Abstract
The evolving field of plasmonics has enabled the rise of engineered plasmonic nanomaterials to improve neural interface performance. Plasmonic nanostructures such as nanoparticles, if appropriately designed, can act as mediators to efficiently deliver light to target cells for less-invasive modulation with high spatial resolution than common electrical methods. Also, originating from either excitation of surface plasmons alone or in combination with thermoplasmonic effects, they can improve the performances of nanotools in neuroengineering. Here, we review plasmonic-based modalities and explore recent developments, advantages and limitations for minimally invasive neuromodulation, central nervous system disease diagnosis and therapy, and smart carrier-drug delivery toward the brain. The subject of the study stands at the interface of neuroscience and engineering. Thus, within the scope of this study, we provide background information about the nervous system and its underlying basic biology, types of neural interfaces, as well as the physics of surface plasmons and thermoplasmonic phenomena.
Similar content being viewed by others
Introduction
In 2007, the World Health Organization (WHO) reported that neurological disorders affect up to one billion people worldwide, across people of different age, sex, education, and income1. In the US, the most burdensome neurological disorders are stroke, Alzheimer’s disease and other dementias, and migraine, with an increasing number of affected people from 1997 to 2017, attributed to the aging US population and population growth2.
To address these disorders, we need to improve the understanding of neural information processing. This will allow us to enhance treatment of neurological disorders, and improve functional recovery in the central nervous system (CNS). Neuroscience relies on monitoring the electrical activities of neurons within functioning brains and has advanced through steady improvements in underlying observational tools.
Neurotechnologies capable of stimulating or recording specific populations of neurons have been initially employed as tools for basic scientific research to study how the brain works. However, the complexity of the nervous system requires tools with a high spatiotemporal resolution for probing targeted neurons.
In the realm of nanotools for neuromodulation, plasmonic materials such as gold and silver offer exciting prospects. They can significantly enhance light–matter interactions when the oscillation of the surface electrons is coupled with light at the resonance condition. At resonance, optical signals are significantly enhanced as the absorption is maximized. The amount of absorbed energy is relaxed nonradiatively, which results in the rapid release of heat energy to the surroundings. Photothermal effect as a result of the light-to-heat conversion process, also known as the thermoplasmonic effect, is highly localized near the nanosized plasmonic structures. Hence, plasmonic particles can be considered as energy sources capable of generating a controllable amount of localized heat3. This localized effect could be used in targeting specific cells with high spatiotemporal resolution in neuromodulation4 as well as in thermally-mediated drug delivery or therapy5.
With the rise of nanotechnology, the emerging field of engineered plasmonic nanostructures has improved the performance of neural interface tools. Neuroplasmonics, generally refers to the application of plasmonics in neuroengineering6, holds promising potential in both research and clinical applications. These include extracellular vesicle detection and characterization7,8,9, neuroimaging10,11, neuromodulation via optogenetics12 and label-free approaches13,14,15, treatment of neurological disorders16, relief of chronic neuropathic pain, and treatment of central nervous system injuries17.
Emerging technologies for sensing brain activity generally anticipate challenges for finding the best transition process from academic research labs to useful products for patients18,19. Plasmonic-based approaches and devices in particular require careful consideration of their technological limitations and constraints, including excessive tissue temperature rise, toxicity of plasmonic materials, and long-term durability20.
As a step in that challenging transition, we report progress on the current use of plasmonics to design strategies that improve the performance of neural interfaces. We discuss the advantages and limitations of neuroplasmonics for neuromodulation, CNS disease diagnosis and therapy, and drug delivery toward the brain. In “Control and readout of neurons”, approaches for monitoring neural activity are briefly reviewed with an emphasis on introducing photo-assisted strategies employing plasmonics and its derivative effects. Specific subsections of “ Electrical neural interfaces”, “Optical neural interfaces”, and “Hybrid neural interfaces” provide the relevant details. Within the subsection “Optical neural interfaces”, detailed discussions are specifically categorized within optogenetics (via photoupconversion), and nongenetic photo-assisted strategies (photothermal, photochemical, and photoacoustic). In “Surface Plasmon Resonance (SPR) and Thermoplasmonic Effects”, the physics behind localized surface plasmon resonances (LSPR) and associated thermal effects (Thermoplasmonics: Thermal Effects in Plasmonic Structures) are introduced and discussed. “Plasmonic Nanostructures and Nanotools for Neuroengineering” reviews the applications of plasmonics/thermoplasmonics in the development of engineered nanostructures and nanotools for neural engineering, including prevalent areas of nanoparticle-assisted neuromodulation approaches (Hybrid plasmonic nanoparticle-coated microelectrodes), on-fiber modalities (On-fiber plasmonic modalities), and on-chip modalities (On-chip plasmonic modalities). We also discuss the prospects for clinical applications (Prospects for clinical applications), specially within the categories of diagnosis and therapy, and smart carrier–drug delivery toward the brain. The last section, “Summary and outlook”, summarizes the review and discusses the outlook for the area of plasmonics for neuroengineering.
Control and readout of neurons
Neurons form highly complex circuits with vast cell variation based on morphological, biochemical, and physiological properties. In humans and other vertebrates, the nervous system can be divided into the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS includes the brain, which is separated from the rest of the body by the blood–brain barrier (BBB), and spinal cord nerves. The brain and the spinal cord nerves are safely contained within the skull and vertebral canal of the spine.
Neurons communicate with each other through axons and dendrites. Synapses, where two neurons communicate, are categorized into two types, supporting the main modalities of synaptic transmission: 1) chemical synapses and 2) electrical synapses. More details about the CNS and synaptic communications can be found in Supplementary Note 1: Central Nervous System (CNS).
The nervous system is connected to the outside world through the engineered connecting tools that are called neural interfaces21. These tools can manipulate and read neuronal activities via electrical22, optical23,24, or hybrid modalities (combining electrical and optical)25. Other cross-modalities can also be found via combinations of the electrical/optical strategies with magnetic26, chemical27, acoustic28,29, or their derivative strategies.
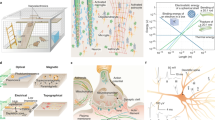
Plasmonic materials as stimulus-responsive mediators have played a significant role to improve the performance of interfaces in many ways. With light as a stimulus, photo-assisted plasmonic-based strategies, via labeled or label-free approaches, can be realized as photothermal (Fig. 1a), photochemical (Fig. 1b), photoacoustic (Fig. 1c), and photoupconversion (Fig. 1d) strategies. Also, the stimulus can be an alternating magnetic field, for magnetic nanoparticles (MNPs) as the mediators, based on which magnetothermal and magnetomechanical neuromodulation strategies can be realized. These MNP-based strategies are introduced within hybrid neural interfaces. In the following, we review various neural interfaces and how these strategies can improve their performances.
Application of plasmonic nanomaterials for manipulation of neurons including labeled and label-free neuromodulation through triggering ion channels of various types. The common strategies can be classified into four groups. a Photothermal strategy with two process variants: activating temperature-sensitive ion channels or thermo-transient receptor potentials (thermo-TRPs) and photothermal-driven optocapacitive strategy based on induced capacitive current activating voltage-gated channels65. For photothermal therapy, photomagnetic-driven photothermal conversion process can be used for design of drug-carriers to promote both cellular uptake and crossing blood–brain barrier (BBB)67,68,69, 137, 142 (* with a potential for triggering thermo-sensitive ion channels due to magnetoplasmonic effect85). b Photochemical strategy via opto-uncaging inducing rapid thermal expansion (or collapse) of polymeric cage73, 98, and nanomechanical forces that lead to chemical neuromodulation45, 75. Additionally, silicon nanowires could elicit AP via atomic gold-enhanced photoelectrochemical process76. c Photoacoustic strategy inducing acoustic waves and triggering mechanosensitive ion channels46. d Photoupconversion strategy using upconversion nanoparticles (UCNPs) for optogenetics under low-energy and deeply penetrative NIR light exposure that converts into high-energy short wavelengths (VIS)143 for activating opsins. The low efficiency of the upconversion process can be enhanced by combining UCNPs with designed plasmonic materials51,52,53.
Electrical neural interfaces
Electrical neural interfaces directly connect the nervous system to a peripheral device. Based on the principles of electrical stimulation of neural tissues30, 31, neural interfaces have been mainly implemented in the form of microelectrodes and nanoelectrodes (for both stimulation and recording) with steady progress toward better performance32.
To detect the spiking activity of individual neurons in the brain, electrical interfaces are typically used to make a connection between the electrolyte solution in the brain with metal or organic electrodes via associated electronics to convert the submillivolt voltages into electronic signals. A typical electrical interface consists of an implanted device, integrated circuits and mechanisms to transmit signals from and to the implant, and specialized algorithms to process these signals. The devices are capable of applying direct current to the cell as well as reading its response with the help of an amplifier to magnify the signals for further data processing. To prevent tissue damage, a stimulating electrode delivers current on the order of microamperes with a maximum to ~1 mA, although this value is empirically derived.
The primary strength of the electrophysiological approach is direct connection to neurons without the need for a membrane potential reporter. The combination of time resolution and sensitivity with high signal-to-noise ratio (SNR) allows the temporal pattern of neuronal signals to be precisely determined over multiple orders of magnitude. Direct electrical stimulation and recording of neurons by implanted micro- or thin-film electrodes in intact brain tissue provide many insights into the function of neural circuits. A connection, however, requires direct physical contact to the neurons, which can be a weak point for the electrophysiology strategy.
Among the shortcomings of electrophysiology are low spatial precision (particularly at a single-cell level), low selectivity due to electrical current leakage to neighboring tissues causing simultaneous stimulation of multiple cell types (see Fig. 2a), weak mechanical stability of the devices and invasiveness of the implantable electrodes, which may induce an injury with a subsequent inflammatory response, and artifacts that complicate neural activity recordings33. Some of the challenges are addressed with technological progress, for instance by engineering extremely small electrodes (7 μm in diameter) or hybrid ultraflexible electrodes22,32.
a Electrical stimulation that affects all cell types vs. optical stimulation via genetic targeting of (channelrhodopsin (ChR2) or halorhodopsin (NpHR))-optogenetic-constructs which is a cell-specific neuromodulation. b Optogenetic tool families47,144 allowing induction of action potentials, silencing of neurons, or modulation of intracellular signaling cascades (Panel reprinted with permission from ref. 34). c Classification of opsin genes (Reprinted (adapted) with permission from ref. 48), and d Using minimally invasive NIR light (780 nm) and gold nanorods (AuNRs) to activate channel thermo-TRPs (photoactivation of TRPV1-overexpressing HEK293T cells). Schematic representation of the localized photothermal heating of a TRPV1-expressing plasma membrane bearing plasma membrane-targeted gold nanorods (pm-AuNRs) (Reprinted with permission from ref. 4).
Electrophysiology remains a prevailing strategy for many applications, such as probing speedy interactions between neurons or brain areas and for simultaneous recording of activity across multiple neural circuits.
