Abstract
Protein-based fibers combine unique mechanical properties with biocompatibility and biodegradability, and often outperform polymer-based fibers. Furthermore, a growing need for sustainable materials has triggered a revival in the study of protein fibers, including keratin, collagen, elastin, and silk, which do not require environmentally damaging petrochemicals for their synthesis. Nowadays, bioinspired research intends to mimic the underlying proteins as well as their natural assembly or spinning processes, to achieve fibers with properties equivalent to those of their natural counterparts. Protein-based fibers can also be used to mimic functions in nature, which can otherwise not be achieved with synthetic polymer-based fibers. Here, we review promising protein fibers, their synthesis, and applications, such as air and water filtration, energy conversion, smart textiles, and in biosensoring and biomedical fields.
Similar content being viewed by others
Introduction
Finding green, sustainable and environmentally friendly materials with excellent performance is one big challenge in the present days1. Since several decades, polymers reflected the holy grail for several applications, but since the early 2000s, issues concerning the fragmentation of plastic waste into microplastics and its omni-presence in the environment are constantly rising2. In an increasing number of applications, protein-based fibers could be considered as replacements for polymer-based fibers, especially when taking the natural functions of protein fibers into account. For instance, one wide-spread use of silk fibers in nature is the protection of animals descendants or to form a cocoon for the pupa to develop from the larval to the adult stage3. If smart scaffolds, tough webs or protective covers are intended to be used for human applications, it is of utmost importance to understand the natural function, but also the hierarchical structure of protein-fibers as well as their natural assembly processes. With this knowledge it will be possible to produce man-made protein fibers with similar properties like the natural ones4. Such protein fibers can then be tested as replacement materials for polymer fibers in existing, but also in new, so far unreachable applications. In the biomedical field, natural protein-based fibers are abundant in the extracellular matrix (ECM) of vertebrate tissues providing mechanically robust and often “smart” scaffolds for cells. The understanding of the natural systems allows here to transfer the properties of several fibrous proteins to artificial scaffolds resulting in systems for biomedical applications with similar properties as the natural ones5. This review aims to provide an overview over some well-established protein-based fibrous systems, their assembly behavior as well as the transfer of this knowledge to enable explicit applications.
Natural protein-based fibers
Natural protein-based fibers show properties useful for a myriad of applications. Most protein-based fibers are soft, show high moisture absorbance and outstanding mechanical properties4. The investigation of natural fiber assembly/spinning processes is necessary to develop technical processes (which can be scaled-up) for producing protein-based fibers for any application. Here, we will focus on four well examined fibrous protein families namely silk (spider silk and Bombyx mori silk), collagen, keratin, and elastin.
Silk
Silk is a natural protein fiber produced by many arthropods. The best-characterized silk is produced by the silkworm Bombyx mori (B. mori). This silk is used by mankind for more than 5000 years in textile applications due to its properties and availability6, since it can easily be harvested from a silkworm’s cocoon. Single B. mori silk fibers can reach lengths within 700 to 1500 m, regarding the mechanical properties, the strength of silkworm silk ranges from 300 to 700 MPa7. Silkworm silk is spun into a cocoon to protect the larva during their transformation into the adult moth. The silk threads of the cocoon consist of a fiber core and a protein skin, made of sericin, covering two fibroin filaments (Fig. 1a)8. In order to obtain the core fibers made of silk fibroin, sericin has to be removed by a degumming process9. The fibers show a substructure with oriented β-sheet-rich nanofibrils with mean diameters of 90 to 170 nm and consist of a heavy chain fibroin (~390 kDa) and a light chain fibroin (~26 kDa) cross-linked by a disulfide bond10. Silk fibroins can exist in two conformational states: Silk I and Silk II. Silk II represents the solid form found in the spun silk, while Silk I is the dissolved, metastable structure stored in the silk gland8.
a B. mori fibers contain a core of nanofibrils with β-sheet structure forming crystallites responsible for the mechanical strength of the fiber. In between are amorphous parts responsible for fiber elasticity. The nanofibrils are covered by layers consisting of silk fibroins, and sericin. b On the macroscopic level, spider silk fibers also possess a skin-core structure, with a core formed by nanofibrils. The fibrils contain often β-sheet crystals aligned with the fiber axis responsible for the strength of the fiber. The amorphous phase is formed by GPGXX and GGX motifs being necessary for the elasticity of the fibers. c Keratin fibers show a hierarchical structure consisting of a cortex and a cuticle. Within the cuticle there are spindle-shaped macrofibrils based on two main structures, the protofibrils and the matrix. The protofibrils are formed by a mostly α-helical protein with low cysteine content in case of α-keratin or a β-sheet-rich structure with high cysteine content in case of β-keratin. d Each collagen molecule is made of three α-chains forming the ~300 nm long triple helical collagen molecule. Upon lateral and longitudinal assembly fibrils are achieved, which assemble into the final collagen fiber. e Elastin fibers are part of the extracellular matrix (ECM) and responsible for the elasticity of tissues. Elastin fibers are composed of microfibrils consisting of tropoelastin. The fibers are formed by enzymatically driven cross-linking of the microfibrils.
Spider silk, as another silk protein fiber, is recently gaining more and more interest due to its extraordinary mechanical properties. Orb weaving spiders produce up to seven different types of silk, which differ in their composition and in their mechanical properties. The most investigated spider silk type is the major ampullate silk (MA silk) forming the radii and the frame of the web as well as the lifeline to escape from predators11. The maximum strength of MA-silk fibers is up to 1.7 GPa, which is in the range of synthetic high-tech materials. However, such spider silk fibers have much higher toughness and extensibility than e.g., Kevlar and carbon fibers12. Spider silk fibers also possess a hierarchical structure consisting of a skin (consisting of silk, glycoproteins and lipids)13 and a core which comprises β-sheet-rich nanofibrils (Fig. 1b). Spider silk fibers are composed of one or more proteins, called spidroins. All spidroins comprise a large central domain of repeated sequence motifs flanked by non-repetitive (NR) domains, consisting of 100 to 140 amino acids, which are highly conserved between different spider species and silk types. Within the amino acid sequence, GPGXX and GGX motifs are responsible for the elasticity of the fiber. The terminal domains of MaSps are essential during storage and for the fiber formation process, acting as control units of self-assembly upon external triggers like pH, ion exchange and shear force14. The motifs of the central domain consist of 40 to 200 amino acids, which are often repeated up to 100 times15. MA-silk fibers mainly consist of two protein classes named MaSp (Major ampullate Spidroin) 1 and 2 differing in proline content (MaSp1 low, MaSp2 high). In some spider species, additional MaSps are found, such as MaSp3, which lacks poly-alanine motifs, typical for MaSp1 and MaSp2. Further, MaSp3 also contains more polar amino acids than the other MaSps. MaSp4, for instance, contains an abundant amount of proline residues (even more than MaSp2)16.
Keratin
Keratins are structural proteins in epithelial cells forming a cytoplasmic network of intermediate filaments of 10–12 nm in diameter17. Besides, the term keratin also includes intermediate filament-associated proteins. Keratins are used extracellularly by vertebrates in nails, hair, feathers, horns, hooves and claws18. The wide occurrence of keratin is resulting in various characteristics and functions. Keratin fibers show a hierarchical structure consisting of a cortex and a cuticle. The structure of keratins can be divided into alpha-keratins, where the polypeptide chains are arranged in alpha-helices, and into beta-keratins forming beta-sheets (Fig. 1c)19. Two twisted polypeptide chains in alpha-keratin form coiled-coil structures. Alpha-keratin filaments have a diameter of 7–10 nm, while beta-keratin ones are 3 to 4 nm in diameter.
Both classes can also be subdivided in hard and soft keratins, resulting in acidic-hard, basic-hard, acidic-soft and basic-soft keratins20. The amino acid composition is influencing the mechanical properties of the keratin. The occurrence of cysteine and disulfide bridges is influencing the hardness of keratins. A high amount of cysteine is resulting in certain properties like higher stability once assembled and lower solubility, like in wool keratin21. A high amount of sulfhydryl groups (5% or more) leads to hard keratins, whereas a low amount (less than 1% cysteines) is resulting in soft ones. Type I keratins, exemplified by K31–K40, are acidic, while type II keratins, including K1–K8, are basic or neutral18,22. The influence of Keratin I’s acidity, with an isoelectric point below 5, extends to fiber mechanics, involving charge repulsion, structural modifications, and heightened thiol reactivity. In contrast, Keratin II’s alkalinity, marked by an isoelectric point above 7, contributes to fiber cohesion, stability, and potential swelling or shrinking responses. This intricate interplay between keratin types and their acid-base characteristics underscores the diverse mechanical properties and applications of keratinaceous materials18,19. In addition to keratin I and II, less prominent type III keratins occur specifically in cells of mesenchymal origin (e.g., smooth cardiac and skeletal myogenic cells), while type IV and type V chains are found in neuronal cells and in the nuclear envelope of mammalian cells, respectively17.
Collagen
Collagens represent a family of ECM-proteins containing at least one triple helical domain23. More than 50 different types of collagen have been described so far, being either globular or fibrillar24. Most collagens form supramolecular assemblies, and can be divided into fibril-forming, basement membrane-forming, microfibrillar, anchoring fibril, hexagonal network forming, fibril-associated transmembrane and multiplexins ones. Collagens are found in tendons, ligaments, skin, organs, and bones. Besides being a building block for most organs and tissue, collagens also play an important role in the process of cell growth and differentiation. Collagens are considered as viscoelastic materials with high tensile strength and low extensibility. In nature, collagen can be obtained from animals as well as plants and bacteria. Most common sources are bovine, porcine, human collagens and fish skin25. Fibrillar collagens co-assemble into banded fibrils and fibers in tissues. They are arranged in longitudinally staggered arrays of molecules of a length that is a non-integer multiple of the stagger between next neighbors (Fig. 1d)26.
Elastin
Elastin is a fibrous protein of the extracellular matrix of vertebrates and responsible for the elasticity and resilience of tissues27. It can be found in different organs like the lung as well as skin and tendons28. Further, it is highly abundant in ligaments and the walls of arteries, mostly in tissues which require rapid extension and complete recovery. In comparison to collagens, it is 1000 times more elastic29. Elastin forms an insoluble protein-network based on its soluble precursor tropoelastin produced by fibroblasts, smooth muscle cells and endothelial cells. Tropoelastin contains alternating hydrophobic domains interrupted by hydrophilic parts30. The hydrophobic domains are responsible for elasticity and are involved in cell interactions. In contrast, the hydrophilic domains are involved in amine-dependent cross-linking. Cleavage of signal peptides is finally resulting in insoluble elastin31. In its mature form, elastin is hydrophobic and completely water insoluble. Due to the large number of cross-links, elastin is highly resistant to proteolysis and provides a stable and persistent structure (Fig. 1e)32.
Natural fiber assembly and fiber spinning
Collagens, elastins and keratins are responsible for functions like supporting the mechanics of bones, cartilage, hair and muscles. Their fibrous networks are formed by controllable self-assembly, being highly influenced by protein sequence as well as concentration, temperature and ions present in the surrounding solution33.
