5, 584–587 (2015).
Ise, T., Dunn, A. L., Wofsy, S. C. & Moorcroft, P. R. High sensitivity of peat decomposition to climate change through water-table feedback. Nat. Geosci. 1, 763–766 (2008).
Eliasson, P. E. et al. The response of heterotrophic CO2 flux to soil warming. Global Change Biol. 11, 167–181 (2005).
Luo, Y., Wan, S., Hui, D. & Wallace, L. L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625 (2001).
Bradford, M. A. et al. Cross-biome patterns in soil microbial respiration predictable from evolutionary theory on thermal adaptation. Nat. Ecol. Evol. 3, 223–231 (2019).
Dacal, M., Bradford, M. A., Plaza, C., Maestre, F. T. & García-Palacios, P. Soil microbial respiration adapts to ambient temperature in global drylands. Nat. Ecol. Evol. 3, 232–238 (2019).
Charman, D. et al. Climate-related changes in peatland carbon accumulation during the last millennium. Biogeosciences 10, 929–944 (2013).
Loisel, J. & Yu, Z. Surface vegetation patterning controls carbon accumulation in peatlands. Geophys. Res. Lett. 40, 5508–5513 (2013).
Robroek, B.M.J. et al. Taxonomic and functional turnover are decoupled in European peat bogs.Nat. Commun. 8, 1161 (2017).
Piao, S. et al. Forest annual carbon cost: a global-scale analysis of autotrophic respiration. Ecology 91, 652–661 (2010).
Dorrepaal, E. et al. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic.Nature 460, 616–619 (2009).
Wardle, D. Communities and Ecosystems: Linking the Aboveground and Belowground Components (Princeton University Press, 2002).
Hodgkins, S. B. et al. Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 9, 3640 (2018).
Dise, N. Peatland response to global change. Science 326, 810–811 (2009).
Singh, B. K., Bardgett, R. D., Smith, P. & Reay, D. S. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 8, 779–790 (2010).
Walker, L. R. & Wardle, D. A. Plant succession as an integrator of contrasting ecological time scales. Trends Ecol. Evol. 29, 504–510 (2014).
Koch, A. L. Oligotrophs versus copiotrophs. Bioessays 23, 657–661 (2001).
Fierer, N., Bradford, M. A. & Jackson, R. B. Toward an ecological classification of soil bacteria. Ecology 88, 1354–1364 (2007).
Bradford, M. A., Davies, C. A., Frey, S. D. & Maddox, T. R. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol. Lett. 11, 1316–1327 (2008).
Gallego-Sala, A. V. et al. Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nat. Clim. Change 8, 907–913 (2018).
Wardle, D. A. et al. Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633 (2004).
Friedlingstein, P. et al. Climate–carbon cycle feedback analysis: results from the C4MIP model intercomparison. J. Clim. 19, 3337–3353 (2006).
Whitehead, D. R. Late-pleistocene vegetational changes in Northeastern North-Carolina. Ecol. Monogr. 51, 451–471 (1981).
Bragazza, L. A decade of plant species changes on a Mire in the Italian Alps: vegetation-controlled or climate-driven mechanisms? Clim. Change 77, 415–429 (2006).
Thormann, M. N. Diversity and function of fungi in peatlands: a carbon cycling perspective. Can. J. Soil Sci. 86, 281–293 (2006).
Williams, R. T. & Crawford, R. L. Microbial diversity of Minnesota peatlands. Microb. Ecol. 9, 201–214 (1983).
Rosling, A. et al. Archaeorhizomycetes: unearthing an ancient class of ubiquitous soil fungi. Science 333, 876–879 (2011).
Clemmensen, K. E. et al. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 205, 1525–1536 (2015).
Levy-Booth, D. J. et al. Seasonal and ecohydrological regulation of active microbial populations involved in DOC, CO2, and CH4 fluxes in temperate rainforest soil. ISME J. 13, 950–963 (2019).
Lamit, L.J. et al. Patterns and drivers of fungal community depth stratification in Sphagnum peat.FEMS Microbiol. Ecol. 93, fix082 (2017).
Chroňáková, A., Bárta, J., Kaštovská, E., Urbanová, Z. & Picek, T. Spatial heterogeneity of belowground microbial communities linked to peatland microhabitats with different plant dominants.FEMS Microbiol. Ecol. 95, fiz130 (2019).
Freeman, C., Ostle, N. & Kang, H. An enzymic ‘latch' on a global carbon store—a shortage of oxygen locks up carbon in peatlands by restraining a single enzyme. Nature 409, 149–149 (2001).
Hättenschwiler, S. & Vitousek, P. M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 15, 238–243 (2000).
Hartman, W. H., Richardson, C. J., Vilgalys, R. & Bruland, G. L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl Acad. Sci. USA 105, 17842–17847 (2008).
Wilson, R.M. et al. Stability of peatland carbon to rising temperatures.Nat. Commun. 7, 13723 (2016).