Optical neural interfaces
Optical modulation (monitoring or stimulating)34 of neurons avoids some of the challenges imposed by electrical strategies.
The wavelength of light can be specifically set for passing through anatomical barriers to access neurons. With the exception of the photoreceptors in the eye, most neurons are not generally activated by exposure to light. Light has the capacity to precisely affect only particular neurons, for example, those that are transfected with opsins, a group of light-sensitive membrane proteins (Fig. 2a–c)35, 36. Additionally, light can reduce the constraint level of size and multiplexing in electrical neural probes to create ultraminiature multichannel brain sensors37.
Optical measurement of neural activities is indirect, which means it requires reporters to translate membrane potentials to optical signals, which in turn allows monitoring of previously inaccessible variables with spatial resolution and limited invasiveness. Optical neural interfaces include the methods for both control and readout of the signals. Without the need for gene modification, the methods are known as intrinsic.
If prior genetic coding is used, the methods are categorized as extrinsic. Based on the physiological parameters in the brain (blood cell velocity, level of cerebral blood oxygenation, cellular volume, and membrane potential), in vivo measurement of brain intrinsic optical signals can be performed via four main methods: 1) laser doppler flowmetry, 2) near-infrared (NIR) spectroscopy38,39, 3) functional optical coherence tomography40, and 4) surface plasmon resonance (SPR)41,42,43. Extrinsic optical signals, on the other hand, are monitored by genetic or chemical changes in neurons using fluorescence signals with the use of commonly used probes of calcium indicators or voltage-sensitive fluorescent proteins44.
With a particular focus on plasmonic effects, in the following, the commonly known optical interfaces are reviewed via the two main categories of: i) optogenetics via photoupconversion strategy, and ii) nongenetic photo-assisted approaches based on photothermal, photochemical45, and photoacoustic46 strategies (see Fig. 1).
Optogenetics
Commonly used electrical stimulation can effectively modulate neural activity; however, single-cell type modulation is limited due to the heterogeneity of brain tissue. Additionally, unwanted signals from cells other than the targeted one are induced due to the leakage of the current to neighboring cells. Optogenetics as labeled optical strategy can overcome these shortcomings. Optical modalities based on fluorescent reporters to monitor and control the function of groups of cells with light, often in the intact animal, rely on the introduction of exogenous light-responsive biological compounds, ion channels, or ion pumps47 (see Fig. 2b, c).
The tools operating by optogenetic constructs provide many advantages, including the allowance of cell-type-specific excitation and inhibition of neurons and modulation of intracellular signaling cascades34. An important constraint of optogenetics is the limited tissue penetration (~1 mm) of the operational wavelength of opsins, which is in the visible range, and no NIR-activated opsin has yet been developed. Also, implanted optical fibers which are the typical light delivery tools to deeper regions impose possible risk of infection, and tissue damage, as well as inconveniences associated with the wired fibers such as artifacts, and unstable signals due to displacement of the fiber48.
In the absence of NIR-activated opsins, upconversion nanoparticles (UCNPs) can be used as light-emitting transducers which were first demonstrated for nanoparticle-mediated optogenetics in 201149. By utilizing low-energy NIR illumination, UCNPs emit high-energy light to facilitate the absorption of different opsins within their operational wavelength. UCNP-mediated optogenetics has gained attention due to its advantages, including access to deeper regions via larger tissue penetration, minimized photodamage, and the provision of remote and less invasive stimulation. They offer high spatiotemporal resolution enabling targeted manipulation of cellular activities in deep tissues and living animals within optogenetic strategies50.
UCNPs are often composed of an inorganic photostable host matrix, a sensitizer, and an activator. The photoupconversion process relies on the sensitizer to absorb energy from the incident light source and then transfer it to the activators, leading to the emission of higher-energy photons. Optimizing chemical composition, size, morphology, structure, and surface modification enhance the low efficiency of photoupconversion. Improvement of upconversion efficiency of UCNPs is essential for successful biophotonic applications, which remains challenging due to conflicting demands.
For instance, to improve their limited brightness, small particles are highly desirable which exhibit high brightness under low excitation power density. However, the efficiency of upconversion luminescence depends on the power density of the excitation light, which should not exceed 4 W. cm−2 to ensure the safety of tissues and cells. On the other hand, larger particles improve photoupconversion efficiency. However, to completely excrete particles post-examination in diagnostics applications, particles of around 20 nm in size are favorable which can be eliminated through the lymphatic system. To reduce thermal damage from light exposure, one needs to enhance the photoupconversion efficiency of UCNPs or shift their excitation wavelength to the NIR spectrum, where water absorption is low51.
An effective method for enhancing the luminescence properties of UCNPs is to integrate them with plasmonic materials of various structures, including continuous metallic films, nanoparticles, or nanostructured metals12. Utilizing plasmon resonances, it becomes possible to increase both the intensity of incident electromagnetic fields and the rates at which radiative emission occurs (see Fig. 1d).
The luminescence enhancement strategy relies on the nanostructure’s geometry and dielectric properties of the surrounding medium. Various geometries like nanowires, nanorods, hole arrays, and pillar arrays have been employed. To achieve optimal enhancement of upconversion luminescence, precise adjustment of metal particle size and geometry is important to optimize the spectral overlap between the LSPR and the absorption or emission of the UCNPs52. For instance, using 20 nm discontinuous gold islands instead of a continuous gold film enhanced the luminescence by up to 12 times. Similarly, decorating UCNPs with 90 nm gold nanorods and applying 2 W NIR power yielded a remarkable 27-fold enhancement in the 800 nm emission. In addition to gold, silver nanoparticles have also demonstrated the ability to promote upconversion luminescence through plasmonic effects53.
Limited in vivo animal studies54 and in vitro studies suggest that opsin activation efficiency by NIR stimulation via UCNP-mediated optogenetics is relatively comparable to traditional visible light optogenetics, although typically the latter may require less laser power. These findings demonstrate the potential of NIR activation of photoreceptor ChRs using photoupconverted light emission from UCNPs. However, the challenges for clinical translational research yet to be addressed including improvement of emission efficiency with low-power NIR, cell-type-specific targeting, and long term biocompatibility.
Nongenetic photo-assisted strategies
Approaches that require no prior genetic modifications are label-free strategies alternative to optogenetics55,56. Nongenetic modulation is relatively simple and fast compared to optogenetics, as there is no waiting time, days or weeks, for the virus transfection period. Hence, stimulation and recording can be performed immediately after the insertion of the tools. These strategies can be classified into four groups as follows.
Photothermal
Direct infrared neural stimulation (INS) is one important subset of photothermal and label-free neuromodulation strategy.
The use of low-level pulsed infrared light in neural activation in vivo to evoke compound nerve and muscle potentials in mammalian peripheral nerves has been a breakthrough in the field57. Infrared light (~780–2100 nm) can be used for the noninvasive and direct modulation (stimulation or inhibition) of neurons without genetic or chemical premodification58. However, in the INS approach, the optimal condition pertaining to safety and efficacy should be found which is the interplay between various parameters, such as wavelength, radiant exposure, and optical spot size59.
To demonstrate the mechanism behind INS, four biophysical hypotheses based on the electromagnetic field of light, photoelectrochemical, photomechanical, and photothermal properties were examined60. The first three were found to be null, but the photothermal mechanism explained the thermal nature of the direct INS, i.e., triggering the thermosensitive ion channels or thermo-transient receptor potentials (thermo-TRPs) (Fig. 1a). Moreover, the finding that the temporally and spatially mediated temperature gradient at the axon level accounted for the direct or indirect activation of transmembrane ion channels and not the absolute value of the temperature rise, provided a key understanding of the stimulation mechanism.
To date six thermo-TRPs that are sensitive to temperature, from painful heat, moderate warmth to cold and painful cold have been characterized61. More information about the noninvasive mechanisms for manipulation of various types of ion channels, including thermosensitive and mechanosensitive channels, are provided in ref. 48.
Water is an important component in infrared neuromodulation. For certain ranges of wavelengths in infrared wavelength ranges where water is highly absorptive, water enhances the heat generation at the target. However, over near-infrared (NIR) ranges, water has low absorption and scattering yielding a higher tissue penetration depth. For instance, compared to the light of the visible spectrum, interrogation by light of near-infrared (NIR-I, ~650–950 nm and NIR-II, 900–1700 nm) of biological tissue composed of several substances has a lower degree of light attenuation and higher tissue penetration.
Light in the NIR-I range propagates relatively deeply (a few cm) into biological tissue, as it is only weakly absorbed by water, hemoglobin, collagen and proteins; hence, it has long been used for fNIRS and fNIRI62,63. Additionally, NIR-II is of special interest because it is able to penetrate deeper into soft tissue with reduced scattering and is less autofluorescent. This window also takes advantage of the water transparency window, a wavelength range in which water absorption is negligible (800–1400 nm) compared to other substances with low concentrations, although there are several wavelengths with absorption peaks.
Apart from directly triggering thermo-TRPs, a rapid change in the optocapacitance of the membrane can also induce action potentials indirectly. Optocapacitive neuromodulation relies on light-to-heat conversion processing by the membrane via an appropriate energy transducer such as plasmonic nanoparticles (PNPs). The change in the capacitance and dielectric characteristics of the cell membrane due to a rapid and transient temperature increase upon light irradiation induces a capacitive current across the membrane. This in turn, leads to depolarization of an electrogenic cell such as the neuron64 (see Fig. 1a).
Neuromodulation via the optocapacitive mechanism requires a high rate of temperature change in the membrane, which is related to the amount of energy absorbed by the membrane. Optocapacitive mechanism is nonspecific due to tissue light scattering, which limits light focusing. Further, the abundance of water in biological systems can limit the spatial specificity of optocapacitive neuromodulation with lasers of water-absorbing wavelengths, thus requiring appropriate transducers.
To efficiently transfer the light energy to the membrane PNPs are employed based on which light-based thermally-mediated modulation strategies can be designed. One successful example of neuromodulation using PNPs via thermoplasmonic effects is the design of surface-engineered plasma membrane-targeted gold nanorods (pm-AuNRs) that exhibited high-efficiency cell binding without cytotoxicity. The pm-AuNRs could induce action potentials without the need for prior genetic engineering of the target cells. The thermosensitive TRPV1 cation channel in single neuronal cells was activated via a nanophotothermal effect that generated local heat upon illumination with NIR4 (Fig. 2d).
The light in the visible wavelength range where water has minimum absorption can also be used employing PNPs. To evoke action potentials, particles are conjugated to the proteins providing stable photothermal performance as well as increasing the efficiency of the light-to-heat conversion processes. For instance, using visible light (~532 nm), neurons were stimulated by conjugating gold nanoparticles (AuNPs) of 20 nm to antibody molecules. As the AuNPs were placed close to the neuronal membrane, diffusion of the NPs away from the neuron could be avoided. The excitation of the neurons was due to heat-induced changes in membrane capacitance and did not involve temperature sensitivity of any membrane proteins65. This approach offers an alternative path to optogenetics in fundamental research as well as in human therapeutics based on photostimulation, for instance, in patients who suffer from degenerative photoreceptor diseases such as age-related macular degeneration or retinitis pigmentosa.