It is important to mention that only a few organisms are able to actively spin protein-based fibers. Spinning involves storage of fibrous proteins in a soluble form until the initiation of assembly during the spinning process, leading to the final water-insoluble fiber. Prominent examples for fiber spinning organisms are the silkworm B. mori as well as spiders34. The key elements of self-assembly of collagens, elastins and keratins and the fiber spinning of silk fibers are highlighted in Fig. 2.
a Images of natural silk spinning glands. Upper panel: left: Spinning gland of B. mori divided into anterior (ASG), middle (MSG) and posterior (PSG) silk gland. Right: Spinning gland of spiders, divided into duct, sac and tail. Adapted and modified with permission from ref. 47, licensed under CC BY 4.0. Lower panel: In spiders, the silk proteins are secreted in the tail and undergo a liquid-liquid phase separation yielding micelle-like droplets within the ampulla. In the duct, chaotropic salts (NaCl) are exchanged by kosmotropic ones (K2PO3), and a pH shift occurs (indicated in blue and orange). Upon elongational flow, the fiber is formed and pulled out of the spinning duct. b α-and β-keratins form dimers, which assemble into protofilaments, further assembling into intermediate filaments. c The assembly of collagens starts with procollagen. The N- and C-terminal domains are degraded by proteases, initiating self-assembly. Lysyloxidase is cross-linking the tropocollagens resulting in collagen fibrils, which assemble into fibers. d The assembly process of elastins is similar to that of collagens, starting with the precursor tropoelastin, which is degraded enzymatically. Fibulin interacts with the precursor initiating the self-assembly to microfibrils. Lysyloxidase is cross-linking the microfibrils resulting in elastic elastin-fibers.
Assembly of keratin
Assembly of keratin intermediate filaments is a dynamic process, characterized by the continuous incorporation of keratin subunits into existing filament networks35. α- and β-keratins form a central rod consisting of four helical bundles. The amino acid sequences are arranged as heptad repeats35. The domain forming the central rod is flanked by N- and C-terminal domains, which are divided into subdomains. While the rod domain is important for the interaction during the intermediate filament formation, the terminal domains stabilize the assembled filament and are responsible for the functional characteristics of the filament, like elasticity and flexibility36. The first step of assembly is the parallel association of type I and type II keratin proteins, resulting in a “coiled-coil” heterodimer. Hydrophobic residues are stabilizing the interaction. Afterwards, the association of up to four heterodimers takes place. The final keratin filaments contain ~20,000 to 30,000 keratin molecules36,37,38.
Extracellular keratin of hairs or nails represent hard keratin. These keratins contain more cysteine residues in comparison to other keratins39. The stiffness derives from cornification. Cornification is the combination of intermediate filaments of keratin, keratin-associated proteins (KAPs), and corneous proteins (CPs) forming a corneous material. KAPs and CPs form chemical bonds with the meshwork of IF-keratins determining the bundling and cross-linking, resulting in softer corneous layers in the epidermis and hard corneous layers in the skin appendages40. The most characterized cross-links within keratin fibers are based on covalent disulfide bonds41. Nevertheless, like in all proteins, there are also some noncovalent bonds42.
Assembly of collagen and elastin
Fibrillar collagens are made as procollagens, containing an uninterrupted triple helix with globular domains at each end43. In collagen triple helices, two identical chains (α1) are associated with a third chain (α2). Staggering the molecules with a periodicity of 67 nm lead to assembled fibrils showing diameters of few hundred nanometers44. The globular domains comprising the N- and the C-propeptides limit self-assembly of collagens. C-terminal domains are important for the initiation of lateral collagen assembly, while the N-terminal domains are important for fibrillogenesis45. Enzymatic degradation of the terminal extensions by proteinases is the first step within fibril assembly, cleaving the terminal propeptides yields tropocollagen. Tropocollagen contains short helical structures at the ends of the triple helix based on telopeptides, which are necessary for collagen cross-linking mediated by lysyloxidase. Tropocollagens are covalently crosslinked in a staggered manner resulting in collagen fibrils, which are ten to hundreds of nanometers in diameter and microns in length. In tissues, collagens co-assemble into fibers26.
Like collagen, elastin is synthesized as a tropoelastin monomer46. However, tropoelastin does not contain any N- or C-terminal propeptides, being responsible for the alignment of the monomers. Tropoelastin is produced by different cells like fibroblasts, smooth muscle cells or endothelial cells. Self-assembly of tropoelastin yields spherules within the extracellular space. Lysyloxidase deaminates specific lysine residues forming allysines condensing with other allysines or lysine residues forming intra- and intermolecular crosslinks. These cross-links are responsible for insolubility, mechanical stability as well as for the protease resistance and allow the growth of a elastin fiber network within the ECM46.
Silk fiber spinning
B. mori silk fibers
The silk glands of B. mori originate from salivary glands and are divided into three parts: the posterior silk gland (PSG), the middle silk gland (MSG) and the anterior silk gland (ASG). Within the PSG, fibroins are produced and stored at high concentrations in the MSG47. In the MSG of B. mori sericin is produced, surrounding the fibroin. Upon transport through the ASG, the proteins change their conformation influenced by several factors, such as pH gradients, ion composition as well as shear forces. The pH in the glands of B. mori is proposed to decrease from 6.9 in the PSG, to around 5 in the MSG and down to 4.8 in the ASG. Like the pH, also the influence of ions is highly important. In B. mori, the copper ion concentration differs in the different parts of the gland, and the concentrations of potassium, magnesium and zinc increase from the PSG to the ASG47. Within the ASG, the diameter of the lumen decreases resulting in shear forces necessary for fiber assembly. Lastly, within the ASG water is removed from the dope contributing to the fiber formation.
Spider silk fibers
In contrast to B. mori, spiders can produce several different silk types. Therefore, they have different gland types47. The focus of research is set on the MA silk gland, producing MA silk. MA glands of spiders are divided into tail, sac and duct. Within the sac and the duct carbonic anhydrase is produced, being responsible for a pH gradient. In contrast to B. mori, the pH in the ampullate silk gland of spiders drops from 7.2 to 5.748. Upon acidification, the N-terminal domain of dimeric spidroins (parallel dimers induced by the C-terminal domain) is forming dimers of dimers in an anti-parallel manner due to the protonation of glutamic acid residues. The C-terminal dimerization domain is destabilized by the pH shift, and hydrophobic parts of the sequence are exposed leading to parallel alignments of the core domains. The anti-parallel (N-terminal) and parallel (C-terminal) alignments result in a spidroin network. In the spinning duct of spiders also an exchange of chaotropic salts like sodium and chloride to kosmotropic salts, like potassium, occurs. Finally, shear forces are necessary for fiber formation caused by drawing the fibers out of the spinning duct47.
Man-made protein fibers
Although protein-based fibers (such as silk or wool) are applied by mankind since millennia, man-made protein fibers were introduced only about 70 years ago, but at that time did not find entrance in industrial applications49. Due to nowadays emerging issues with polymer fibers, based, e.g., on microplastic pollutions, the production of man-made protein fibers as well as the development of strategies to recombinantly produce fibrous proteins has nowadays led to a renaissance in considering protein-based fibers for various applications (Table 1).
Protein sources: natural vs. recombinant
Proteins used for man-made fiber spinning originate in many instances from natural sources (Fig. 3). In terms of spider silk, the silk proteins could be obtained by collecting natural occurring webs or “forced silking” of the spider followed by denaturation of the fibers, or extracting soluble proteins from the silk glands50. Other proteins are extracted from animal-based sources, for instance skin and other tissues for collagen and elastin51,52, or regenerated from wool, feathers and hair in case of keratin53 and cocoons to gain B. mori silk54,55. The extraction usually includes cutting and washing the raw material. Depending on the protein, heating and washing steps as well as enzymatic and chemical (acidic or basic) treatments are used to remove animal by-products49,54,56. Recently, ionic liquids have been used to increase the purification yields of hard-to-achieve proteins such as cysteine-rich keratins56.
a Proteins can be collected (e.g., cocoons, hair, wool, spider silk) or extracted from natural sources, like vertebrate tissues (rat-tail, fish skin). To dissolve the assembled fibers, several organic agents can be used. Dissolved proteins are dialysed against water and lyophylized. b Fibrous proteins can be recombinantly produced using different expression systems, e.g., yeast (Pichia pastoris) or bacteria (Escherichia coli). Recombinant production needs the knowledge of the DNA sequence encoding the fibrous protein. The plasmid with the respective (often engineered) DNA is transformed into the expression host, followed by induction of recombinant protein production. Fermentation allows the scale-up of protein production. Finally, the fibrous proteins have to be purified prior to processing.
Regenerated proteins pose several challenges, such as the need to disrupt naturally occurring protein structures, potential contamination with inflammatory impurities, uncertain quality, low yields, and frequently limited scalability in the regeneration process57,58. Consequently, advanced biotechnological procedures have been devised to enable the efficient production of recombinant fibrous proteins in substantial quantities with consistent quality (Fig. 3).
In this context, recombinant variants of silk, keratin, collagen, and elastin have been developed. Spider silk proteins and collagen variants have been produced using various hosts, including bacteria, yeasts, plants, mammalian and insect cells, as well as transgenic animals to find the best host for yielding significant amounts of the aimed proteins59,60,61. In contrast, elastin62,63,64,65 production in bacterial systems worked well, however, some groups employ tobacco plants as an expression host due to unlimited scalability and reduced costs66,67,68,69. Keratins are also mostly produced in bacterial systems70,71,72,73,74. Generally, the bacterial expression system offers advantages such as well-established genetic tools, cost-effectiveness, and promising high yields with fast growth, making it time-efficient75.
These processes afford the opportunity for genetic-level modifications of the protein, presenting a distinct advantage over proteins from natural sources75. Notably, recombinant elastin proteins incorporating additional lysine residues, produced using Escherichia coli (E. coli), have been industrially produced and then spun into robust and resilient fibers76. These lysine-rich recombinant proteins have been combined with nanocellulose, resulting in a composite fiber with enhanced mechanical properties77. Additionally, wet-spun fibers, obtained from recombinant proteins combining squid ring teeth protein with an elastin-like polypeptide, have been successfully engineered to produce micron-sized fibers. The mechanical performance was significantly enhanced with the increase of molecular weight from a 12mer to a 36mer. The breaking strength of the 36mer fibers were 550 MPa, in comparison, the 12mer fibers showed a breaking strength of 208 MPa78,79.
Useful methods for protein fiber spinning
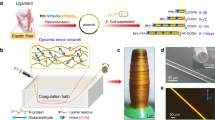
Natural protein-based fibers are used for heat insulation (keratin fibers)21, as protection from the environment (cocoons)8 or for capturing prey (spider web)3, and, therefore, they show often interesting features, which can not be found in synthetic polymer fibers. In order to mimic the properties of such protein fibers for technical approaches, several spinning techniques for protein spinning have been employed such as electro-spinning, wet-spinning, dry-spinning and microfluidic spinning (Fig. 4).
a For wet-spinning a protein solution (dark blue) is extruded into a coagulation bath (light blue) leading to the solidification of the fiber. Afterwards, the fibers are collected using a winder. b In microfluidic spinning an additional chip with defined channels is used in order to pre-assemble and align the fibers in a controlled manner prior to coagulation. c Within dry-spinning, a protein solution (dark blue) is extruded directly into air and is collected by a winder. d During electro-spinning a protein solution (dark blue) is extruded into an electric field. During the extrusion, the solvent evaporates and a solid fiber is formed.
Wet-spinning
Wet-spinning is a well-known technique developed in the 1930s80 and mostly used in the textile industry for producing synthetic fibers like nylon, but can also be employed for proteins81. The process is based on the extrusion of a protein solution (ranging from 1% to 30% (w/V)) into a coagulation bath. Within the wet-spinning process, the fiber is forming due to a loss of solvent, which is caused by the exchange of the solvent with the liquid of the coagulation bath, yielding a coagulated filament. The filament solidifies as a fiber with diameters of tens to hundreds of micrometers. Post-processing steps, like stretching, washing and drying are necessary to obtain fibers with the aspired features80. Usually, organic solvents are used, for instance formic acid (FA) or dimethyl sulfoxide (DMSO)65,69, due to their ability to solve and denature proteins as well as to induce secondary structure formation82. It is also possible to use aqueous buffers with the advantage of being less toxic and environmentally friendly, further they can maintain the biological activity of the protein83,84. Different liquids are used for the coagulation bath, usually alcohols like ethanol or methanol but also ammonium sulfate solutions13,85. The wet-spinning process offers the advantage of producing a variety of fiber cross-sectional shapes and sizes86.