Fenner, N. & Freeman, C. Woody litter protects peat carbon stocks during drought. Nat. Clim. Change 10, 363–369 (2020).
Freeman, C., Fenner, N. & Shirsat, A.H. Peatland geoengineering: an alternative approach to terrestrial carbon sequestration.Philos. Trans. A 370, 4404–4421 (2012).
Carter, M. R. Soil Sampling and Methods of Analysis (Lewis Publishers, 1993).
Bálint, M., Schmidt, P.-A., Sharma, R., Thines, M. & Schmitt, I. An illumina metabarcoding pipeline for fungi.Ecol. Evol. 4, 2642–2653 (2014).
Abarenkov, K. et al. The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytol. 186, 281–285 (2010).
Bokulich, N. A. & Mills, D. A. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl. Environ. Microbiol. 79, 2519–2526 (2013).
Gregorich, E. G., Wen, G., Voroney, R. P. & Kachanoski, R. G. Calibration of a rapid direct chloroform extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 22, 1009–1011 (1990).
Anderson, J. P. E. & Domsch, K. H. Quantification of bacterial and fungal contributions to soil respiration. Arch. Mikrobiol. 93, 113–127 (1973).
Tortora, G. J., Berdell, R. F. & Case, C. L. Microbiology: An Introduction (Benjamin-Cummings Pub. Co., 1998).
Maheshwari, R. Fungi: Experimental Methods in Biology. 2nd edn. (CRC Press, 2016).
Ghuneim, L.-A. J., Jones, D. L., Golyshin, P. N. & Golyshina, O. V. Nano-sized and filterable Bacteria and Archaea: biodiversity and function.Front. Microbiol. 9, 1971 (2018).
Anusha, N., Umikalsom, M., Ling, T. & Ariff, A. Relationship between fungal growth morphologies and ability to secrete lipase in solid state fermentation. Asian J. Biotechnol. 4, 15–29 (2012).
Bakken, L. R. & Olsen, R. A. Buoyant densities and dry-matter contents of microorganisms: conversion of a measured biovolume into biomass. Appl. Environ. Microbiol. 45, 1188–1195 (1983).
Dijksterhuis, J. Fungal spores: highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 81, 2–11 (2019).
Golan, J. J. & Pringle, A. Long-distance dispersal of fungi. In The Fungal Kingdom (eds. Heitman, J. et al.) Ch. 14, 309–333 (ASM Press, 2017).
Reponen, T., Hyvärinen, A., Ruuskanen, J., Raunemaa, T. & Nevalainen, A. Comparison of concentrations and size distributions of fungal spores in buildings with and without mould problems. J. Aerosol Sci. 25, 1595–1603 (1994).
Domeignoz-Horta, L. A. et al. Microbial diversity drives carbon use efficiency in a model soil.Nat. Commun. 11, 3684 (2020).
Acknowledgements
We would like to thank Drs. David J. Levy-Booth and William W. Mohn at University of British Columbia and Dr. Joel E. Kostka at Georgia Institute of Technology for sharing their published data, Dr. Xianyu Huang at China University of Geosciences for sample collection in Dajiuhu peatlands, Dr. William H. Schlesinger at the Cary Institute of Ecosystem Studies for his detailed comments on experimental design and data interpretation, Dr. Jeff Chanton at Florida State University, Dr. Dorothy Peteet at Columbia University, Dr. Christopher W. Schadt at Oak Ridge National Laboratory, Dr. Scott Neubauer at Virginia Commonwealth University, and Dr. Louis James Lamit at Syracuse University for their comments, Dr. Neal Flanagan for providing water levels, Belen de la Barrera for laboratory measurement, Dr. Randy Neighbarger for technical editing. US DOE Office of Science, Terrestrial Ecosystem Sciences (DE-SC0012272), Key Research Program of Frontier Sciences, CAS (QYZDB-SSW-DQC007), Jilin Provincial Science and Technology Development Project (Grant No. 20190101025JH), the Duke University Wetland Center Endowment, and China Scholarship provided financial support.
Author information
Authors and Affiliations
Contributions
H.W., J.T., and C.J.R. conceived the ideas and designed this research; C.J.R., H.W., R.V., and H.C. obtained funding; H.W., M.H., C.J.R., H.C., and Z.B. collected field samples; J.T., H.C., and X.L. did microbial measurement and analyzed microbial community data; H.W. measured soil chemistry and conducted incubation experiments; M.H. and Z.B. analyzed plant information. H.W. and J.T. compiled data of soil respiration from literatures; H.W. wrote the manuscript with J.T. and C.J.R.; and all other authors discussed results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary handling editor: Joe Aslin.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Tian, J., Chen, H. et al. Vegetation and microbes interact to preserve carbon in many wooded peatlands. Commun Earth Environ 2, 67 (2021). https://doi.org/10.1038/s43247-021-00136-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-021-00136-4
This article is cited by
-
Response of fungal communities to fire in a subtropical peatland
Plant and Soil (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.