Photothermal effect can also be induced using magnetoplasmonic particles66, or light-responsive magnetic particles which exhibit plasmonic effects67 (Fig. 1a). Photomagnetic effect can assist design of drug-carriers toward brain, as well as promoting the celluar uptake of nanomedicines68,69.
Photochemical
Gold’s tunable optical properties, including resonant wavelengths and biodegradability as composites70, make it suitable for smart carriers of bioactive molecules. It enables remote targeting and controlled release of cargoes to target cancer cells71, or precise ejection of neuromodulators to target neurons with sub-second precision (~0.1 ms)72.
Photochemical neuromodulation via plasmonic effects can be achieved through two mechanisms: uncaging bioactive molecules designed to trigger a cellular response, or direct depolarization of cells through atomic gold-induced photoelectrochemical currents. Uncaging of bioactive substances occurs either through the photothermal effect or the photomechanical effect (see Fig. 1b).
In uncaging based on the photothermal effect, a temperature-responsive polymer, coated on gold nanocages, undergoes collapse upon exposure to light matching the nanocages’ absorption peak70. This light-to-heat conversion process causes a local temperature increase, resulting in the collapse of the polymer chains and the subsequent release of the encapsulated substances. When the laser is turned off, the heating process ceases, and the polymer returns to its original conformation, closing the nanocage pores and stopping the release. The release dosage can be regulated by adjusting the power density and irradiation time. This approach is suitable for in vivo studies due to the high transparency of soft tissue in the near-infrared (NIR) region72.
Similarly, photo-sensitive microgels have been used for the controlled release of neurotransmitters, such as glutamate. NIR-triggered delivery of glutamate in the rat auditory cortex demonstrated synchronized spiking activity, enabling remote control of brain activity without genetic modifications73.
NIR-stimulated remote photorelease of biomolecules in deep brain tissue is hindered by the toxicity of high doses of surface light energy required for energy preservation at depth. Plasmonic materials offer a solution by serving as nanotransducers, enabling rapid and localized release of biological compounds for investigating cell signaling processes, including neurotransmission45.
In photothermal uncaging, substances are released through the accumulation of sufficient heat by plasmonic particles. However, this heat accumulation can cause tissue damage and irreversible membrane rupture, leading to the release of the payload. Additionally, the slow heat-induced uncaging process can also result in protein damage.
Uncaging based on the photomechanical effect could address some of the limitations. It relies on the generation of plasmonic nanobubbles or cavities through clusters of gold nanoparticles upon exposure to a near-infrared laser pulse. These nanobubbles mechanically rupture the host cancer cell or disrupt the liposome and endosome, leading to the release of the drug into the cytoplasm74.
For neuromodulation, gold cages have been employed to design light-stimulated, mechano-responsive encapsulated compounds. These cargoes could access deep brain regions in rodents (up to 4 mm) using significantly lower laser power, reduced by 40 times compared to traditional gold-coated liposomes75.
Lastly, atomic gold-enhanced photoelectrochemical currents demonstrated the potential to locally modulate target neurons. This was exemplified by optically modulating the excitability of rat dorsal root ganglion neurons through light stimulation of coaxial p-type/intrinsic/n-type silicon nanowires. The modulation was achieved by inducing a cathodic process at the n-shell, resulting in membrane depolarization. The photothermal effect had a minimal contribution, indicating that it was not the primary mechanism. The depolarization mechanism in this case differs from the photo-driven optocapacitive mechanism, which relies on a light-to-heat conversion process and requires a rapid, high, and transient temperature change. Instead, it was hypothesized that the presence of atomic gold on Si nanowire surfaces lowered the kinetic barrier for photoelectrochemical current, leading to eliciting action potentials76. However, plasmon-induced resonant cavities were observed in Si nanowires, revealing two types of resonant modes: discrete modes along the nanowire length as small as 30 nm, and localized waves along the nanowire diameter of every size77. Also, the nanowire showed intensity modulation similar to longitudinal wave quantization, and the wavelength of longitudinal plasmons compressed towards the nanowire tips.
Photoacoustic
The photoacoustic mechanism primarily triggers mechanosensitive ion channels via nanomechanical forces such as pressure waves and nanoscale cavitation which are generated by acoustic waves. In photoacoustic modulation, light energy absorption raises the particle’s temperature. An increase in milli-Kelvin range is sufficient to generate pressure waves causing thermoelastic expansion. The waves propagate as sound waves at the speed of sound. The nanomechanical forces generated by these acoustic waves primarily activate mechanosensitive ion channels (refer to Fig. 1c). For photoacoustic mechanism to dominate neuromodulation, stress confinement is required. For instance, a laser pulse duration shorter than 67 ps for nanoparticles smaller than 100 nm ensures efficient pressure wave generation, while thermal confinement requires a laser excitation pulse width longer than the thermal diffusion time constant29. This sets the photoacoustic mechanism apart from the photo-assisted optocapacitive method, which requires a rapid and significant temperature gradient.
Plasmonic materials can be engineered as photoacoustic transducers to enhance the generation of acoustic waves at resonance78,79. Optoacoustic imaging, based on the detection of ultrasound waves generated by light-matter interaction, has significantly advanced neuroimaging and understanding of the brain. By quantifying different contrast agents, it has bridged the gap between neuronal and neural network theories. Photoacoustic imaging, specifically, detects ultrasound waves produced by endogenous tissue chromophores or exogenous reporters upon exposure to short-pulsed lasers.
Gold nanoparticles have been used as contrast agents for imaging, but recent advancements include semiconducting polymer-based particles known as photoacoustic nanotransducers for neuromodulation. Surface-modified photoacoustic nanotransducers selectively bind to neurons and absorb nanosecond-pulsed lasers in the NIR-II window (1030 nm), generating localized acoustic waves. Successful in vitro and in vivo experiments using photoacoustic nanotransducers injected into the mouse brain cortex demonstrated submillimeter spatial resolution and minimal heat deposition29.
NIR-interrogation of plasmonic nanoparticles enables localized and efficient rapid thermal expansion, leading to the generation of acoustic waves through the photoacoustic effect. These acoustic waves exert nanomechanical forces on mechanosensitive channels, allowing for selective activation or inhibition of specific neurons or neural circuits by controlling the intensity and timing of laser pulses80.
Ekeroth et al. developed a radioplasmonic system that combined plasmonic and acoustic resonances46. The plasmonic resonance facilitated electromagnetic absorption and heat generation, while the acoustic resonance enabled resonant thermal expansion. The synergy between these two resonances resulted in efficient conversion of energy into acoustic vibrations or light-to-sound conversion. This approach allowed for total-body thermo-acoustic imaging with single-cell resolution.
Hybrid neural interfaces
One strategy to improve the overall performance of neural interfaces while avoiding some of the challenges in all-electrical or all-optical strategies is the development of hybrid strategies that mainly combine optical and electrophysiological modalities, although other cross-modal strategies such as magnetothermal81, magnetomechanical82, magnetoplasmonic83, and sonoelectrical84 also exist.
Magnetoplasmonic modulation occurs when both magneto-optical effects and surface plasmon polaritons (SPPs) are stimulated in structures that possess both plasmonic and magneto-optical capabilities85. This combination leads to the generation of localized light and amplification of magneto-optical signals in appropriately arranged nanostructures. Moreover, the enhanced electromagnetic field resulting from SPP excitation in plasmonic nanomaterials extends into an adjacent magnetic layer, thereby increasing its activity in response to magnetic fields86.
A hybrid electroplasmonic platform capable of spatially and temporally precise neurostimulation was designed to resolve ineffective spatial resolution in the electrical approach as well as collateral heating of neighboring tissue in INS. A reliable and repeatable single-cell hybrid activation with a subthreshold-level short-duration electrical current was reported through an empirical cellular study of the membrane action potential. No damage was observed compared with optical stimulation alone14.
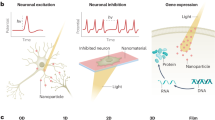
Other hybrid platforms include those based on magnetic field-responsive transducers. These platforms utilize magnetic nanoparticles (MNPs) for neuromodulation through the generation of heat or force in response to an alternating magnetic field (Fig. 3a). Magnetothermal neuromodulation, driven by an alternating magnetic field, is a label-free and thermally-driven approach. Deep-penetrating MNPs enable minimally invasive and wireless deep brain stimulation. This mechanism involves heat generation by MNPs, resulting in a transient increase in the local temperature of the membrane. Heat-sensitive TRPV1 channels can detect this temperature change, leading to the induction of action potentials26 (Fig. 3b).
a Magnetic manipulation using heat or force that are generated by the presence of magnetic materials in an alternating magnetic field (Reprinted (adapted) with permission from ref. 48). b Schematics of wireless thermally-mediated neuroactivation by a magnetic field to generate heat using magnetic nanoparticles (MNPs) that activate channel thermo-TRPs (Reprinted with permission from ref. 26). c Working principles of magnetothermal and magnetochemical strategies which are nongenetic and non-optical strategies.
Another label-free and non-thermal approach to neuromodulation is the magnetomechanical strategy. In this approach, the presence of MNPs in an alternating magnetic field produces a force that activates mechanosensitive ion channels. The working principles of magnetothermal and magnetomechanical strategies are demonstrated in Fig. 3c).
The use of magnetic-driven techniques offers benefits such as remote neuromodulation and a deeper understanding of the brain’s microcircuitry in both normal and disease states. However, when considering clinical applications, a primary concern is safety due to potential heating, the presence of magnetic particles in the brain, and the use of viral tools. Additionally, there is uncertainty whether the nanoparticles respond to other magnetic fields encountered in daily life87.
The key element in neuroplasmonics is the ability of plasmonic materials to generate a controllable amount of heat or force in response to light as an external stimulus. In order to understand the parameters that contribute to highly efficient and less invasive photo-assisted neuromodulation, the background physics of surface plasmon excitation and resulting heat generation are reviewed in the following.
Surface plasmon resonance (SPR) and thermoplasmonic effects
In 1902, Wood was the first who reported observation of the surface oscillations of free electrons which were optically excited88, and Liedberg demonstrated the first SPR-based biosensor in 198389. Since then, SPR has been widely applied in biosensing, enabling the detection of single-molecular concentrations with significant practical implications.
SPPs are electromagnetic waves composed of charges that propagate along the interface of a metal and a dielectric material. The optical excitation of SPPs is known as SPR. Supplementary Note 2: Physics of Surface Plasmons provides further explanations on the ways to excite SPRs, including prism-coupled and fiber-optic coupling, with applications for biosensing.
When SPRs are excited, they become localized in PNPs, resulting in the generation of heat in the vicinity of these particles. The amount of produced heat energy can be controlled through appropriate selection of materials, the geometry of the nanoparticles, and their design. In this context, we will provide a brief overview of the localized SPR phenomenon and the underlying mechanism responsible for heat generation by plasmonic materials.