Microfluidic spinning
Microfluidic spinning (aka microfluidics), in general, allows the processing of small amounts of fluids using channels with diameters of tens to hundreds of micrometer87. Microfluidics have a great potential for producing fibers in the micro- and nanoscale. Usually, microfluidic devices use laminar flow conditions, allowing to control fiber formation akin to processes as found e.g., in silk fiber spinning arthropods. The typical set-up of microfluidics for fiber production includes several channels for the fiber-forming protein and a sheath solution. Both solutions have their own input ports, generating a core-sheath flow88. In microfluidic spinning the protein solution can also be extruded into a coagulation bath like in wet-spinning. Size and shape of the fibers can be modulated by the flow rates as well as by the arrangement and the dimensions of the channels89. The dope concentrations necessary for microfluidics vary from 1% (w/V) to almost 50% (w/V), which make microfluidics useable for a broad range of proteins90. Further, microfluidics provide advantages, such as the control of the fiber diameter allowing to produce nanofibers. The usage of a chip with different channels also allows the mimicry of natural assembly and spinning processes, like changes in the pH or ion concentrations by changing the buffer system. Further, the chip can mimic the shape of the natural silk glands, e.g., that of spiders or B. mori. Like for wet spinning, post-processing leads to mechanical properties similar to that of natural fibers48,91.
Dry-spinning
For dry-spinning, proteins have to be dissolved in a volatile solvent. The protein solution is pumped through the spinneret directly into the air. The solvent usually has a low boiling point, low latent heat and appropriate volatility92. The solidification of the fiber occurs during the evaporation of the solvent, which is achieved upon contact with, e.g., hot gas during spinning. The drying starts on the surface of the fiber, resulting in the formation of a skin, and upon further evaporation, the fiber solidifies resulting in a dumbbell shape. The dumbbell shape of the fibers is due to solvent diffusion and evaporation. The inhomogeneous evaporation from the outside to the inside of the fiber is resulting in the collapse of the outer part93. Post-stretching allows further alignment of the fibers. There are several parameters controlling fiber morphology and quality, including concentration of the dope ranging from 15% to 40% (w/V), swelling behavior in the solvent, skin friction, as well as the temperature of the gas flow ranging from 100 to 250 °C93. The molecular weight of the protein is highly influencing the viscosity of the spinning dope and the solidification of the material93,94. Post-processing of dry-spun fibers, e.g., stretching, can yield mechanical properties like found in natural fibers95,96.
Electrospinning
Electrospinning enables processing of continuous protein fibers with diameters in the submicron to nanometer range with a large surface-to-volume ratio97. The basic principle of this technique is the uniaxial stretching of a viscoelastic solution within an electric field. The electric field increases the repulsive force of the solution resulting in a jet. There are several parameters influencing the morphology of the produced fiber, e.g., viscosity of the spin dope (ranging from 1% to 20% (w/V)), conductivity of the solvent, applied voltage, distance between tip and collector and humidity98. Further, fiber assembly can be controlled by choosing different collecting devices. Usually, a static collection plate is used resulting in non-woven fiber meshes, whereas dynamic collection devices, for instance a rotating drum, allow the collection of aligned fibers. Besides organic solvents like 1,1,1,3,3,3-hexafluoroisopripanol (HFIP), also aqueous solutions can be used for electrospinning of proteins99. The chosen solvent will have a high impact on the achieved protein structure within the fiber. For instance, fluorinated alcohols are known to weaken hydrophobic interactions while strengthening electrostatic interactions of the protein due to the low polarity, thus stabilizing secondary structures, particularly α-helices82. Other organic solvents, like FA, are known to dissolve proteins and are inducing β-sheets in the spun fibers due to the dehydration of the proteins100. The benefits of electrospinning are its simplicity as well as its versatility101. Electro-spun structures can closely mimic the hierarchical architecture and fibrous features of extracellular matrices (ECM)102.
Post-processing of man-made protein fibers
Post-processing of man-made protein fibers is often necessary to result in mechanical properties similar to that of the natural ones103. In terms of collagen, the post processing includes for example cross-linking using UV-light (254 nm) as well as thermal treatment. Both increase the strength as well as strain at failure of the collagen fibers. The tensile strength in case of collagen increased up to ~50 MPa in comparison to untreated fibers94.
For B. mori silk fibers, the effect of pre-extension on the mechanical properties was tested, using a short-term stress-relaxation process. Preliminary extension of >3% shifted the yield point from ~5% to 6–8%, while the stress at rupture also increased to around 10–20% after preliminary extension of >10%104.
In case of spider silk there are several publications dealing with the effect of post-spin stretching on the produced protein fibers13,105,106,107,108. A variety of post-spin treatments have been tested. The as-spun fibers were placed in various solutions (H2O, isopropanol, ethanol, methanol and ammonium sulfate) and stretched from both ends using tweezers, improving the mechanical properties of the artificial spider silk fibers. As-spun fibers showed a stress of 10.4 MPa and a strain of 1.5%, whereas the post-stretched fibers showed an increased stress of 29 MPa and strain of 27.3%105. The discovery aligns with native silk spinning, where the fiber is not extruded, but pulled out109.
Bioinspired protein fiber production
Silk, collagen and keratin fiber mimicry
In order to mimic the natural silk spinning process as well as artificial collagen spinning91,110,111,112,113,114,115,116,117 microfluidic-spinning and dry-spinning are appropriate techniques. In case of mimicking spider silk fibers, both the underlying proteins as well as the spinning process are in the focus of research13,48,85,118,119,120,121,122,123.
B. mori fibroin has been spun into fibers showing almost the same properties as natural ones by either using dry-spinning, wet-spinning or microfluidics90,124,125,126,127,128,129,130,131. In these approaches factors like solvent and pH were used to influence the performance of the fiber. Further, it is essential to induce the formation of β-sheets within the fiber based on the protein sequence8,132. Man-made fibers were produced by degumming and solving B. mori cocoons followed by dry-spinning. Afterwards, the fibers were post-processed by immersing in 80% ethanol and post-drawing, resulting in fibers with a strength of 252 MPa (Fig. 5a). Degummed silk fibers, in comparison, showed a tensile strength about 354 MPa133. In another example, dry-spun fibers demonstrated a strength within the range of 295 MPa to 614 MPa and a toughness of 55 MJm−3 to 155 MJm−3 134. Moreover, B. mori silk fibers wet-spun from an all-aqueous solution into a coagulation bath containing an aqueous buffer exhibited a strength ranging from 162 MPa to 370 MPa and a toughness of 45 MJm−3 to 189 MJm−3. In contrast, wet-spun fibers using organic solvents displayed strength values ranging from 38 MPa to 508 MPa and toughness values from 2.5 MJm−3 to 102 MJm−3. Once again, the utilization of aqueous-based dopes resulted in improved mechanical properties135.
Mimicking natural protein fiber production can imply either the used protein, the establishment of a spinning solution, or the spinning process, or any combination thereof. a Regenerated B. mori fibroin biomimetically spun using dry-spinning, resulted in µm-sized fibers with a tensile stress of ~250 MPa. Adapted and modified with permission from ref. 133, copyright American Chemical Society, 2016. b In terms of spider silk, recombinant proteins mimicking the natural ones have been processed into a biomimetic spinning dope (BSD) based on native-like liquid-liquid phase separation. In addition, classical spinning dopes (CSD) were prepared by dehydration using polyethylene glycol (PEG). The dopes were then spun using a microfluidic set-up inspired by the natural spinning process (including pH shift, elongational flow and ion exchange), resulting in um-sized fibers with a maximum stress of ~420 MPa. Adapted and modified with permission from ref. 136, copyright American Chemical Society, 2023. c Spinning solutions of keratin were formed by coacervation, and the wet-spinning process involves ion exchange and a pH drop. The mimicry of the different natural factors like the spinning solution as well as the spinning conditions are resulting in micrometer scale fibers with excellent mechanical properties. Adapted and modified with permission from ref. 137, licensed under CC BY 4.0. d Collagen was extracted from natural sources like rat-tail, solved under acidic conditions, and spun using a microfluidic-spinning process, resulting in fibers with a diameter of 3.6 µm and a tensile strength of 284 MPa. Adapted and modified with permission from ref. 91, copyright American Chemical Society, 2016.
Our group works with recombinant spider silk proteins, based on two different MaSp2 proteins of Araneus diadematus. Like in nature, both MaSp2-derived engineered proteins, namely eADF3((AQ)12)NR3 and eADF4(C16)NR4, were recombinantly produced via co-expression in E. coli yielding a mixture of homo- and heterodimers48. Interestingly, both sequences can also be hybridized yielding a two-in-one spidroin136.The preparation of a biomimetic spider silk fibers starts with the preparation of a biomimetic spinning dope. Potassium phosphate was used to induce liquid-liquid phase separation, like in the spinning duct13,48. A microfluidic chip with three channels enabled to mimic the naturally occurring pH drop necessary for proper fiber assembly. The resulting fibers showed a strength of 834 MPa and a toughness of 143 MJm−3 (Fig. 5b). These properties were achieved by utilizing a mixture of MaSps. In contrast, fibers made of just a single MaSp exhibited lower strength (329 MPa and 602 MPa) and toughness (137 MJm−3 and 32 MJm−3)48.
Regenerated keratin derived from wool or feathers can be used for man-made fiber spinning137,138,139, but the disadvantage is the complex extraction process complicated by highly cross-linked cysteine residues resulting in strong intra- and intermolecular interactions4. Coacervation in combination with wet-spinning was used to produce keratin fibers with improved mechanical properties after post-stretching (Fig. 5c)137. The dissolved keratin was acidified to a pH between 1.0 and 1.7 resulting in phase separation and wet-spun into a coagulation bath containing 1% of H2O2. The final fibers showed an increased toughness of 50 MJm−3. This improvement of the mechanical properties of the fibers is speculated to be attributed to changes in the conformation of the proteins. A significant aspect of recombinant keratins is their tunability in mechanical properties through modifications of the amino acid sequence. This unique feature allows for precise control over the mechanical characteristics of the fibers. Similar to the other proteins mentioned, the mechanical properties of keratin fibers are influenced by factors such as the amino acid sequence, composition, and nanostructure22,140. In terms of man-made keratin fibers, there is the chemical cross-linking, using cross-linking agents like a mixture containing polyol and citric acid141, mercuric acid142, dithiols143 or glutaraldehyde144 can be used to fine-tune mechanical properties.
Collagens can be spun using microfluidics91 and wet-spinning110 to mimic some of the natural properties of collagen fibers, however, with the benefit of producing endless fibers, which are not existent in nature. The staggering of 67 nm of the triple helices is important for the mechanical properties of collagen fibers110. Factors like a pH shift from 3 to 9 occurring in the natural fiber assembly process of collagen are mimicked in artificial fiber spinning of collagen. A microfluidics chip was used to simplify the exchange of buffers and to induce a pH change to more basic conditions resulting in the formation of microfibrils. The chosen process allowed the production of homogenous fibers with diameters between 3 µm to 16 µm depending on the collection rate91. Further, the mechanical properties showed a tensile strength of 383 MPa being superior to that of tendons. Classically wet-spun collagen fibers, in contrast, showed a tensile strength of 241 MPa (Fig. 5d)91.
Mimicry of natural functions using man-made protein fibers
Mimicking natural functions of fibers is most often of interest in the field of biomedical research. In this context, electro-spun nanofibers can mimic several features of the extracellular matrix. For mimicking the function of the ECM, fiber diameter, stiffness, pore size of fiber meshes and cell attachment play an important role. Electro-spun non-woven meshes provide nanoscale scaffolds with interconnecting pores, resembling some features found in the natural ECM in tissues (Fig. 6)145.
The ECM has several properties important for the attachment and proliferation of cells, like fiber diameter, pore size, topography, as well as stiffness. The SEM image in the top lane is representative for the ECM, in this case showing the myocardium of a rat heart. Adapted with permission from ref. 216, licensed under CC BY 4.0. Electrospun fibrous proteins are able to mimic the properties of the natural ECM. Exemplarily, electrospun non-woven meshes derived from (a) recombinant spider silk (seeded with mouse fibroblasts) is used as scaffold for different cell types. Adapted and modified with permission from ref. 146, copyright John Wiley and Sons, 2011. b Cell adhesion was evaluated after 4 h on electrospun meshes made of recombinant spider silk proteins with different fiber diameters (150, 250, 480 and 680 nm). As control, a treated cell culture plate was used. The adhesion test showed that meshes with a larger fiber diameter are better suited for fibroblast adhesion than smaller fiber diameters. Adapted and modified with permission from ref. 146, copyright John Wiley and Sons, 2011. c Regenerated B. mori fibers are used as scaffold for human oral keratinocytes (NHOK). Adapted and modified with permission from ref. 217, copyright Elsevier, 2004. d The spreading behavior of the set-up of (c) was investigated. Polystyrene (used as control) as well as the pure B. mori silk fibers showed less cell spreading, whereas collagen I coated silk fibroin fibers were showing a good cell spreading behavior. Adapted and modified with permission from ref. 217, copyright Elsevier, 2011. e Electrospun collagen fibers were seeded with human osteoblasts. Adapted with permission from ref. 218, copyright John Wiley and Sons, 2009. f In the set-up of (e) F-actin was stained in red using phalloidin, and the nuclei were stained in blue by using DAPI. The collagen fibers enhanced cell growth regardless of the fiber diameter. Adapted with permission from ref. 218, copyright John Wiley and Sons, 2009. g Human mesenchymal stem cells were seeded on extracted keratin fibers. Adapted and modified with permission from ref. 160, copyright Elsevier, 2015. h Cell viability of human mesenchymal stem cells on different keratin scaffolds of the set-up in (g), as well as different calcium phosphate (CaP) coated fibers was determined by using a PicoGreen assay, measuring the amount of double-stranded DNA after 4 days of culturing. Keratin containing scaffolds (cKeratin-PCL (crosslinked Keratin-PCL; poly-ε-caprolactone) and sCaP-cKeratin-PCL (CaP surface coated PCL–cKeratin scaffold) showed increased cell proliferation in comparison to the controls (PCL and CaP surface coated PCL scaffolds). Adapted and modified with permission from ref. 160 copyright Elsevier, 2015.
Mimicking the extracellular matrix was tested with all of the mentioned fibrous proteins (spider silk146,147,148,149, B. mori silk150,151,152,153, collagen154,155,156,157,158,159, keratin160,161,162,163,164,165 and elastin64,164,166,167,168,169). For instance, e-spun spider silk fibers were used as scaffold for different cell types, and the influence of different fiber diameters regarding the spreading behavior was investigated (Fig. 6a, b). Additionally, B. mori fibers were tested as scaffold for human oral keratinocytes (NHOK) by analyzing the spreading behavior of the cells (Fig. 6c, d). In the same way also e-spun keratin and collagen fibers were analyzed regarding their suitability as ECM-mimicking scaffold (Fig. 6e–h). Interestingly, in some approaches even several proteins have been combined, like silk proteins with elastin as well as silk proteins with collagen, resulting in higher tensile strength as well as elasticity170.
Sarrami et al.171 produced electro-spun nanometer-sized keratin fibers mixed with nanohydroxyapatite particles, creating a scaffold for bone tissue engineering. Mimicking the properties of the ECM, like fiber diameter and pore size, the keratin fibers enabled cell adhesion and viability. In another example, Nivison-Smith et al.62 produced aligned electro-spun fibers having a ribbon-like structure, used as scaffold for smooth muscle cells62.
Applications of bioinspired protein fibers
Protein based fibers in textiles
Wool (keratin) fibers are used for textile applications since 10,000 BC. In ancient times, woollen fibers were short and, therefore, much rougher than today. The scaly surface allowed textiles to be manufactured without weaving. The first textiles based on woven wool fibers are dated back to 1800–1500 BC. The first usage of silk from silkworm cocoons as textiles traces back to 5000–3000 BC and was found in China within the Yangshao Culture. Silk was brought to Europe by the crusades, and different manufacturing techniques (e.g., spinning wheels) were developed during the middle ages. The industrial revolution changed the silk industry, because innovations made the spinning of cotton cheaper and more profitable172.
Nowadays, wet-spun recombinant spider silk fibers could be used for the production of a prototype of sneakers173. Besides sneakers, a prototype jacket was produced by emulated spider silk of golden orb spiders174. Furthermore, the silk of Argiope bruennichi was spun into a yarn used for the production of a sustainable non-petroleum-based prototype tennis dress175. Commercially available products are a wrist for watches made from wet-spun recombinant spider silk fibers (Fig. 7a)176, or a hoodie containing around 12% of spider silk in combination with cotton177.
a Commercially available wrist band for watches made from wet-spun recombinant spider silk fibers. b Regenerated collagen type I fibers have been used as suture materials for medical device manufacturing. (i) SEM image of an extruded single fiber. (ii) The photograph depicts 56 collagen fiber strands braided and knotted together forming a fiber bundle. (iii) Stress-strain curve of a fiber bundle. (iv) Confocal images show human tenocytes attached to the microfibers. Living cells were stained with CMFDA (5-chloromethylfluorescein diacetate, green), the nuclei were stained with DAPI (blue). Adapted and modified with permission from ref. 179, copyright Elsevier, 2021. c Wet-spun collagen fibers used for muscle tissue engineering. (i) Fibers showed the typical collagen band pattern with a periodicity of 67 nm. (ii) The fibers showed a maximum stress of ~241 MPa and a strain of ~17%. (iii) The wet-spun fibers were highly processable, like in flat woven structures. (iv) The wet-spun collagen fibers were used for cultivating myoblasts (muscle cells, green) and fibroblasts (endothelial cells, red) in co-culture, showing collagen fibers to be a versatile scaffold for the tendon-muscle interface. Adapted and modified with permission from ref. 110, copyright John Wiley and Sons, 2021. d Electrospun recombinant elastin yarns, manually woven to a frame with pore sizes of 1 mm2 were used for the cultivation of dermal fibroblasts. (i) Scheme of the production of elastin yarns and the final woven topography. (ii) SEM image of an elastin-yarn. (iii) Stress-strain curve of dry and hydrated tropoelastin-silk yarns at a strain rate of 100 mm/min. (iv) Merged images of F-actin staining (yellow), nuclei (blue) and the yarn (green). (v) Representative hematoxylin and eosin staining of tropoelastin-silk meshes after 8 weeks of implantation in mice. Adapted and modified with permission of ref. 63, copyright Elsevier, 2019.
In modern technically-oriented textile applications, B. mori silk fibers have been combined with carbon nanotubes to yield a composite fiber. The composite materials are twisted into a yarn morphology and woven to textile mats, which were used for sneakers with water repellent properties due to the hydrophobicity of the carbon nanotubes178.
Protein-based fibers in biomedicine
In the realm of biomedical applications, individual fibers or yarns have the potential to be used as sutures. In these applications, polymer-based sutures are often non-degradable and non-biocompatible, necessitating their removal several days post-surgery. Protein-based sutures derived from silk, collagens, elastin, and keratins circumvent these drawbacks. For instance, wet-spun collagen microfibers, exemplifying their application as suture material (Fig. 7bi, ii), were braided and knotable. The cytotoxicity of these extruded fibers was assessed by seeding human tenocytes onto them, revealing an elongated cellular morphology (Fig. 7biv). In a clinical viability study, these collagen fibers were implanted in rats to assess stability. Results demonstrated suture stability for up to 6 months in vitro and 4 weeks in vivo, accompanied by a pro-regenerative response179.
Besides sutures, protein-based fibers are a promising material in ligament replacement due to their light-weight, extensibility as well as high tensile strength. Bombyx mori fibroin fibers find application in ligament replacement, particularly for anterior cruciate ligaments, owing to their mechanical properties180,181,182,183,184. Silk fibers braided by Pagán et al.181 were mechanically analyzed, exhibiting mechanical properties equivalent to the natural human anterior cruciate ligament, thus presenting a promising material for tendon replacement181.
In tissue engineering, protein-based woven meshes of silk, keratin, elastin and collagen are in use due to their adaptable mechanical properties as well as pore sizes. Exemplarily, endless-collagen fibers were knitted and then tested in cell culture with muscle cells showing cell alignment, myotube formation and myogenesis within the woven structures (Fig. 7c)110. The collagen fibers were wet-spun and exhibited a characteristic D-pattern. Fibers showed mechanical properties of 241 MPa, aligning with values documented in the literature. In contrast to cross-linked fibers (383 MPa), the tensile strength of the non-crosslinked fibers was comparatively lower91. However, the lack of crosslinking showed other advantages, especially for biomedical applications110. The mechanical robustness of the collagen fibers further enabled their weaving (Fig. 7ciii), subsequently, employed for the cultivation of myoblasts and fibroblasts. Following a 7-day cultivation period, the myoblasts (depicted in green) and fibroblasts (depicted in red) demonstrated complete coverage of the scaffold (Fig. 7civ), providing the basis for engineering a muscle-tendon interface implant110.
Single fibers have also been innovatively employed as biosensors. Liu et al.185 developed a biosensing, antibacterial suture based on a wet-spun silkworm fibroin fiber. This core fiber, coated with macrophage stimulating factors to enhance cell growth and healing, also featured a coating of graphene quantum dots to improve conductivity and antibacterial activity. In cell and animal studies, the resulting core-shell fiber demonstrated a robust antibacterial effect against E. coli. Moreover, in vivo assessments revealed enhanced wound healing properties, and the fibers acted as a sensor for wound stress185.
Weiss et al.63 employed electrospinning techniques to fabricate yarns from recombinantly produced tropoelastin, combined with regenerated Bombyx mori fibroin. Manual weaving of the resulting yarns yielded meshes with pore sizes of 1 mm² (Fig. 7di). Notably, the tropoelastin-silk yarns exhibited significant disparities in mechanical properties between dry and wet states. In the dry state, these yarns displayed a narrow elastic region, culminating in failure at 9% strain. Conversely, in the hydrated state, the yarns demonstrated increased elasticity. The Young’s modulus of the dry fibers, initially measuring 354 MPa (Fig. 7diii), markedly decreased to 8.6 MPa in the wet state. The tropoelastin-silk scaffolds, characterized by their distinctive mechanical behavior, were utilized for the cultivation of fibroblasts, facilitating cell attachment, proliferation, and alignment along the direction of the yarns (Fig. 7div). Notably, in vivo studies spanning 8 weeks underscored the scaffolds’ resilience and revealed significant cellular infiltration into the structure (Fig. 7dv)63.