Localized surface plasmon resonance (LSPR)
Metal NPs, such as gold and silver, exhibit strong optical extinction and can generate LSPRs in the visible and near-infrared (NIR) wavelength range when their diameter (d) is smaller than the incident light wavelength (λ)90 (see Fig. 4a, b).
a Enhanced local EM field around a plasmonic nanoparticle (NP) under resonance conditions. The collective electron oscillations are in phase with the incident light frequency that yields a sharp spectral response. b Absorption and scattering are dominant, respectively, for NPs smaller than 15 nm and larger than 15 nm (Reprinted with permission from145). c The light-to-heat conversion process of gold nanoparticles (AuNPs) under resonance conditions yields a temperature profile around AuNPs. The profile under femtosecond pulsed illumination has a steep gradient compared to that under continuous wave (CW) illumination (Reprinted with permission from ref. 95). d Illustration of LSPR photoexcitation, relaxation of photoexcited LSPR and release of thermal energy to the surrounding medium. The time scale of the energy exchanges is shown on a logarithmic time scale (Reprint permitted under Term of Use from ref. 96).
The resonant wavelength at which surface plasmon polaritons are excited depends on the refractive index (RI) of the surrounding medium. Changes in RI caused by modifications or molecule adsorption on the NP surface can be detected by measuring redshifts in the LSPR spectral position. This label-free approach is useful in biomedical applications such as intracellular drug delivery, where adjusting the resonance peak from visible to NIR region is necessary91. This tuning can be achieved by manipulating the morphology and composition of NPs with various structures like core–shells, nanorods, or nanoprisms92. Hence, it is possible to select an appropriate light wavelength for a specific neuroengineering application considering tissue safety.
An example of using the SPR phenomenon to directly record neural activities is the development of an all-optical refractive index (RI)-based modality that could simultaneously stimulate and detect neural activity41. To explain the biophysical mechanism underlying this approach and establish a connection between optical signals and neural activity spikes, the stimulation process was modeled using three molecular-scale phenomena: i) Poisson-Boltzmann equations, analyzed numerically with the finite element method (FEM), ii) Drude-Lorentz electron model, analyzed numerically with Green’s method, and iii) Fresnel’s 3-layered model93. The theoretical model demonstrated strong agreement with previous experimental data obtained by Kim et al.41.
Thermoplasmonics: thermal effects in plasmonic structures
The large optical cross-section and nonradiative relaxation of absorbed light by PNPs generate significant localized heating energy. PNPs convert light energy to plasmonic photothermal (PPT) heat energy known as thermoplasmonic effect94, making them a stable in situ heat source for controllable and uniform thermal processing.
Under resonance conditions, highly energetic photoexcited electrons quickly release thermal energy to the surroundings. This creates a temperature profile strongly influenced by the medium properties, particle shape and composition, and characteristics of the light source (continuous or pulsed wave)95 (see Fig. 4c). Pulsed waves result in a steep temperature profile, advantageous for stimulating thermosensitive ion channels, as discussed earlier.
The light-to-heat conversion process occurs in three steps with different time scales: i) electron–electron collisions in ~10 fs ≤ τ ≤ 100 fs, ii) relaxation through the electron–phonon interaction (e–ph) within 100 fs ≤ τ ≤ 1 ps, and iii) transfer of thermal energy to the metal-medium interface with cooling of NP via phonon–phonon collisions (ph–ph) in the range of 10 ps ≤ τ ≤ 10 ns (Fig. 4d)96.
Given their heat-generating properties, PNPs have garnered significant interest in the field of thermoplasmonics, finding applications in diverse areas of science, such as nanomedicine (intracellular drug delivery both in general therapeutic and precision applications)97 based on photothermal effects98, cell biology, remotely controlled photothermal therapy99, neuroimaging and neuromodulation4,100. The heat dissipation of excited plasmonic nanoparticles in the medium also leads to a change in refractive index (RI), which can be utilized in designing RI-based biosensors.
Plasmonic nanostructures and nanotools for neuroengineering
Engineered nanostructures, such as nanoparticles (NPs) or nanofilms, have been extensively utilized to enhance neural interface performance. These nanostructures have great potential in various neuroengineering applications, including optical modulation and readout of neural activity for research purposes, as well as clinical applications such as diagnosis, therapy, stem cell differentiation enhancement, smart drug delivery, especially for crossing the BBB, and imaging97,101,102,103.
Plasmonics advancements are also expanding the capabilities of optical tweezers which enabled studies on neuronal growth, function, and communication across different scales. This allows label-free trapping and sensing of a wider range of molecules, and when combined with fiber optics or tweezers-in-tweezers technologies, they offer potential applications for deep brain optical trapping and precise molecule delivery to neurons104,105.
Materials capable of converting light to heat encompass various structures, such as metallic/semiconductor materials, carbon-based materials, organic polymers, and two-dimensional materials, with a wide range of applications106. Among these, inorganic NPs such as gold, silver, platinum, zinc, metal alloys, and heavily doped semiconductors107 hold particular interest due to their unique optical properties, including absorption and thermo-optical effects108.
AuNPs are the most well-studied plasmonic materials in bio-related applications since they show better biocompatibility, stability, and electroconductivity91. They have been employed in many biomedical applications, including tumor diagnosis and therapy109 and neuromodulation110.
There are many different kinds of plasmonic nanostructures and nanotools for neuroengineering. Here we will discuss three of the most prevalent: hybrid plasmonic nanoparticle-coated micro-electrodes, on-fiber plasmonic modalities, and on-chip plasmonic modalities, and their advantages and limitations. Prospects for clinical application including diagnosis and therapy, and smart carrier-drug delivery toward brain are also highlighted.
Hybrid plasmonic nanoparticle-coated microelectrodes
Plasmonic nanomaterials can be used to improve the spatial resolution and specificity of electrical neural interfaces. Proposing 3D plasmonic nanostructures on multielectrode arrays, Dipalo et al. reported successful long-term and stable recordings of both intracellular and extracellular electrical spiking activity in primary mammalian neurons and cardiac-derived HL-1 cells (see Fig. 5a–c)111. The efficacy of the proposed method was demonstrated with the recording of spontaneous and unperturbed electrical activity with a high SNR. The key point in this achievement was the combination of vertical nanoelectrodes structured on planar microelectrodes with plasmonic optoporation.
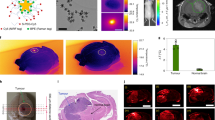
Schematic illustration of the plasmonic optoporation platform integrated with a hippocampal neuronal culture system: a SEM image of an electrode with plasmonic 3D nanoelectrodes. b SEM cross-section view of a neuronal process (blue) engulfing two 3D nanoelectrodes (yellow). c Representation of neurons on a microelectrode array (MEA) with 3D nanoelectrodes. The 3D nanoelectrode excited with a laser records intracellular activity, while the rest of the electrode catches extracellular signals. (Reprint permitted under Term of Use from ref. 111). Gold nanograin microelectrode: d SEM image of morphologies of Au grains on an electrode with a diameter of 10 μm. e Phase-contrast optical image of live neural networks cultured on an MEA. The inset shows a representative extracellular action potential detected from a nanograin microelectrode. f Stimulation and recordings of neuronal networks in vitro (Reprinted with permission from ref. 112). Electroplasmonic nanoantenna: g SEM image of cardiomyocyte cells cultured on an array of electroplasmonic nanoantennas (height of 45 nm, diameter of 90 nm). h Side and top views of near-field enhancement ∣E/E0∣2 along the pristine nanoantenna at 678.8 nm. Finite-difference time-domain (FDTD) simulations show that plasmonic excitations lead to strong confinement of the light within the 20-nm-thick electrochromic layer. i High SNRs (~60–220) are shown for the electroplasmonic nanoantenna even for low field values (2 × 10E2 to 8 × 10E2 V/cm). j Electron micrograph of human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (iCMs) (purple) cultured on an electroplasmonic nanoantenna array. k Differential scattering signal in response to electrogenic activity of a network of cardiomyocyte cells. A strong far-field signal allowing label-free and real-time optical detection of the electrogenic activity of iCMs is obtained from substrates with electroplasmonic nanoantennas (red curve). Control measurements are performed to verify the origin of the electro-optic signal. In the absence of electroplasmonic nanoantennas, no far-field signal is detected (blue curve) (Modified and Reprinted with permission under Term of Use from ref. 13). Hybrid nanoplasmonic microelectrode: l Schematic illustration. m SEM images of a microelectrode having a tip coated with ~20 nm diameter colloidal gold nanoparticles (AuNPs). LSPR absorption of AuNps is shown in the inset (Reprinted (adapted) with permission from ref. 14).
Nanogold grains could improve performance of a neuroelectrode: a single-cell-sized microelectrode was fabricated with a 500 nm-gold nanograin deposition at its center (shown in Fig. 5d–f). Performance testing using rat hippocampal neuronal culture showed lower device-tissue interface impedance (a 69-fold decrease) and higher electrical charge injection for stimulation (a tenfold increase) compared to a microelectrode with a flat gold layer. Additionally, a very low noise level (2.89 μV(RMS)) yielding a high SNR for low-amplitude action potentials (18.6–315 μV) was reported, suggesting the potential application of the construct for cell-based biosensors or clinical neural prosthetic devices112.
To precisely modulate neural activity using light of visible spectrum, a nanoelectrode made up of a glass micropipette coated by AuNPs of ~20 nm diameter was used for localized activation and inhibition of electrically excitable cells by a 532 nm green laser. The experimental in vitro studies served as proof of concept that wireless nanoelectrodes in combination with visible light can be used for precise temporal modulation of neural and cardiac cellular responses instead of electrical electrodes or infrared lasers113.
The lack of electro-optical translators for the efficient conversion of electrical activity into high photon-count optical signals was successfully overcome by Habib et al.13. A noninvasive electroplasmonic nanoantenna for extracellular, high SNR and real-time optical recording of electrophysiological signals from electrogenic cells (neurons and heart cells) (Fig. 5g–k) was developed. The electroplasmonic optical probe offered ultrasensitive and label-free detection of electrical signals at kilohertz frequencies. It was reported that the probe could be used in vivo via integration of plasmonic devices on flexible substrates and optical fibers.
Figure 5l, m shows the design of a hybrid electroplasmonic stimulation platform for spatially and temporally precise neural excitation. The visible light pulses were aimed at a gold nanoparticle-coated nanoelectrode placed alongside the neuron within a distance of 2 μm. The improved results of a single-cell hybrid activation were reliable and repeatable, without any damage as observed with pure optical stimulation. Thus, the hybrid neurostimulation method holds promise for creating personalized high-acuity neural modulation prosthetic devices. It could serve as a preferable alternative to conventional electrical stimulation technologies14.