Protein-based fibers for water collection
Silk microfibers, characterized by wet-rebuilt periodic spindle-knots, exemplify a design inspired by natural spider webs to capture water from humid air, where the natural model efficiently collects water droplets from drizzle, fog, or condensation. The hydrophobic nature of the man-made fiber surface plays a pivotal role in enabling the collection of water droplets, mirroring the water collection principle exhibited by natural spider silk fibers, attributed to their distinctive spindle knot structure186. For the creation of artificial fibers imitating this spindle knot structure, three primary methodologies have been employed. Firstly, electrospinning was utilized to generate beaded fibers, where the beading was intricately influenced by factors such as dope viscosity, jet characteristics, and surface tension186. Secondly, dip-coating emerged as a prevalent technique for spindle-knot fiber production. The process involves immersing a regular protein fiber in another protein solution and subsequent horizontal drawing after a designated period. The breaking of the protein film enveloping the fibers resulted in the attachment of tiny drops around the fiber, thus forming the spindle knot structure. Thirdly, microfluidics offered an alternative approach for achieving the spindle knot fiber morphology, involving the use of a PDMS chip with multiple holes to allow two alternating flows, yielding fibers with the desired structure186,187. For instance, dip coating was used to generate silkworm silk-based spindle-knot-fibers (Fig. 8a). Therefore, degummed silkworm silk derived from cocoons was dip-coated with gelatin derived from porcine skin. The gelatin-coated fiber showed enhanced mechanical properties (Fig. 8aiii) and a high efficiency in water collection (Fig. 8aiv)170. In another example a B. mori fibroin fiber, extracted from cocoons, was dip-coated with recombinant spider silk proteins. After drying of the silk solution on the fiber surface, spindle-knot fibers were obtained. Regarding water collection, the protein-based fiber showed much higher efficiency than a nylon-based system (Fig. 8c)188.
a Regenerated B. mori silk fibers dip-coated with gelatin. (i) Scheme of the production of the spindle knot fibers. (ii) SEM images of the morphology of the fabricated fibers coated with 10% gelatin. (iii) Stress-strain curves of degummed silk fibroin fibers (red) and various treated gelatin-coated fibers. Black: freshly prepared gelatin-coated fiber; blue: treated gelatin-coated fiber stored at 40 °C and 30% humidity (15 days); green: gelatin-coated fiber stored for 1 year. (iv) Water collecting properties of the gelatin-coated fiber at ~95% relative humidity. Adapted and modified with permission from ref. 170, copyright Springer nature 2023. b B. mori fibers dip-coated with spider silk proteins. A core-shell silk fiber consisting of B. mori silk protein (core) coated with recombinant major ampullate silk protein (MaSp2) allowed the formation of knots on the fiber surface after drying. These fibers enabled water collection from the surrounding environment. Adapted and modified with permission from ref. 188, copyright John Wiley and Sons, 2020.
Protein-based fiber meshes for filtration applications
Keratin and silk non-woven meshes have also been used as filter membranes due to their sustainability and biodegradability, referring to the possibility of natural decomposition of the keratin- and silk-based fibers by enzymes. This transformative process converts complex organic compounds into simpler, environmentally benign substances, including water, carbon dioxide, methane, biomass, and inorganic compounds189,190.
The big advantage of using fiber meshes in filtration is their big surface-to-volume ratio and their permeability, especially for gas or liquid flow-through applications. B. mori fibroin, extracted from cocoons, was prepared as aqueous solution and electrospun into nanofiber meshes, using polyethylene glycol (PEO) as additive. The obtained filter layers were compared to commercially available systems regarding filtration efficiency and resistance of air flow. Polluted air was flown through an acrylic pipe and purified by the air filter. In general, the filter efficiency was superior in comparison to that of artificial ones191. Electrospun spider silk mats (Fig. 9aii) were also used for air filtering membranes192. Applying centrifugal electrospinning allowed high throughput filter membrane production within a roll-to-roll process (Fig. 9aiv)193. Due to their nanometer size, the filter efficiency of the spider silk meshes was significantly higher than that of a commercial filter system (Fig. 9aiii)193. Electrospun keratin fibers extracted from wool were used to produce electrospun meshes with fibers having diameters in the nanometer range and a varying pore size due to the duration of the electrospinning process. These meshes were used as air filters to remove solid particles with micron size e.g., bacteria194.
a Filtering membranes made of recombinantly produced spider silk proteins. (i) Scheme of the spider silk non-woven mesh used as filter membrane. (ii) SEM image of an electrospun spider silk protein filter mat. (iii) The e-spun meshes were tested in air filtering applications, showing a higher filter efficiency than tested commercial filters. (iv) Filter mat production was scaled-up using centrifugal electrospinning together with a roll-to-roll process. Model showing the spinning device: scaffold support bracket (1), spinning chamber (2), collector (3) and winding unit (4). Adapted and modified with permission from ref. 193, licensed under CC BY 4.0. b E-spun silk fibroin (B. mori) non-woven meshes spray-coated with TiO2-nanoparticles for photocatalytic water treatment. (i) Scheme of the production and application of the composite meshes. (ii) SEM image of the particle-loaded e-spun fibers. (iii) degradation kinetics of pyraclostrobin upon photolysis. Adapted and modified with permission of ref. 200, copyright Royal Society of Chemistry, 2020.
Protein-based fiber meshes for catalytic applications
In order to use protein-based fiber meshes regarding water treatment, fibers were modified using inorganic nanoparticles, either immobilized by chemical binding on the fiber surface or by a spray-coating process195. Combining electrospun fiber meshes consisting of B. mori silk fibroin with TiO2 and ZnO nanoparticles, a hybrid mesh was created used for the treatment of pesticide polluted water. The particles had no considerable influence on the mechanical properties of the fibers, but the hybrid materials showed a significant degradation activity on pesticides upon irradiation with sun light (Fig. 9b)195. Additionally, keratin, extracted from wool, was blended with polyethylene terephthalate (PET) and electrospun into nanofibers with diameters ranging from 310 nm to 790 nm. The final composite material was placed in an Cr(VI) solution, showing an increasing adsorption ability with increasing keratin content, due to the disulfide bonds and the redox reaction with the cystine oxide196.
Conclusion and outlook
In conclusion, protein-based fibers and fiber systems are promising materials for a multitude of applications. The understanding of the natural assembly and spinning processes is resulting in fibers with mechanical properties sometimes as good as in nature sometimes even better. Due to the properties of being biodegradable and biocompatible, protein-based fibers are used to replace polymer-based ones in applications as filters as well as scaffolds in catalytic reactions for water treatment. In the field of biomedicine, protein-based fibers are used as sutures and scaffolds in tissue engineering. However, there are some problems that must be overcome in the future. First, the lack of mass production of fibrous proteins is a major drawback at the moment. The extraction and the collection of naturally derived proteins is yet not as (cost) efficient as the production of synthetic polymers. Therefore, the focus of research is on the scale-up and the price-reduction of the production of recombinant proteins. Further, degradation and long-term stability are issues which protein fibers face, which can be beneficial in terms of environmental impact, but detrimental in the durability of a product. There is still the necessity of posttreatment of the fibers with chemicals or the mixing with other polymers to render the protein fibers stable against UV-light or even water. But the versatility and multifunctionality as well as the easy modification of protein fibers makes them valuable and promising for a myriad of applications. Due to further knowledge-gain regarding the natural systems and the mimicking thereof in man-made fiber spinning set-ups, we assume that protein-based fibers will play an increasingly important role in the future. Especially, regarding the raising demand for green materials as well as for alternative energy sources and the reduction of microplastics, protein-based fibers have already shown their potential as one material of choice.
References
Arent, D. J., Wise, A. & Gelman, R. The status and prospects of renewable energy for combating global warming. Energy Econ. 33, 584–593 (2011).
Shim, W. J. & Thomposon, R. C. Microplastics in the ocean. Arch. Environ. Contam Toxicol. 69, 265–268 (2015).
Humenik, M., Scheibel, T. & Smith, A. Spider silk: understanding the structure-function relationship of a natural fiber. Prog. Mol. Biol. Transl. Sci. 103, 131–185 (2011).
Poole, A. J., Church, J. S. & Huson, M. G. Environmentally sustainable fibers from regenerated protein. Biomacromolecules 10, 1–8 (2009).
Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
Mark, H. F. (ed.). Encyclopedia of Polymer Science and Technology (John Wiley & Sons Inc, 2014).
Chen, S., Liu, M., Huang, H., Cheng, L. & Zhao, H.-P. Mechanical properties of Bombyx mori silkworm silk fibre and its corresponding silk fibroin filament: a comparative study. Mater. Des. 181, 108077 (2019).
Pereira, R. F. P., Silva, M. M. & de Zea Bermudez, V. Bombyx mori silk fibers: an outstanding family of materials. Macromol. Mater. Eng. 300, 1171–1198 (2015).
Panilaitis, B. et al. Macrophage responses to silk. Biomaterials 24, 3079–3085 (2003).
Guo, C. et al. Structural comparison of various silkworm silks: an insight into the structure–property relationship. Biomacromolecules. https://doi.org/10.1021/acs.biomac.7b01687 (2018).
Rhisiart, A. & Vollrath, F. Design features of the orb web of the spider, Araneus diadematus. Behav. Ecol. 5, 280–287 (1994).
Heim, M., Keerl, D. & Scheibel, T. Spider silk: from soluble protein to extraordinary fiber. Angew. Chem. Int. Ed. Engl. 48, 3584–3596 (2009).
Heidebrecht, A. et al. Biomimetic fibers made of recombinant spidroins with the same toughness as natural spider silk. Adv. Mater. 27, 2189–2194 (2015).
Motriuk-Smith, D., Smith, A., Hayashi, C. Y. & Lewis, R. Analysis of the conserved N-terminal domains in major ampullate spider silk proteins. https://doi.org/10.1021/bm050472b (2005).
Ayoub, N. A., Garb, J. E., Tinghitella, R. M., Collin, M. A. & Hayashi, C. Y. Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PLoS ONE 2, e514 (2007).
Garb, J. E. et al. The transcriptome of Darwin’s bark spider silk glands predicts proteins contributing to dragline silk toughness. Commun. Biol. 2, 275 (2019).
Plowman, J. E. The proteomics of keratin proteins. J. Chromatogr. B 849, 181–189 (2007).
Bragulla, H. H. & Homberger, D. G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 214, 516–559 (2009).
Lee, H. et al. Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng. Regen. Med. 11, 255–265 (2014).
Yu, J., Yu, D., Checkla, D. M., Freedberg, I. M. & Bertolino, A. P. Human hair keratins. J. Invest. Dermatol. 101, S56–S59 (1993).
Lazarus, B. S. et al. Engineering with keratin: a functional material and a source of bioinspiration. iScience 24, 102798 (2021).
Basit, A., asghar, F., Sadaf, S. & Akhtar, M. W. Health improvement of human hair and their reshaping using recombinant keratin K31. Biotechnol. Rep. 20, e00288 (2018).
Exposito, J.-Y., Valcourt, U., Cluzel, C. & Lethias, C. The fibrillar collagen family. Int. J. Mol. Sci. 11, 407–426 (2010).
Owczarzy, A. et al. Collagen—structure, properties and application. https://doi.org/10.34821/ENG.BIOMAT.156.2020.17-23 (2020).
Avila Rodríguez, M. I., Rodríguez Barroso, L. G. & Sánchez, M. L. Collagen: a review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 17, 20–26 (2018).
Birk, D. E. & Bruckner, P. in Collagen, Vol. 247 (eds Brinckmann, J., Notbohm, H. & Müller, P. K.) 185–205 (Springer, 2005).
Mithieux, S. M. & Weiss, A. S. Elastin. in Advances in Protein Chemistry: Fibrous Proteins: Coiled-Coils, Collagen and Elastomers, Vol. 70 (eds Parry, D. & Squire, J.) 437–461 (Academic Press, 2005).
Debelle, L. & Tamburro, A. M. Elastin: molecular description and function. Int. J. Biochem. Cell Biol. 31, 261–272 (1999).
Anwar, R. A. Elastin: a brief review. Biochem. Educ. 18, 162–166 (1990).
Parks, W. C., Pierce, R. A., Lee, K. A. & Mecham, R. P. Elastin. Adv. Mol. Cell Biol. 6, 133–181 (1993).
Karamanos, N. K. et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 288, 6850–6912 (2021).
Kristensen, J. H. & Karsdal, M. A. Elastin. in Biochemistry of Collagens, Laminins and Elastin. Structure, Function and Biomarkers (ed. Karsdal, M. A.) 197–201 (Academic Press, 2016).
Ferraro, V., Anton, M. & Santé-Lhoutellier, V. The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: functionality, bioactivity and trends of application. Trends Food Sci. Technol. 51, 65–75 (2016). This review compares the assembly processes of collagen, elastin and keratin and also highlights their usage as biomaterials.
Teulé, F. Spinning of fibers from protein solutions. in Biologically Inspired Textiles 1st edn (eds Abbott, A., Ellison, M. & Abbott, A. G.) 44–73 (CRC Press, 2008).
Smack, D. P., Korge, B. P. & James, W. D. Keratin and keratinization. J. Am. Acad. Dermatol. 30, 85–102 (1994).
Steinert, P. M. et al. Glycine loops in proteins: their occurence in certain intermediate filament chains, loricrins and single-stranded RNA binding proteins. Int. J. Biol. Macromol. 13, 130–139 (1991).
Conway, J. F. & Parry, D. A. Intermediate filament structure: 3. Analysis of sequence homologies. Int. J. Biol. Macromol. 10, 79–98 (1988).
Hatzfeld, M. & Weber, K. Modulation of keratin intermediate filament assembly by single amino acid exchanges in the consensus sequence at the C-terminal end of the rod domain. J. Cell Sci. 99, 351–362 (1991).
Singh, V., Wang, S. & Ng, K. W. 2.25 Keratin as a biomaterial. in Comprehensive Biomaterials II (eds Ducheyne, P., Healy, K., Hutmacher, D. E., Grainger, D. W. & Kirkpatrick, C. J.) 542–557 (Elsevier Science, 2017).
Alibardi, L. Chapter six—the process of cornification evolved from the initial keratinization in the epidermis and epidermal derivatives of vertebrates: a new synthesis and the case of sauropsids. Int. Rev. Cell Mol. Biol. 327, 263–319 (2016).
Kharaziha, M., Scheibel, T. & Salehi, S. Multifunctional naturally derived bioadhesives: from strategic molecular design toward advanced biomedical applications. Prog. Polym. Sci. 101792. https://doi.org/10.1016/j.progpolymsci.2024.101792 (2024).
Ziegler, K. Crosslinking and self-crosslinking in keratin fibers. in Chemistry of Natural Protein Fibers (ed. Asquith, R. S.) 267–300 (Springer, 1977).
Revell, C. K. et al. Collagen fibril assembly: new approaches to unanswered questions. Matrix Biol. 12, 100079 (2021).
Fratzl, P. Cellulose and collagen: from fibres to tissues. Curr. Opin. Colloid Interface Sci. 8, 32–39 (2003).
Silver, F. H., Freeman, J. W. & Seehra, G. P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 36, 1529–1553 (2003).
Wang, R., Ozsvar, J., Yeo, G. C. & Weiss, A. S. Hierarchical assembly of elastin materials. Curr. Opin. Chem. Eng. 24, 54–60 (2019).
Andersson, M., Johansson, J. & Rising, A. Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 17. https://doi.org/10.3390/ijms17081290 (2016). This study delineates the natural assembly processes of spider silk and Bombyx mori silk fibers, emphasizing their distinct characteristics.
Saric, M., Eisoldt, L., Döring, V. & Scheibel, T. Interplay of different major ampullate spidroins during assembly and implications for fiber mechanics. Adv. Mater. 33, 2006499 (2021). The authors co-expressed different spider silk proteins resulting in artificial fibers with outstanding mechanical properties.
Gillespie, J. S. Progress in man-made protein fibers1. Text. Res. J. 26, 881–888 (1956).
Römer, L. & Scheibel, T. The elaborate structure of spider silk: structure and function of a natural high performance fiber. Prion 2, 154–161 (2008).
Karami, A. et al. Extraction and characterization of collagen with cost-effective method from human placenta for biomedical applications. World J. Plast. Surg. 8, 352–358 (2019).
Zhang, J. et al. Robust biological fibers based on widely available proteins: facile fabrication and suturing application. Small 16, e1907598 (2020).
Chilakamarry, C. R. et al. Extraction and application of keratin from natural resources: a review. 3 Biotech 11, 220 (2021).
Sah, M. K. & Pramanik, K. Regenerated silk fibroin from B. mori silk cocoon for tissue engineering applications. IJESD 404–408. https://doi.org/10.7763/IJESD.2010.V1.78 (2010).
Xiao, Y. et al. Bioinspired tough and strong fibers with hierarchical core–shell structure. Adv. Mater. Interfaces 10 https://doi.org/10.1002/admi.202201962 (2023).
Shavandi, A., Silva, T. H., Bekhit, A. A. & Bekhit, A. E.-D. A. Keratin: dissolution, extraction and biomedical application. Biomater. Sci. 5, 1699–1735 (2017).
Mathew-Steiner, S. S., Roy, S. & Sen, C. K. Collagen in wound healing. Bioengineering 8 https://doi.org/10.3390/bioengineering8050063 (2021).
Eisoldt, L., Smith, A. & Scheibel, T. Decoding the secrets of spider silk. Mater. Today 14, 80–86 (2011).
Whittall, D. R., Baker, K. V., Breitling, R. & Takano, E. Host systems for the production of recombinant spider silk. Trends Biotechnol. 39, 560–573 (2021).
Wang, T., Lew, J., Premkumar, J., Poh, C. L. & Win Naing, M. Production of recombinant collagen: state of the art and challenges. Eng. Biol. 1, 18–23 (2017).
Yanagisawa, S. et al. Improving cell-adhesive properties of recombinant Bombyx mori silk by incorporation of collagen or fibronectin derived peptides produced by transgenic silkworms. Biomacromolecules 8, 3487–3492 (2007).
Nivison-Smith, L. & Weiss, A. S. Alignment of human vascular smooth muscle cells on parallel electrospun synthetic elastin fibers. J. Biomed. Mater. Res. Part A 100A, 155–161 (2012).
Aghaei-Ghareh-Bolagh, B. et al. Fabricated tropoelastin-silk yarns and woven textiles for diverse tissue engineering applications. Acta Biomater. 91, 112–122 (2019). This paper summarizes the production of tropoelastin-silk yarns and their application in the field of tissue engineering.
Li, M. et al. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 26, 5999–6008 (2005).
Qiu, W., Teng, W., Cappello, J. & Wu, X. Wet-spinning of recombinant silk-elastin-like protein polymer fibers with high tensile strength and high deformability. Biomacromolecules 10, 602–608 (2009).
Patel, J. et al. Elastin-like polypeptide fusions enhance the accumulation of recombinant proteins in tobacco leaves. Transgenic Res. 16, 239–249 (2007).
Trabbic-Carlson, K., Liu, L., Kim, B. & Chilkoti, A. Expression and purification of recombinant proteins from Escherichia coli: comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 13, 3274–3284 (2004).
Collins, T. et al. Batch production of a silk-elastin-like protein in E. coli BL21(DE3): key parameters for optimisation. Micro. Cell Fact. 12, 21 (2013).
Tronci, G. et al. Wet-spinnability and crosslinked fibre properties of two collagen polypeptides with varied molecular weight. Int. J. Biol. Macromol. 81, 112–120 (2015).
Gao, F. et al. Recombinant human hair keratin nanoparticles accelerate dermal wound healing. ACS Appl. Mater. Interfaces 11, 18681–18690 (2019).
Kan, J. et al. Study of mechanisms of recombinant keratin solubilization with enhanced wound healing capability. Chem. Mater. 32, 3122–3133 (2020).
Hatzfeld, M. & Weber, K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J. Cell Biol. 110, 1199–1210 (1990).
Paladini, R. D., Takahashi, K., Gant, T. M. & Coulombe, P. A. cDNA cloning and bacterial expression of the human type I keratin 16. Biochem. Biophys. Res. Commun. 215, 517–523 (1995).
Herrmann, H., Wedig, T., Porter, R. M., Lane, E. B. & Aebi, U. Characterization of early assembly intermediates of recombinant human keratins. J. Struct. Biol. 137, 82–96 (2002).
Salehi, S., Koeck, K. & Scheibel, T. Spider silk for tissue engineering applications. Molecules 25, https://doi.org/10.3390/molecules25030737 (2020).
Sun, J. et al. Protein fibers with self-recoverable mechanical properties via dynamic imine chemistry. Nat. Commun. 14, 5348 (2023).
Zhao, L. et al. Biosynthetic protein and nanocellulose composite fibers with extraordinary mechanical performance. Nano Today 44, 101485 (2022).
Li, Y. et al. Bioinspired and mechanically strong fibers based on engineered non-spider chimeric proteins. Angew. Chem. Int. Ed. Engl. 59, 8148–8152 (2020).
Wan, S. et al. Biological composite fibers with extraordinary mechanical strength and toughness mediated by multiple intermolecular interacting networks. Nano Res. 15, 9192–9198 (2022).
Puppi, D. & Chiellini, F. Wet-spinning of biomedical polymers: from single-fibre production to additive manufacturing of three-dimensional scaffolds. Polym. Int. 66, 1690–1696 (2017).
Doblhofer, E., Heidebrecht, A. & Scheibel, T. To spin or not to spin: spider silk fibers and more. Appl. Microbiol. Biotechnol. 99, 9361–9380 (2015).
Hong, D.-P., Hoshino, M., Kuboi, R. & Goto, Y. Clustering of fluorine-substituted alcohols as a factor responsible for their marked effects on proteins and peptides. J. Am. Chem. Soc. 121, 8427–8433 (1999).
Köhler, T., Peterek, S. & Gries, T. Wet spinning PAN-fibres from aqueous solutions of ZnCl 2 and NaSCN. IOP Conf. Ser. Mater. Sci. Eng. 254, 82016 (2017).
Rangtong, L. et al. Structure and properties of wool keratin/poly (vinyl alcohol) blended fiber. Adv. Polym. Technol. 37, 2756–2762 (2018).
Andersson, M. et al. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 13, 262–264 (2017).
Zhang, D. (ed.). Advances in Filament Yarn Spinning of Textiles and Polymers (Elsevier Science, 2014).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Pullagura, B. K. & Gundabala, V. Microfluidics-based on-demand generation of nonwoven and single polymer microfibers. Langmuir 36, 1227–1234 (2020).
Daniele, M. A., Boyd, D. A., Adams, A. A. & Ligler, F. S. Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications. Adv. Healthc. Mater. 4, 11–28 (2015). This review article summarizes the latest progress within microfluidic fiber spinning and highlights the usage of the spun fibers in tissue engineering.
Peng, Q., Shao, H., Hu, X. & Zhang, Y. Role of humidity on the structures and properties of regenerated silk fibers. Prog. Nat. Sci. Mater. Int. 25, 430–436 (2015).
Haynl, C., Hofmann, E., Pawar, K., Förster, S. & Scheibel, T. Microfluidics-produced collagen fibers show extraordinary mechanical properties. Nano Lett. 16, 5917–5922 (2016).
Temesgen, S., Rennert, M., Tesfaye, T. & Nase, M. Review on spinning of biopolymer fibers from starch. Polymers 13, 1121 (2021).
Imura, Y., Hogan, R. M. C. & Jaffe, M. Dry spinning of synthetic polymer fibers. in Advances in Filament Yarn Spinning of Textiles and Polymers 187–202 (Woodhead Publishing, 2014).
Weadock, K. S., Miller, E. J., Bellincampi, L. D., Zawadsky, J. P. & Dunn, M. G. Physical crosslinking of collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 29, 1373–1379 (1995).
Yue, X. et al. A novel route to prepare dry-spun silk fibers from CaCl2–formic acid solution. Mater. Lett. 128, 175–178 (2014).