Near-infrared stimulation (800 nm) combined with a gold nanorod-coated microelectrode was used to develop a closed-loop thermoplasmonic neural stimulation platform. The goal was to investigate whether using a photothermal inhibition mechanism in a closed-loop system enables a controllable neural spike rates. The thermoplasmonic-based feedback controller showed precise neuromodulation in vitro. The technology was suggested to be helpful in studying neuronal network dynamics and developing new neuromodulation techniques for clinical applications15.
On-fiber plasmonic modalities
Optical fibers are one the most commonly used light delivery tools, for instance, in neuroimaging or optogenetics. However, the merging characteristics of an optical fiber platform and thermoplasmonic effect can be a basis for developing light-triggered active lab-on-fiber probes114. With careful design of the plasmonic structure and the optical properties of the source and the fiber, a thermoplasmonic-assisted lab-on-fiber can be used as optrodes in biomedical applications.
For neuroengineering applications, careful analysis of the plasmonic fiber-based device is essential in order to control temperature rise in the target due to the thermoplasmonic effect, in order to supply the required heat for neuromodulation while avoiding excessive heating which could potentially damage the tissues. In terms of tissue safety analysis, a specific scenario involving the photothermal effect of the thermoplasmonic fiber optic design for in vivo neural stimulation was numerically examined. The study took into account the complexity of physical structure of the brain (Fig. 6a)115. Modeling the two actual experimental conditions for photothermal neuromodulation, acute or long-term, two cases were considered: exposing the brain to air and closing the brain from the outside after fiber implantation.
a Numerical investigation of the effects of photothermal stimulation on brain modulation by a thermoplasmonic optical fiber (Reprint permitted under Term of Use from ref. 115). b Illustration of a thermoplasmonic fiber tip for NIR in vitro localized photothermal neural stimulation on microelectrode array (MEA) chips. Direct inhibition of the network of neurons occurred as a result of localized heat generated by the gold nanorod (GNR)-coated fiber tip. Neurons far from the fiber tip remained unaffected. c Extinction spectrum and infrared thermographic image of the fiber delivering NIR light. The white arrow in the inset shows the localized temperature change at the tip (Reprinted with permission from ref. 117). d A schematic of the SPR system for neuromodulation and detection of the reflection intensity of the laser beam through a multichannel photodetector array (Reprinted with permission from ref. 41). e Distributed gold nanorods (GNRs) in the vicinity of the plasma membrane of nerve tissues absorb light energy at 980 nm. Pulsed infrared illumination photothermally heats GNRs, increasing the local temperature at the membrane and inhibiting neural activity. TEM image of a cross-sectional view of rat sciatic nerve after injecting GNRs. Compound nerve action potentials recorded from a rat sciatic nerve in vivo with and without gold nanorods, upon exposure energy of 0.641 J. cm−2 (Reprinted with permission from ref. 120). f Schematics of the inkjet-printed thermoplasmonic interface for patterned neuromodulation (Reprinted with permission from ref. 122). g Schematic showing the enhancement of the fluorescence spectra of voltage-sensitive dye on a layer-by-layer (LbL) substrate when neurons are on a GNR-coated nanoplasmonic substrate. The corresponding peak values of fluorescence of voltage-sensitive dye with respect to the different numbers of LbL are plotted (Reprinted with permission from ref. 124). h Simultaneous microelectrode recording and calcium imaging from cultured neurons with NIR light-sensitive GNRs during photothermal illumination (Reprinted with permission from ref. 100). i Scanning electron microscopy images (SEM) images of Au nanowire (AuNW) electrodes obtained with anodic aluminum oxide templates. (A) Top, and (B) cross-section view of an ordered network of parallel AuNWs of 1.2 μm in length and 160 nm in diameter standing over a flexible Au base. (C) SEM images from primary neural cell cultures at high cell seeding conditions. SEM images from cultures at low cell seeding conditions on top (D) and 45∘ tilted (E) views. Scale bars: 2 μm (A), 0.5 μm (A-inset), 5 μm (B) and 1 μm (B-inset), 200 μm (C, left), 100 μm (C, middle), 10 μm (C, right), 4 μm (D and E, left) and 400 nm (D and E, right) (Reprinted with permission from ref. 133).
Below a penetration depth of 1 mm, a noticeable disparity in temperature rise was observed between the two cases: the skull open to air and the skull covered. In the case of the brain open to air (e.g., acute experimental condition), it was found that the laser power needed to be significantly reduced when stimulating the brain’s surface. However, for the covered brain, a slight increase in laser power was required to achieve the same thermal stimulation effect. This difference was attributed to the limited areas of heat transfer and air convection. Furthermore, the study found that the daily variations in cerebral blood flow had a negligible impact on temperature changes.
Although fiber-optic photothermal neuromodulation is relatively more invasive than ultrasound-based neural stimulation, it offers advantages such as precise targeting within the submillimeter range and the ability to produce versatile stimulation effects, whether excitatory or inhibitory.
Considering the thermoplasmonic effect of resonant materials, Principe et al. designed a thermoplasmonics-assisted active lab-on-fiber probe where a gold square lattice of nano-holes was directly integrated onto the cleaved facet of an optical fiber tip116. They studied temporal heating and cooling processes both experimentally and numerically. The rapid time dynamics of the device together with the possibility of controlling the temperature distribution were demonstrated. By careful design of a plasmonic nanostructure allowing wavelength selectivity and low power loss, the device’s performance could be improved. This opens up possibilities for future advanced applications, including drug delivery, photo-acoustic imaging probes, and nano-heaters for biochemical applications.
In 2012, a fiber-based SPR sensor was designed for the in vivo detection of brain activity42. The setup involved dividing a 635 nm diode laser using a 2 × 2 fiber coupler to direct half of the laser energy to a multimode fiber coated with a 50 nm gold layer, generating SPR waves, while the other half was directed to a photodetector. The sensor measured the shift in SPR signal intensity relative to changes in refractive index (Δn) in refractive index units (RIU).
The performance of a fiber-based SPR sensor as an all-optical, less-invasive method for monitoring neural activity was computationally analyzed with different available geometries using the FEM. It was shown that the optical response of the proposed sensor tracked the action potential of the neuron in its vicinity43.
As an alternative to optogenetic for optical neuromodulation without genetic modification, Kang et al. proposed a thermoplasmonic-based optical fiber probe for neural stimulation (see Fig. 6b, c). They demonstrated the ability of this approach to modulate neural activity locally in vitro. They further simulated the spatiotemporal temperature change by the thermoplasmonic optical fiber and analyzed its applicability to in vivo animal models117.
On-chip plasmonic modalities
Prism coupled-SPR is a basic chip platform for biosensing, however, its application for neuromodulation has been limited so far. In 2008, Kim et al. developed a low noise prism coupled-SPR sensor chip for in vitro recording of extracellular neural activity41. The sample was a natural neuronal tissue of sciatic nerves from the knee (distal) to the special cord (proximal) of a rat. A glass slide of BK7 prism coated with a 50 nm-thick gold film was used as a chamber for optical recording. A low-noise laser diode of 635 nm and a beam diameter of 100 μm used as a source (Fig. 6d). Simultaneous recording of electrical and intensity-based optical signals in response to electrical stimulation was reported to be label- and artifact-free. The reported limitation of this system was the required close proximity of the metal surface to the nerve.
Using a LSPR modality with a gold nanoparticle-embedded probe, Zhang et al. could successfully detect hippocampal neural spiking activity in real time118. Inspired by this work, a similar probe was numerically studied to enhance the sensitivity. The proposed modified structure showed better performance in terms of sensitivity in a broader range of refractive index changes119.
As noted earlier, tissue damage is an important risk in many optical neuromodulation techniques. To address this concern in infrared neural stimulation (INS), plasmonic gold nanorods were employed. By harnessing the localized photothermal effect in the plasma membrane, the action potentials of in vivo peripheral neural tissues were triggered using pulsed infrared light and gold nanorods. This could increase neural responsivity while significantly lowering the threshold stimulation level compared to conventional INS. The requisite radiant exposure is thus reduced, along with the concern about tissue damage (see Fig. 6e)120.
By integrating nanomaterials as mediators into optical neural stimulation, Wang et al. improved the responsivity and sensitivity of INS to achieve spatial specificity, energy efficiency, and safety by significantly lowering the light power121.
To make personalized implants that can be remotely controlled to alter brain activity, inkjet printers were utilized (see Fig. 6f). The inkjet-printed thermoplasmonic patterns of gold nanorods could generate heat at micron resolution over a large area and selectively modulate neuronal network activities. The printed heat patterns selectively and instantaneously inhibited the activities of cultured hippocampal neurons upon NIR light illumination. It was concluded that the inkjet printing process could be a universal method for biofunctional thermoplasmonic interfaces in various bioengineering applications. Because the printing process is applicable to thin and flexible substrates, the technology was suggested to be easily applicable to implantable neurological disorder treatment devices and wearable devices122.
Plasmonic gold nanorods were recently used to stimulate neurons via the thermoplasmonic effect, followed by successful demonstration of both induction and suppression of action potentials123. Hong et al. designed a neural chip platform using a gold nanorod-assisted thermoplasmonic interface on a multielectrode array for in situ neuro-manipulation. They could successfully manipulate a cultured neuronal network both structurally and functionally. The advantages of the design were maximizing the photothermal conversion efficiency at the desired wavelength and the NIR operation range of wavelength, which is less harmful to the cells.
Plasmonic gold nanorods have the additional capability of improving fluorescence signals in optical imaging of neural activity. To enhance the fluorescence effect of low SNR voltage-sensitive dyes, a nanoplasmonic chip incorporating gold nanorods was developed. The chip’s performance was tested using cultured rat hippocampal neurons and a widely used type of voltage-sensitive dye, di-8-ANEPPS. The fluorescence signal reached its maximum level with nine layers of polyelectrolyte spacer, resulting in enhanced membrane potential imaging (Fig. 6g)124. Simultaneous fluorescence imaging and electrical recording confirmed the suppression of neural spiking activity at the single-cell level in photothermal neuromodulation (Fig. 6h)100.
Prospects for clinical applications
There are general considerations for clinical translations of research technologies. Neuroplasmonic approaches need to be validated in long-term trials in large animal models such as nonhuman primates that are closer to humans in terms of brain size, peripheral nerve anatomy, and relevant autonomic nerve stimulation condition. Such trials would characterize the cell and tissue response to foreign materials and identify any potential for inflammation, fibrosis, or toxicity.
In optical approaches, one main concern is the potential thermal tissue damage. Furthermore, inspection of the long-term robustness of devices after implantation is required for device optimization125,126. Securing regulatory approval for neuroplasmonic therapies will also entail navigating pathways optimized distinctly for devices and drugs. Many neuroplasamonic technologies will likely transcend this traditional boundary, and early engagement with regulators, such as the FDA, would be critical.