Wei, W., Zhang, Y., Shao, H. & Hu, X. Posttreatment of the dry-spun fibers obtained from regenerated silk fibroin aqueous solution in ethanol aqueous solution. J. Mater. Res. 26, 1100–1106 (2011).
Teo, W. E. & Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 17, R89–R106 (2006).
Tan, S.-H., Inai, R., Kotaki, M. & Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 46, 6128–6134 (2005).
DeSimone, E., Aigner, T. B., Humenik, M., Lang, G. & Scheibel, T. Aqueous electrospinning of recombinant spider silk proteins. Mater. Sci. Eng. C Mater. Biol. Appl. 106, 110145 (2020).
Borkner, C. B., Elsner, M. B. & Scheibel, T. Coatings and films made of silk proteins. ACS Appl. Mater. Interfaces 6, 15611–15625 (2014).
Bhardwaj, N. & Kundu, S. C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 28, 325–347 (2010).
Su, Y. et al. 3D electrospun synthetic extracellular matrix for tissue regeneration. Small Sci. 1, 2100003 (2021).
Ha, S.-W., Tonelli, A. E. & Hudson, S. M. Structural studies of Bombyx mori silk fibroin during regeneration from solutions and wet fiber spinning. Biomacromolecules 6, 1722–1731 (2005).
Aksakal, B., Tsobkallo, E. & Darvish, D. Mechanical properties of Bombyx mori silk yarns studied with tensile testing method. J. Appl. Polym. Sci. 113, 2514–2523 (2009).
Albertson, A. E., Teulé, F., Weber, W., Yarger, J. L. & Lewis, R. V. Effects of different post-spin stretching conditions on the mechanical properties of synthetic spider silk fibers. J. Mech. Behav. Biomed. Mater. 29, 225–234 (2014). This work compares different post-spin stretching conditions and investigates their influence on the mechanical properties of the protein fibers.
Arndt, T. et al. Engineered spider silk proteins for biomimetic spinning of fibers with toughness equal to dragline silks. Adv. Funct. Mater. 32, 2200986 (2022).
Gonska, N. et al. Structure-function relationship of artificial spider silk fibers produced by straining flow spinning. Biomacromolecules 21, 2116–2124 (2020).
Schmuck, B. et al. Strategies for making high‐performance artificial spider silk fibers. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202305040 (2023).
Eberhard, W. G. Spider Webs. Behavior, Function, and Evolution (The University of Chicago Press, 2020).
Koeck, K. S., Salehi, S., Humenik, M. & Scheibel, T. Processing of continuous non‐crosslinked collagen fibers for microtissue formation at the muscle‐tendon interface. Adv. Funct. Mater. 32, 2112238 (2022). The authors produced non-crosslinked, wet spun collagen fibers which were used for co-culture with the possible application as muscle tendon implant.
Kivanany, P. B. et al. An in vitro model for assessing corneal keratocyte spreading and migration on aligned fibrillar collagen. J. Funct. Biomater. 9, 54 (2018).
Zeugolis, D. I., Paul, G. R. & Attenburrow, G. Cross-linking of extruded collagen fibers—a biomimetic three-dimensional scaffold for tissue engineering applications. J. Biomed. Mater. Res. Part A 89, 895–908 (2009).
Arafat, M. T., Tronci, G., Yin, J., Wood, D. J. & Russell, S. J. Biomimetic wet-stable fibres via wet spinning and diacid-based crosslinking of collagen triple helices. Polymer 77, 102–112 (2015).
Dunn, M. G., Avasarala, P. N. & Zawadsky, J. P. Optimization of extruded collagen fibers for ACL reconstruction. J. Biomed. Mater. Res. 27, 1545–1552 (1993).
Wang, M. C., Pins, G. D. & Silver, F. H. Collagen fibres with improved strength for the repair of soft tissue injuries. Biomaterials 15, 507–512 (1994).
Zeugolis, D. I., Paul, R. G. & Attenburrow, G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: animal species and collagen extraction method. J. Biomed. Mater. Res. Part A 86, 892–904 (2008).
Kato, Y. P. et al. Mechanical properties of collagen fibres: a comparison of reconstituted and rat tail tendon fibres. Biomaterials 10, 38–42 (1989).
Rising, A. & Johansson, J. Toward spinning artificial spider silk. Nat. Chem. Biol. 11, 309–315 (2015).
Peng, Q. et al. Recombinant spider silk from aqueous solutions via a bio-inspired microfluidic chip. Sci. Rep. 6, 36473 (2016).
Schmuck, B. et al. High-yield production of a super-soluble miniature spidroin for biomimetic high-performance materials. Mater. Today 50, 16–23 (2021).
Renberg, B., Andersson-Svahn, H. & Hedhammar, M. Mimicking silk spinning in a microchip. Sens. Actuators B Chem. 195, 404–408 (2014).
Lazaris, A. et al. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science 295, 472–476 (2002).
Shao, Z., Vollrath, F., Yang, Y. & Thøgersen, H. C. Structure and behavior of regenerated spider silk. Macromolecules 36, 1157–1161 (2003).
Xie, F., Zhang, H., Shao, H. & Hu, X. Effect of shearing on formation of silk fibers from regenerated Bombyx mori silk fibroin aqueous solution. Int. J. Biol. Macromol. 38, 284–288 (2006).
Zhou, G., Shao, Z., Knight, D. P., Yan, J. & Chen, X. Silk fibers extruded artificially from aqueous solutions of regenerated bombyx mori silk fibroin are tougher than their natural counterparts. Adv. Mater. 21, 366–370 (2009).
Wei, W., Zhang, Y., Zhao, Y., Shao, H. & Hu, X. Studies on the post-treatment of the dry-spun fibers from regenerated silk fibroin solution: post-treatment agent and method. Mater. Des. 36, 816–822 (2012).
Wei, W. et al. Bio-inspired capillary dry spinning of regenerated silk fibroin aqueous solution. Mater. Sci. Eng. C 31, 1602–1608 (2011).
Jin, Y., Zhang, Y., Hang, Y., Shao, H. & Hu, X. A simple process for dry spinning of regenerated silk fibroin aqueous solution. J. Mater. Res. 28, 2897–2902 (2013).
Luo, J. et al. Tough silk fibers prepared in air using a biomimetic microfluidic chip. Int. J. Biol. Macromol. 66, 319–324 (2014).
Yan, J., Zhou, G., Knight, D. P., Shao, Z. & Chen, X. Wet-spinning of regenerated silk fiber from aqueous silk fibroin solution: discussion of spinning parameters. Biomacromolecules 11, 1–5 (2010).
Fang, G. et al. Insights into silk formation process: correlation of mechanical properties and structural evolution during artificial spinning of silk fibers. ACS Biomater. Sci. Eng. 2, 1992–2000 (2016).
Asakura, T., Kametani, S. & Suzuki, Y. Silk. in Encyclopedia of Polymer Science and Technology (ed. Mark, H. F.) 1–19 (John Wiley & Sons Inc, 2014).
Zhang, C., Zhang, Y., Shao, H. & Hu, X. Hybrid silk fibers dry-spun from regenerated silk fibroin/graphene oxide aqueous solutions. ACS Appl. Mater. Interfaces 8, 3349–3358 (2016). The authors fabricated regenerated B. mori silk into fibers through dry spinning and examined how the addition of graphite nanoparticles affected the mechanical properties of the fibers.
Peng, Q., Shao, H., Hu, X. & Zhang, Y. Microfluidic dry-spinning and characterization of regenerated silk fibroin fibers. JoVE. https://doi.org/10.3791/56271 (2017).
Koeppel, A. & Holland, C. Progress and trends in artificial silk spinning: a systematic review. ACS Biomater. Sci. Eng. 3, 226–237 (2017).
Saric, M. & Scheibel, T. Two-in-one spider silk protein with combined mechanical features in all-aqueous spun fibers. Biomacromolecules 24, 1744–1750 (2023). Mimicking the natural spinning parameters of spider silk results in artificial man-made fibers with outstanding mechanical properties.
Sun, J. et al. Bioinspired processing of keratin into upcycled fibers through pH-induced coacervation. ACS Sustain. Chem. Eng. 11, 1985–1994 (2023). The authors used coacervation for the production of keratin based fibers. Further, it is shown how pH influences the mechanical properties.
Bayanmunkh, O. et al. Fabrication of wet-spun wool keratin/poly(vinyl alcohol) hybrid fibers: effects of keratin concentration and flow rate. ACS Omega 8, 12327–12333 (2023).
Zhu, J. et al. Reinforced wool keratin fibers via dithiol chain re-bonding. Adv. Funct. Mater. 2213644 https://doi.org/10.1002/adfm.202213644 (2023).
Chambre, L., Martín-Moldes, Z., Parker, R. N. & Kaplan, D. L. Bioengineered elastin- and silk-biomaterials for drug and gene delivery. Adv. Drug Deliv. Rev. 160, 186–198 (2020).
Song, K., Xu, H., Mu, B., Xie, K. & Yang, Y. Non-toxic and clean crosslinking system for protein materials: effect of extenders on crosslinking performance. J. Clean. Prod. 150, 214–223 (2017).
Ghosh, R. C., Holker, J. R. & Speakman, J. B. The reactivity of keratin. Text. Res. J. 28, 112–119 (1958).
Zhu, J. et al. Reinforced wool keratin fibers via dithiol chain re‐bonding. Adv. Funct. Mater. 33, 2213644 (2023).
Xu, X. et al. Reconstructed hierarchically structured keratin fibers with shape-memory features based on reversible secondary-structure transformation. Adv. Mater. 35, e2304725 (2023).
Jun, I., Han, H.-S., Edwards, J. R. & Jeon, H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int. J. Mol. Sci. 19 https://doi.org/10.3390/ijms19030745 (2018).
Leal-Egaña, A. et al. Interactions of fibroblasts with different morphologies made of an engineered spider silk protein. Adv. Eng. Mater. 14, B67–B75 (2012).
Zhu, B., Li, W., Lewis, R. V., Segre, C. U. & Wang, R. E-spun composite fibers of collagen and dragline silk protein: fiber mechanics, biocompatibility, and application in stem cell differentiation. Biomacromolecules 16, 202–213 (2015). This paper combines the advantages of collagen fibers being part of the ECM and spider silk fibers showing high tensile strength to create a hybrid material suitable for stem cell proliferation.
Zhu, B. et al. Optimization of glutaraldehyde vapor treatment for electrospun collagen/silk tissue engineering scaffolds. ACS Omega 2, 2439–2450 (2017).
Zhu, B. et al. A study of unidirectionally aligned collagen-silk composite fibers and the application in hdpPSC neural differentiation. Microsc Microanal. 20, 1436–1437 (2014).
Meechaisue, C. et al. Preparation of electrospun silk fibroin fiber mats as bone scaffolds: a preliminary study. Biomed. Mater. 2, 181–188 (2007).
Keirouz, A. et al. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 112, 110939 (2020).
Soffer, L. et al. Silk-based electrospun tubular scaffolds for tissue-engineered vascular grafts. J. Biomater. Sci. Polym. Ed. 19, 653–664 (2008).
Wei, K., Kim, B.-S. & Kim, I.-S. Fabrication and biocompatibility of electrospun silk biocomposites. Membranes 1, 275–298 (2011).
Yoo, J. J. et al. Electrospinning fabrication of collagen-based scaffolds for vascular tissue engineering. FASEB J. 20, A1101 (2006).
Boland, E. D. et al. Electrospinning collagen and elastin: preliminary vascular tissue engineering. Front. Biosci. 9, 1422–1432 (2004).
Blackstone, B. N., Gallentine, S. C. & Powell, H. M. Collagen-based electrospun materials for tissue engineering: a systematic review. Bioengineering 8, 39 (2021).
Chen, Z. G., Wang, P. W., Wei, B., Mo, X. M. & Cui, F. Z. Electrospun collagen-chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 6, 372–382 (2010).