Photothermal imaging, cancer therapy, i.e., hyperthermia98, and selective destruction of antibiotic-resistant bacteria16 are examples of clinical applications of plasmonic biomaterials. While neuromodulation technologies remain in pre-clinical testing, certain electrical stimulation strategies have been successfully implemented in clinical applications for the treatment of brain disorders such as Parkinson’s disease, epilepsy, and major depression. Devices used in these cases are generally electrodes connected to co-implanted electrical stimulators and have favorable safety profiles with low chances of hardware failure over the years-long implantation period.
Researchers hoping to translate technologies developed in the laboratory would benefit from early engagement with clinicians and clinical partners to identify indications that would stand to benefit from new therapeutic modalities. In addition, initiatives that bring together researchers, clinicians, and potential industry and venture partners have shown great success in accelerating the translation of new technologies to the clinic.
Optogenetics, for instance, with a transformative effect on basic neuroscience research, has been employed as a study tool in the field of health and disease and has shown its potential for real clinical applications in the areas of drug discovery and gene therapy. However, the strategies are mostly tested on rodents, and critical steps for trials on larger animal models and humans have yet to be undertaken127.
Nanomaterials with plasmonic factors could be interesting candidates for future clinical neuroengineering (neuromodulation, smart nanocarriers for drug delivery toward the brain, etc.) as they are stimuli-responsive and have the ability to act as mediators for efficient light- or heat-assisted neuromodulation while having the privilege of nanosizing, yielding minimally invasive integration with the nervous system or the ability to cross the BBB. The capability of plasmonic biomaterials has been realized in neuroengineering, such as neuronal tissue engineering and regeneration, using engineered scaffolds128, although inorganic plasmonic materials, such as gold and silver, have been less employed for in vivo drug delivery systems as nanocarriers. Despite a few NPs accepted for clinical use, the majority of them are yet to proceed beyond clinical trials101.
Diagnosis and therapy
Plasmonic nanoparticles (PNPs) are tunable for high sensitive optical labels, therefore, they have promising potentials for diagnosis of neurological disorders129. Hyperactive behaviors of neurons and neural circuits that are often found in neurological disorders such as epilepsy could be treated by controlling the activity of brain cells.
Plasmonic extracellular matrix, a promising method to control neuronal activity, can be remotely tuned to enhance cell-based therapies by stimulating or inhibiting neural activity by a source of light17. By modifying and tuning their surface chemistry and remotely activating surface plasmons they can be employed for deep tissue treatment. The tuning ability of metallic NPs allowing modulation of signaling pathways, generation of reactive oxygen species, and regulation of various transcription factors paves the way toward new therapeutic devices for pain management devices to alleviate chronic neuropathic pain17.
Gold nanoclusters were used as the anti-Parkinson’s disease drugs and showed excellent capacity to inhibit aggregation and fibrillation of α-Syn in in vitro experiments130. A biosensing methodology using helically arranged gold nanorods was developed to detect amyloids in Parkinson’s and prion diseases with good agreement between theoretical simulations and experimental findings. Strong dipolar coupling results in an intense chiral response, which makes the detection of nanomolar concentrations of amyloid fibrils possible. The technique successfully identified patients with Parkinson’s disease from human brain homogenates. To confirm the generality of the technique, it was also used for the specific detection of infectious amyloids formed by prion proteins131.
Gold nanorods (AuNRs) are good candidates for neuromodulation, as the plasmonic peak can be tuned by changing its aspect ratio. AuNRs were designed with a resonance peak at 808 nm within the NIR-I spectral window, providing deep tissue penetration and minimum absorption while avoiding the risk of tissue damage by collateral heating. It was shown that they could reversibly modulate the left stellate ganglion function, and inhibit and decrease the occurrence of ventricular arrhythmias in a canine model of acute ischemia. The neuromodulation was based on a photothermal mechanism where genetic transfection is not required132.
Recently, highly ordered nanostructured electrodes composed of densely packed gold vertical nanowires (AuNWs) were designed, and their impact on primary neural cell cultures in vitro and spinal cord tissue in vivo was explored (Fig. 6i). It was found that AuNWs supported the growth of highly viable and dense neural cultures containing a majority of neurons accompanied by glial cells. The gold nanostructure was implanted in an experimental model of rat spinal cord injury, and subacute responses did not significantly differ from those induced by the injury itself, thus highlighting their potential for in vivo applications as neural interfaces in contact with central nervous tissues, including the injured spinal cord133.
In molecular hyperthermia, plasmonic AuNPs were successfully used demonstrating inactivation of an important G-protein coupled receptor for chronic pain sensitization with nanoscale spatio-temporal resolution. The transient photoinactivation of cell membrane protein activity did not require genetic modification, and did not induce global tissue heating as expected in traditional hyperthermia134.
Smart carrier—drug delivery toward brain
Finding efficient passage across the BBB is an important challenge for drug delivery to the brain, for which NPs have shown their potential102,135. In particular, stimuli-responsive PNPs can be considered smart nanocarriers96,103. Gold nanoparticles (AuNPs), in particular, have a localized photoconversion capacity in response to light exposure and hence can act as a carrier for targeted and controllable drug delivery. Drugs or genetic factors that are either directly loaded at the nanoparticle surface or encapsulated in micelles, liposomes, or red blood cells would be released via either bond breakage or phase change due to the temperature increase of PNPs under their resonance condition136.
For instance, a hybrid nanomaterial of palladium plasmonic nanosheets encapsulated by drug responded to an external NIR light stimulus at 808 nm and presented a high photothermal conversion efficiency of 52%. They employed to facilitate the induction of local hyperthermia in photothermal therapies and used as drug-release activation agents137.
Magnetoplasmonic NPs, having both magnetic and plasmonic properties, respond to both magnetic fields and light as remote stimuli; therefore, they have become potential candidates as smart nanocarriers for delivering neuroprotective drugs, genes and growth factors. Light-stimulated superparamagnetic iron oxide-gold core-shell NPs which were functionalized with nerve growth factor were successfully used to promote in vitro neuronal growth and differentiation68. The cellular uptake and crossing of the BBB of magnetoplasmonic NPs can be optimized by tuning the external stimuli and designing structural and surface functionalization, hence, they have been realized as a new therapeutic strategy for neurodegenerative disease138.
Core–shell magnetoplasmonic particles (MNP@Au) were synthesized for multimodal brain imaging and showed potential for targeted drug delivery toward the brain. The particles of ~15 nm exhibited superparamagnetic properties, with a resonance plasmonic peak of gold in the visible range. Enhanced contrast in both MRI and X-ray in the presence of a magnetic field and light exposure was observed along with transmigration of the particles across the BBB without disrupting its integrity66. NIR-responsive nanostars, with a magnetic core and gold-shell structure, exhibited multipolar magnetic plasmon resonance characteristics. The potential of being effective candidates for image-guided drug delivery, as well as having precise multimodal imaging capabilities, was demonstrated. This allows for tunable NIR-triggered on-demand drug release139.
Summary and outlook
Neuroplasmonics, an approach based on plasmonic-assisted neuromodulation, provides various technical benefits in neuroengineering applications over traditional electrostimulation or optogenetics. The optical and thermal properties of plasmonic nanostructures are tunable by design (geometry and material) for a particular need. Further, their responses can be controlled remotely with an external stimulus. Their responsiveness to external stimuli enables us to appropriately engineer them as transducers to control activity of the target cells through several photo-assisted strategies discussed in this review paper. This is the basis for designing wireless platforms in neuroengineering140, which helps prevent many challenges associated with wired devices. For instance, displacements during freely moving experiments and invasiveness related to the implantation of wired devices.
In NIR optogenetic strategy, UCNPs act as light emission transducers where plasmonic materials can help enhance photoupconversion efficiency. Fabricating plasmonic nanoparticles (PNPs) for nongenetic neuromodulation strategies is relatively simple and fast. Also, engineering plasmonic-based neural interfaces are inexpensive compared to other approaches such as optogenetics constructs. UCNP-plasmonic hybrid structures offer improved long-term stability, biocompatibility, and efficiency compared to conventional optogenetic strategies. They also address limitations related to light penetration depth and invasiveness while enhancing upconversion brightness. As a result, these structures are suitable for diverse biomedical applications like drug delivery and NIR-mediated optogenetics.
Optogenetics requires prior gene modifications. The injection of transfect genes in rodents requires days to weeks to be healed before starting the experimentation. Although optogenetics has been used successfully for basic research in neuroscience, it needs to be carefully investigated before translation to humans from the aspect of long-term safety. From this perspective, using light-sensitive biocompatible materials for neuromodulation is advantageous over optogenetics as simplifying clinical translation. However, the limitations of metallic nanoparticles, cell toxicity, and tissue damage, and clinical side effects should also be carefully taken into account.
With the progress in nanofabrication, the stimulus-responsive plasmonic nanostructures can be potentially integrated into an ultra-miniaturized platform seamlessly, as compared to various types of implantable neural interfaces. This avoids implant-driven injuries and inflammations, leading to minimally invasive approaches.
Although there have been some successes in laboratory, it is necessary to gather more data on the safety, side effects, human response, and lifespan of the nanoplasmonic particles as well as required devices when they are tested on larger animals and eventually on humans. Especially, a targeted delivery of nanoplasmonic particles to the site of interest via smart surface chemistry will also be necessary to enable localized neuromodulation. General considerations in clinical translational research are the difficulties in measuring complex samples or tissues, making it hard to apply technological advancements in analysis from the lab to the clinical setting. To enable non-experts to use lab instruments, strict control of processes is necessary to ensure accurate data. Therefore, a simple and reliable automated platform is required to tackle these challenges and optimize lab analysis systems for specific clinical purposes141. Furthermore, a miniaturized portable plasmonic system might enable facile deployment and easier access to clinical settings. A longer time study period would be ideal to comprehensively evaluate the performance of new strategies over extended usage.
Thermal considerations for tissue safety, control of collateral heating of the tissues, cell toxicity of nanoparticles, and efficacy are among the important concerns that should be addressed before these methods are translated from animal studies to the clinic, and ultimately considered as routine medical practices. Robustness of the devices and ease of long-term use in patients who may be ambulatory will have to be established. Approvals by regulatory bodies, and acceptance by the medical profession and by the public will hinge on the results of these steps. As described in this review, the field of plasmonics for neuroengineering is relatively young but holds the promise of new treatment modalities, especially where alternatives do not currently exist or are less attractive.
References
World Health Organization (WHO) report. Neurological disorders affect millions globally https://www.who.int/news/item/27-02-2007-neurological-disorders-affect-millions-globally-who-report (2007).
Collaborators, G. U. N. D. Burden of neurological disorders across the US from 1990–2017: a global burden of disease study. JAMA Neurology 78, 165 (2021).
Baffou, G. & Quidant, R. Thermo-plasmonics: using metallic nanostructures as nano-sources of heat: thermoplasmonics. Laser Photon. Rev. 7, 171–187 (2013).
Nakatsuji, H. et al. Thermosensitive ion channel activation in single neuronal cells by using surface-engineered plasmonic nanoparticles. Angew. Chem. Int. Ed. 54, 11725–11729 (2015).