Xu, Y. et al. ECM-inspired micro/nanofibers for modulating cell function and tissue generation. Sci. Adv. 6 https://doi.org/10.1126/sciadv.abc2036 (2020).
Khajavi, R., Abbasipour, M. & Bahador, A. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 133, n/a–n/a (2016).
Zhao, X. et al. Calcium phosphate coated Keratin-PCL scaffolds for potential bone tissue regeneration. Mater. Sci. Eng. C 49, 746–753 (2015).
Fortunato, G. M. et al. Electrospun structures made of a hydrolyzed keratin-based biomaterial for development of in vitro tissue models. Front. Bioeng. Biotechnol. 7, 174 (2019).
Wan, X. et al. S-nitrosated keratin composite mats with NO release capacity for wound healing. Chem. Eng. J. 400, 125964 (2020).
Jung, S. et al. Transformation of electrospun Keratin/PVA nanofiber membranes into multilayered 3D scaffolds: physiochemical studies and corneal implant applications. Int. J. Pharm. 610, 121228 (2021).
Xu, H., Cai, S., Xu, L. & Yang, Y. Water-stable three-dimensional ultrafine fibrous scaffolds from keratin for cartilage tissue engineering. Langmuir ACS J. Surf. colloids 30, 8461–8470 (2014).
Yen, K.-C., Chen, C.-Y., Huang, J.-Y., Kuo, W.-T. & Lin, F.-H. Fabrication of keratin/fibroin membranes by electrospinning for vascular tissue engineering. J. Mater. Chem. B 4, 237–244 (2016).
Huang, L. et al. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules 33, 2989–2997 (2000).
Daamen, W. F., Veerkamp, J. H., van Hest, J. C. M. & van Kuppevelt, T. H. Elastin as a biomaterial for tissue engineering. Biomaterials 28, 4378–4398 (2007).
Miyamoto, K. et al. Creation of cross-linked electrospun isotypic-elastin fibers controlled cell-differentiation with new cross-linker. Int. J. Biol. Macromol. 45, 33–41 (2009).
Han, J. et al. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 12, 399–408 (2011).
Jiang, Y. et al. Spider-capture-silk mimicking fibers with high-performance fog collection derived from superhydrophilicity and volume-swelling of gelatin knots. Collagen Leather 5, 1–10 (2023).
Sarrami, P., Karbasi, S., Farahbakhsh, Z., Bigham, A. & Rafienia, M. Fabrication and characterization of novel polyhydroxybutyrate-keratin/nanohydroxyapatite electrospun fibers for bone tissue engineering applications. Int. J. Biol. Macromol. 220, 1368–1389 (2022). E-spun keratin fibers were used in combination with nanohydroxyapatite improving the mechanical properties of the fibers as well as being a suitable scaffold for bone tissue engineering.
Sheng, A. Textiles from the silk road. Exped. Mag. 52, 3 (2010).
Götzinger, A. Turnschuhe aus Spinnenseide. Nachr. Chem. 65, 789–790 (2017).
Cumbers, J. New this ski season: a jacket brewed like spider’s silk. Forbes (2019).
Block, I. Lab-grown spider silk used for Adidas x Stella McCartney biodegradable dress. Dezeen (2019).
Bain, M. Synthetic spider silk is finally appearing in products consumers can buy. Quartz (2019).
Xu, T. Biomanufactured materials are coming. Materially Better (2022).
Ye, C. et al. Design and fabrication of silk templated electronic yarns and applications in multifunctional textiles. Matter 1, 1411–1425 (2019).
Dasgupta, A. et al. Comprehensive collagen crosslinking comparison of microfluidic wet-extruded microfibers for bioactive surgical suture development. Acta Biomater. 128, 186–200 (2021). Dasgupta et al. produced microfluidic-spun collagen fibers that are knotable and utilized them as biodegradable sutures.
Altman, G. H. et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 23, 4131–4141 (2002).
Pagán, A., Aznar-Cervantes, S. D., Pérez-Rigueiro, J., Meseguer-Olmo, L. & Cenis, J. L. Potential use of silkworm gut fiber braids as scaffolds for tendon and ligament tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 107, 2209–2215 (2019). The authors used regenerated silkworm silk to produce braided fiber bundles which were used for ligament tissue engineering.
Kwon, S.-Y. et al. Silk and collagen scaffolds for tendon reconstruction. Proc. Inst. Mech. Eng. Part H. J. Eng. Med. 228, 388–396 (2014).
Li, X. & Snedeker, J. G. Wired silk architectures provide a biomimetic ACL tissue engineering scaffold. J. Mech. Behav. Biomed. Mater. 22, 30–40 (2013).
Fan, H., Liu, H., Wong, E. J. W., Toh, S. L. & Goh, J. C. H. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials 29, 3324–3337 (2008).
Liu, M. et al. Biomimicking antibacterial opto-electro sensing sutures made of regenerated silk proteins. Adv. Mater. 33, e2004733 (2021).
Knapczyk-Korczak, J. & Stachewicz, U. Biomimicking spider webs for effective fog water harvesting with electrospun polymer fibers. Nanoscale 13, 16034–16051 (2021).
Chen, W. & Guo, Z. Hierarchical fibers for water collection inspired by spider silk. Nanoscale 11, 15448–15463 (2019).
Venkatesan, H., Chen, J., Liu, H., Liu, W. & Hu, J. A spider‐capture‐silk‐like fiber with extremely high‐volume directional water collection. Adv. Funct. Mater. 30, 2002437 (2020).
Müller-Herrmann, S. & Scheibel, T. Enzymatic degradation of films, particles, and nonwoven meshes made of a recombinant spider silk protein. ACS Biomater. Sci. Eng. 1, 247–259 (2015).
Yamauchi, C., Okazaki, W., Yoshida, T. & Karasawa, A. Enzymatic degradation of keratin films and keratin fibers prepared from human hair. Biol. Pharm. Bull. 31, 994–997 (2008).
Min, K., Kim, S. & Kim, S. Silk protein nanofibers for highly efficient, eco-friendly, optically translucent, and multifunctional air filters. Sci. Rep. 8, 9598 (2018).
Lang, G., Jokisch, S. & Scheibel, T. Air filter devices including nonwoven meshes of electrospun recombinant spider silk proteins. JoVE. https://doi.org/10.3791/50492 (2013).
Müller, F., Zainuddin, S. & Scheibel, T. Roll-to-roll production of spider silk nanofiber nonwoven meshes using centrifugal electrospinning for filtration applications. Molecules 25, 5540 (2020).
Figoli, A. et al. Fabrication of electrospun keratin nanofiber membranes for air and water treatment. Polym. Eng. Sci. 59, 1472–1478 (2019).
Garrido, I. et al. Photocatalytic performance of electrospun silk fibroin/ZnO mats to remove pesticide residues from water under natural sunlight. Catalysts 10, 110 (2020). Here, the authors demonstrate the application of biopolymer-based composite materials as an environmentally friendly alternative for treating wastewater.
Jin, X. et al. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr(VI). Sci. Total Environ. 710, 135546 (2020).
Zainuddin, S. & Scheibel, T. Continuous yarn electrospinning. Textiles 2, 124–141 (2022).
Zhao, L., Chen, D., Yao, Q. & Li, M. Studies on the use of recombinant spider silk protein/polyvinyl alcohol electrospinning membrane as wound dressing. Int. J. Nanomed. 12, 8103–8114 (2017).
Yazawa, K., Mizukami, S., Aoki, M. & Tamada, Y. Electrospinning of spider silk‐based nanofibers. Polym. Adv. Technol. 33, 2637–2644 (2022).
Aznar-Cervantes, S. et al. Electrospun silk fibroin/TiO2 mats. Preparation, characterization and efficiency for the photocatalytic solar treatment of pesticide polluted water. RSC Adv. 10, 1917–1924 (2020).
Wu, R., Bae, J., Jeon, H. & Kim, T. Spider-inspired regenerated silk fibroin fiber actuator via microfluidic spinning. Chem. Eng. J. 444, 136556 (2022).
Kinahan, M. E. et al. Tunable silk: using microfluidics to fabricate silk fibers with controllable properties. Biomacromolecules 12, 1504–1511 (2011).
Sun, M., Zhang, Y., Zhao, Y., Shao, H. & Hu, X. The structure–property relationships of artificial silk fabricated by dry-spinning process. J. Mater. Chem. 22, 18372 (2012).
Ng, P. F. et al. Wet spinning of silk fibroin-based core–sheath fibers. ACS Biomater. Sci. Eng. https://doi.org/10.1021/acsbiomaterials.9b00275 (2019).
Wei, L. et al. Extraction of keratin from pig nails and electrospinning of keratin/Nylon6 nanofibers for copper (II) adsorption. Polymers 15, 467 (2023).
Kammiovirta, K. et al. Keratin-reinforced cellulose filaments from ionic liquid solutions. RSC Adv. 6, 88797–88806 (2016).
Dems, D. et al. Native collagen: electrospinning of pure, cross-linker-free, self-supported membrane. ACS Appl. Bio Mater. 3, 2948–2957 (2020).
Zhou, T. et al. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf. B Biointerfaces 143, 415–422 (2016).
Bi, C. et al. Effect of extraction methods on the preparation of electrospun/electrosprayed microstructures of tilapia skin collagen. J. Biosci. Bioeng. 128, 234–240 (2019).
Tonndorf, R., Aibibu, D. & Cherif, C. Collagen multifilament spinning. Mater. Sci. Eng. C Mater. Biol. Appl. 106, 110105 (2020).
Gwak, H. J. et. al. Preparation of fish-skin-based conjugated collagen fibers and nonwovens for wound dressings. (2022).
Spiaggia, G. et al. Aligned and oriented collagen nanocomposite fibers as substrates to activate fibroblasts. ACS Appl. Bio Mater. 4, 8316–8324 (2021).
Yaari, A., Schilt, Y., Tamburu, C., Raviv, U. & Shoseyov, O. Wet spinning and drawing of human recombinant collagen. ACS Biomater. Sci. Eng. 2, 349–360 (2016).
Yeo, G. C. et al. Fabricated elastin. Adv. Healthc. Mater. 4, 2530–2556 (2015).
Hiramatsu, H. et al. Microfluidics-based wet spinning of protein microfibers as solid scaffolds for 3D cell cultivation. In 2016 International Symposium on Micro-NanoMechatronics and Human Science (MHS) 1–4 (IEEE, 2016).
Nordsletten, D. et al. A viscoelastic model for human myocardium. Acta Biomater. 135, 441–457 (2021).
Min, B.-M. et al. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 25, 1289–1297 (2004).
Hsu, Y.-M., Chen, C.-N., Chiu, J.-J., Chang, S.-H. & Wang, Y.-J. The effects of fiber size on MG63 cells cultured with collagen based matrices. J. Biomed. Mater. Res. Part B Appl. Biomater. 91, 737–745 (2009).
Acknowledgements
Thomas Scheibel acknowledges financial support from the Office of Naval Research Global, Office of Naval Research, and the Air Force Office of Scientific Research, contract number N62909-20-1-2068. Support from the Elite Network of Bavaria is also acknowledged (Tim Schiller).
Author information
Authors and Affiliations
Contributions
Tim Schiller wrote the manuscript. Thomas Scheibel finalized the writing and supervised the project. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing financial interest(s): Thomas Scheibel is the founder and share-holder of AMSilk GmbH. Thomas Scheibel declares to have no competing financial interests or personal relationships that could have appeared to influence the work reported in the present paper. Tim Schiller declares no competing financial interests.
Peer review
Peer review information
Communications Materials thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: John Plummer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schiller, T., Scheibel, T. Bioinspired and biomimetic protein-based fibers and their applications. Commun Mater 5, 56 (2024). https://doi.org/10.1038/s43246-024-00488-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00488-2