Parakhonskiy, B. V., Parak, W. J., Volodkin, D. & Skirtach, A. G. Hybrids of polymeric capsules, lipids, and nanoparticles: Thermodynamics and temperature rise at the nanoscale and emerging applications. Langmuir 35, 8574–8583 (2019).
Sohrabi, F. & Hamidi, S. M. Neuroplasmonics: from kretschmann configuration to plasmonic crystals. Eur. Phys. J. Plus 131, 221 (2016).
Shpacovitch, V. & Hergenröder, R. Optical and surface plasmonic approaches to characterize extracellular vesicles. a review. Anal. Chim. Acta 1005, 1–15 (2018).
Rojalin, T., Phong, B., Koster, H. J. & Carney, R. P. Nanoplasmonic approaches for sensitive detection and molecular characterization of extracellular vesicles. Front. Chem. 7, 279 (2019).
Chin, L. K. et al. Plasmonic sensors for extracellular vesicle analysis: from scientific development to translational research. ACS Nano 14, 14528–14548 (2020).
Boyer, D., Tamarat, P., Maali, A., Lounis, B. & Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 297, 1160–1163 (2002).
Peng, L. et al. Surface plasmon-enhanced NIR-II fluorescence in a multilayer nanoprobe for through-skull mouse brain imaging. ACS Appl. Mater. Interfaces 14, 38575–38583 (2022).
Patel, M., Meenu, M., Pandey, J. K., Kumar, P. & Patel, R. Recent development in upconversion nanoparticles and their application in optogenetics: a review. J. Rare Earths https://www.sciencedirect.com/science/article/pii/S100207212100243X (2021).
Habib, A. et al. Electro-plasmonic nanoantenna: a nonfluorescent optical probe for ultrasensitive label-free detection of electrophysiological signals. Sci. Adv. 5, eaav9786 (2019).
Damnjanovic, R., Bazard, P., Frisina, R. D. & Bhethanabotla, V. R. Hybrid electro-plasmonic neural stimulation with visible-light-sensitive gold nanoparticles. ACS Nano 14, 10917–10928 (2020).
An, Y. & Nam, Y. Closed-loop control of neural spike rate of cultured neurons using a thermoplasmonics-based photothermal neural stimulation. J. Neural Eng. 18, 066002 (2021).
Nguyen, K. T. et al. Strategies of engineering nanoparticles for treating neurodegenerative disorders. Curr. Drug Metab. 18 http://www.eurekaselect.com/149495/article (2017).
Alghazali, K. M. et al. Plasmonic nanofactors as switchable devices to promote or inhibit neuronal activity and function. Nanomaterials 9, 1029 (2019).
Robinson, J. T. et al. Developing next-generation brain sensing technologies—a review. IEEE Sensors J. 19, 10163–10175 (2019).
Benfenati, F. & Lanzani, G. Clinical translation of nanoparticles for neural stimulation. Nat. Rev. Mater. 6, 1–4 (2021).
Colombo, E., Feyen, P., Antognazza, M. R., Lanzani, G. & Benfenati, F. Nanoparticles: a challenging vehicle for neural stimulation. Front. Neurosci. 10. http://journal.frontiersin.org/Article/10.3389/fnins.2016.00105/abstract (2016).
Kozai, T. D. Y. The history and horizons of microscale neural interfaces. Micromachines 9, 445 (2018).
Kim, G. et al. Recent progress on microelectrodes in neural interfaces. Materials 11, 1995 (2018).
Emiliani, V., Cohen, A. E., Deisseroth, K. & Hausser, M. All-optical interrogation of neural circuits. J. Neurosci. 35, 13917–13926 (2015).
Park, Y., Park, S.-Y. & Eom, K. Current review of optical neural interfaces for clinical applications. Micromachines 12, 925 (2021).
Ramezani, Z., Seo, K. J. & Fang, H. Hybrid electrical and optical neural interfaces. J. Micromech. Microeng. 31, 044002 (2021).
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Prominski, A. et al. Porosity-based heterojunctions enable leadless optoelectronic modulation of tissues. Nat. Mater. 21, 647–655 (2022).
Kubanek, J., Shukla, P., Das, A., Baccus, S. A. & Goodman, M. B. Ultrasound elicits behavioral responses through mechanical effects on neurons and ion channels in a simple nervous system. J. Neurosci. 38, 3081–3091 (2018).
Jiang, Y. et al. Neural stimulation in vitro and in vivo by photoacoustic nanotransducers. Matter 4, 654–674 (2021).
Brocker, D. T. & Grill, W. M. Principles of Electrical Stimulation of Neural Tissue, vol. 116, 3–18 (Elsevier, 2013). https://linkinghub.elsevier.com/retrieve/pii/B9780444534972000012.
Ye, H. & Steiger, A. Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J. NeuroEng. Rehabil. 12, 65 (2015).
Luan, L. et al. Recent advances in electrical neural interface engineering: minimal invasiveness, longevity, and scalability. Neuron 108, 302–321 (2020).
Alexander, C. T., Paul, R. S. & E. Duco, J. Optical stimulation of neurons. Curr. Mol. Imaging (Discontinued) 3, 162–177 (2014).
Warden, M. R., Cardin, J. A. & Deisseroth, K. Optical neural interfaces. Annu. Rev. Biomed. Eng. 16, 103–129 (2014).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Scanziani, M. & Häusser, M. Electrophysiology in the age of light. Nature 461, 930–939 (2009).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Villringer, A. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 20, 435–442 (1997).
Roy, C. S. & Sherrington, C. S. On the regulation of the blood-supply of the brain. J. Physiol. 11, 85–158 (1890).
Skala, M. C., Tao, Y. K., Davis, A. M. & Izatt, J. A. Functional optical coherence tomography in preclinical models. Handbook of Biomedical Optics (2011). https://www.routledgehandbooks.com/doi/10.1201/b10951-18.
Ae Kim, S. et al. Optical measurement of neural activity using surface plasmon resonance. Optics Lett. 33, 914 (2008).
Kim, S. A., Kim, S. J., Moon, H. & Jun, S. B. In vivo optical neural recording using fiber-based surface plasmon resonance. Optics Lett. 37, 614 (2012).
Abedini, M., Tekieh, T. & Sasanpour, P. Recording neural activity based on surface plasmon resonance by optical fibers-a computational analysis. Front. Comput. Neurosci. 12, 61 (2018).
Kim, S. A. & Jun, S. B. In-vivo optical measurement of neural activity in the brain. Exp. Neurobiol. 22, 158–166 (2013).
Li, X., Che, Z., Mazhar, K., Price, T. J. & Qin, Z. Ultrafast near-infrared light triggered intracellular uncaging to probe cell signaling. Adv. Funct. Mater. 27, 1605778 (2017).
Abraham-Ekeroth, R. M. & De Angelis, F. Radioplasmonics: plasmonic transducers in the radiofrequency regime for resonant thermo-acoustic imaging in deep tissues. ACS Photonics 8, 238–246 (2021).
Dugué, G. P., Akemann, W. & Knöpfel, T. A comprehensive concept of optogenetics, vol. 196, 1–28 (Elsevier, 2012). https://linkinghub.elsevier.com/retrieve/pii/B978044459426600001X.
Liu, Y., Yi, Z., Yao, Y., Guo, B. & Liu, X. Noninvasive manipulation of ion channels for neuromodulation and theranostics. Acc. Mater. Res. 3, 247–258 (2022).
Deisseroth, K. & Anikeeva, P. Upconversion of light for use in optogenetic methods, United States Patent No. US10252076(B2) https://patents.google.com/patent/US20160317658/en (2011).
Chen, S. Near-infrared Deep Brain Stimulation in Living Mice, vol. 2173, 71–82 (Springer US, New York, NY, 2020). https://link.springer.com/10.1007/978-1-0716-0755-8_4.
Lin, Y. et al. Applications of upconversion nanoparticles in cellular optogenetics. Acta Biomater. 135, 1–12 (2021).
Wu, D. M., García-Etxarri, A., Salleo, A. & Dionne, J. A. Plasmon-enhanced upconversion. J. Phys. Chem. Lett. 5, 4020–4031 (2014).
Wiesholler, L. M. & Hirsch, T. Strategies for the design of bright upconversion nanoparticles for bioanalytical applications. Optical Mater. 80, 253–264 (2018).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–684 (2018).
Chernov, M. & Roe, A. W. Infrared neural stimulation: a new stimulation tool for central nervous system applications. Neurophotonics 1, 011011 (2014).
Zhang, J. et al. Non-invasive, opsin-free mid-infrared modulation activates cortical neurons and accelerates associative learning. Nat. Commun. 12, 2730 (2021).
Wells, J. et al. Optical stimulation of neural tissue in vivo. Optics Lett. 30, 504 (2005).
Fekete, Z., Horváth, C. & Zátonyi, A. Infrared neuromodulation:a neuroengineering perspective. J. Neural Eng. 17, 051003 (2020).
Throckmorton, G. et al. Identifying optimal parameters for infrared neural stimulation in the peripheral nervous system. Neurophotonics 8 https://www.spiedigitallibrary.org/journals/neurophotonics/volume-8/issue-01/015012/Identifying-optimal-parameters-for-infrared-neural-stimulation-in-the-peripheral/10.1117/1.NPh.8.1.015012.full (2021).
Wells, J. et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys. J. 93, 2567–2580 (2007).
Vay, L., Gu, C. & McNaughton, P. A. The thermo-TRP ion channel family: properties and therapeutic implications: The thermo-TRP ion channel family. Br. J. Pharmacol. 165, 787–801 (2012).
Scholkmann, F. et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage 85, 6–27 (2014).
Pinti, P. et al. The present and future use of functional near infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N.Y. Acad. Sci. 1464, 5–29 (2020).
Bondelli, G. et al. Shedding light on thermally induced optocapacitance at the organic biointerface. J. Phys. Chem. B 125, 10748–10758 (2021).
Carvalho-de Souza, J. L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207–217 (2015).
Tomitaka, A. et al. Development of magneto-plasmonic nanoparticles for multimodal image-guided therapy to the brain. Nanoscale 9, 764–773 (2017).
Bornacelli, J. et al. Enhanced ultrafast optomagnetic effects in room-temperature ferromagnetic pt nanoclusters embedded in silica by ion implantation. Nanotechnology 31, 355705 (2020).
Yuan, M., Wang, Y. & Qin, Y.-X. Engineered nanomedicine for neuroregeneration: light emitting diode-mediated superparamagnetic iron oxide-gold core-shell nanoparticles functionalized by nerve growth factor. Nanomed. Nanotechnol. Biol. Med. 21, 102052 (2019).
Han, L. et al. A magnetic polypyrrole/iron oxide core/gold shell nanocomposite for multimodal imaging and photothermal cancer therapy. Talanta 171, 32–38 (2017).
Leung, S. J., Kachur, X. M., Bobnick, M. C. & Romanowski, M. Wavelength-selective light-induced release from plasmon resonant liposomes. Adv. Funct. Mater. 21, 1113–1121 (2011).
Chen, J. et al. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 7, 1318–1322 (2007).
Yavuz, M. S. et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 8, 935–939 (2009).
Li, W. et al. Remote modulation of neural activities via near-infrared triggered release of biomolecules. Biomaterials 65, 76–85 (2015).
Lukianova-Hleb, E. Y. et al. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat. Med. 20, 778–784 (2014).
Xiong, H. et al. Near infrared light triggered release in deep brain regions using ultra photosensitive nanovesicles. Angew. Chem. Int. Ed. 59, 8608–8615 (2020).
Parameswaran, R. et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 13, 260–266 (2018).
Borgh, G. et al. Surface plasmons in silicon nanowires. Adv. Photonics Res. 2, 2100130 (2021).
Prost, A., Poisson, F. & Bossy, E. Photoacoustic generation by a gold nanosphere: from linear to nonlinear thermoelastics in the long-pulse illumination regime. Phys. Rev. B 92, 115450 (2015).
Mantri, Y. & Jokerst, J. V. Engineering plasmonic nanoparticles for enhanced photoacoustic imaging. ACS Nano 14, 9408–9422 (2020).
Ovsepian, S. V., Olefir, I., Westmeyer, G., Razansky, D. & Ntziachristos, V. Pushing the boundaries of neuroimaging with optoacoustics. Neuron 96, 966–988 (2017).
Huang, H., Delikanli, S., Zeng, H., Ferkey, D. M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 5, 602–606 (2010).
Tay, A., Sohrabi, A., Poole, K., Seidlits, S. & Di Carlo, D. A 3d magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. 30, 1800927 (2018).
Cazares-Cortes, E. et al. Recent insights in magnetic hyperthermia: from the “hot-spot" effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Adv. Drug Deliv. Rev. 138, 233–246 (2019).
Marino, A. et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano 9, 7678–7689 (2015).
Multari, C. et al. Magnetoplasmonic nanoparticles for photothermal therapy. Nanotechnology 30, 255705 (2019).
Rizal, C. et al. Magnetophotonics for sensing and magnetometry toward industrial applications. J. Appl. Phys. 130, 230901 (2021).
Temel, Y. & Jahanshahi, A. Treating brain disorders with neuromodulation. Science 347, 1418–1419 (2015).
Wood, R. Xlii. on a remarkable case of uneven distribution of light in a diffraction grating spectrum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 4, 396–402 (1902).
Liedberg, B., Nylander, C. & Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 4, 299–304 (1983).
Chen, Y. & Ming, H. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sens. 2, 37–49 (2012).
Xie, X., Liao, J., Shao, X., Li, Q. & Lin, Y. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci. Rep. 7, 3827 (2017).
Anker, J. N. et al. Biosensing with plasmonic nanosensors. Nat. Mater. 7, 442–453 (2008).
Choi, S. H., Kim, S. J., Im, C.-H., Kim, S. A. & Kim, D. Quantitative model for the change of optical resonance in neural activity detection systems based on surface plasmon resonance. Optics Laser Technol. 43, 938–948 (2011).
Chen, X., Chen, Y., Yan, M. & Qiu, M. Nanosecond photothermal effects in plasmonic nanostructures. ACS Nano 6, 2550–2557 (2012).
Baffou, G. & Rigneault, H. Femtosecond-pulsed optical heating of gold nanoparticles. Phys. Rev. B 84, 035415 (2011).
Guglielmelli, A. et al. Thermoplasmonics with gold nanoparticles: a new weapon in modern optics and biomedicine. Adv. Photonics Res. 2, 2000198 (2021).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Yang, W., Liang, H., Ma, S., Wang, D. & Huang, J. Gold nanoparticle based photothermal therapy: development and application for effective cancer treatment. Sustain. Mater. Technol. 22, e00109 (2019).
Arami, H. et al. Remotely controlled near-infrared-triggered photothermal treatment of brain tumours in freely behaving mice using gold nanostar s. Nat. Nanotechnol. 17, 1015–1022 (2022).
Jung, H. & Nam, Y. Optical recording of neural responses to gold-nanorod mediated photothermal neural inhibition. J. Neurosci. Methods 373, 109564 (2022).
Patra, J. K. et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16, 71 (2018).
Saraiva, C. et al. Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 235, 34–47 (2016).
Rajpoot, K. Nanotechnology-based targeting of neurodegenerative disorders: a promising tool for efficient delivery of neuromedicines. Curr. Drug Targets 21, 819–836 (2020).
Lenton, I. C. D., Scott, E. K., Rubinsztein-Dunlop, H. & Favre-Bulle, I. A. Optical tweezers exploring neuroscience. Front. Bioeng. Biotechnol. 8 https://www.frontiersin.org/articles/10.3389/fbioe.2020.602797 (2020).
Fekete, T. et al. Optically manipulated microtools to measure adhesion of the nanoparticle-targeting ligand glutathione to brain endothelial cells. ACS Appl. Mater. Interfaces 13, 39018–39029 (2021).
Cui, X. et al. Photothermal nanomaterials: a powerful light-to-heat converter. Chem. Rev. 123, 6891–6952 (2023).
West, P. et al. Searching for better plasmonic materials. Laser Photonics Rev. 4, 795–808 (2010).
Jauffred, L., Samadi, A., Klingberg, H., Bendix, P. M. & Oddershede, L. B. Plasmonic heating of nanostructures. Chem. Rev. 119, 8087–8130 (2019).
Li, C., Liu, Z. & Yao, P. Gold nanoparticles coated with a polydopamine layer and dextran brush surface for diagnosis and highly efficient photothermal therapy of tumors. RSC Adv. 6, 33083–33091 (2016).
Paviolo, C. & Stoddart, P. Gold nanoparticles for modulating neuronal behavior. Nanomaterials 7, 92 (2017).
Dipalo, M. et al. Intracellular and extracellular recording of spontaneous action potentials in mammalian neurons and cardiac cells with 3d plasmonic nanoelectrodes. Nano Lett. 17, 3932–3939 (2017).
Kim, R., Hong, N. & Nam, Y. Gold nanograin microelectrodes for neuroelectronic interfaces. Biotechnol. J. 8, 206–214 (2013).
Bazard, P., Frisina, R. D., Walton, J. P. & Bhethanabotla, V. R. Nanoparticle-based plasmonic transduction for modulation of electrically excitable cells. Sci. Rep. 7, 7803 (2017).
Giaquinto, M. (Invited) Stimuli-responsive materials for smart lab-on-fiber optrodes. Results Opt. 2, 100051 (2021).
Hong, W. et al. Computational thermal analysis of the photothermal effect of thermoplasmonic optical fiber for localized neural stimulation in vivo. Electronics 10, 118 (2021).
Principe, S. et al. Thermo-plasmonic lab-on-fiber optrodes. Optics Laser Technol. 132, 106502 (2020).
Kang, H. et al. Thermoplasmonic optical fiber for localized neural stimulation. ACS Nano 14, 11406–11419 (2020).
Zhang, J., Atay, T. & Nurmikko, A. V. Optical detection of brain cell activity using plasmonic gold nanoparticles. Nano Lett. 9, 519–524 (2009).
Mousavi, N. S. S., Moghaddasi, F., Venugopalan, P., Dabirian, A. & Kumar, S. Design of a portable optical fiber-based localized surface plasmon resonance (LSPR) for in-situ biosensing. In: Proc. International Conference in Nanophotonics, Micro-Nano Optics (NANOP2019) (2019). https://zenodo.org/record/7568795.
Eom, K. et al. Enhanced infrared neural stimulation using localized surface plasmon resonance of gold nanorods. Small 10, 3853–3857 (2014).
Wang, Y. & Guo, L. Nanomaterial-enabled neural stimulation. Front. Neurosc. 10, 69 (2016).
Kang, H., Lee, G.-H., Jung, H., Lee, J. W. & Nam, Y. Inkjet-printed biofunctional thermo-plasmonic interfaces for patterned neuromodulation. ACS Nano 12, 1128–1138 (2018).
Hong, N. & Nam, Y. Thermoplasmonic neural chip platform for in situ manipulation of neuronal connections in vitro. Nat. Commun. 11, 6313 (2020).
Kim, R. & Nam, Y. Fabrication of a nanoplasmonic chip to enhance neuron membrane potential imaging by metal-enhanced fluorescence effect. BioChip J. 15, 171–178 (2021).
Yang, X. et al. Nanotechnology enables novel modalities for neuromodulation. Adv. Mater. 33, 2103208 (2021).
Dominguez-Paredes, D., Jahanshahi, A. & Kozielski, K. L. Translational considerations for the design of untethered nanomaterials in human neural stimulation. Brain Stimul. 14, 1285–1297 (2021).
Towne, C. & Thompson, K. R. Overview on research and clinical applications of optogenetics. Curr. Protoc. Pharmacol. 75 https://onlinelibrary.wiley.com/doi/10.1002/cpph.13 (2016).
Borghei, Y.-S., Hosseinkhani, S. & Ganjali, M. R. “plasmonic nanomaterials": an emerging avenue in biomedical and biomedical engineering opportunities. J. Adv. Res. https://www.sciencedirect.com/science/article/pii/S2090123221002162 (2021).
Peng, F., Jeong, S., Ho, A. & Evans, C. L. Recent progress in plasmonic nanoparticle based biomarker detection and cytometry for the study of central nervous system disorders. Cytomet. Part A 99, 1067–1078 (2021).
Gao, G. et al. Gold nanoclusters for Parkinson’s disease treatment. Biomaterials 194, 36–46 (2019).
Kumar, J. et al. Detection of amyloid fibrils in Parkinson’s disease using plasmonic chirality. Proc. Natl Acad. Sci. 115, 3225–3230 (2018).
Ye, T. et al. Precise modulation of gold nanorods for protecting against malignant ventricular arrhythmias via near infrared neuromodulation. Adv. Funct. Mater. 29, 1902128 (2019).
Domínguez-Bajo, A. et al. Nanostructured gold electrodes promote neural maturation and network connectivity. Biomaterials 279, 121186 (2021).
Kang, P. et al. Transient photoinactivation of cell membrane protein activity without genetic modification by molecular hyperthermia. ACS Nano 13, 12487–12499 (2019).
Johnsen, K. B. et al. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 8, 3416–3436 (2018).
Baffou, G., Cichos, F. & Quidant, R. Applications and challenges of thermoplasmonics. Nat. Mate. 19, 946–958 (2020).
Uson, L. et al. Nanoengineering palladium plasmonic nanosheets inside polymer nanospheres for photothermal therapy and targeted drug delivery. Adv. Funct. Mater. 32, 2106932 (2022).
Yuan, M. et al. Progress, opportunities, and challenges of magneto-plasmonic nanoparticles under remote magnetic and light stimulation for brain-tissue and cellular regeneration. Nanomaterials 12, 2242 (2022).
Tomitaka, A. et al. Magneto-plasmonic nanostars for image-guided and NIR-triggered drug delivery. Sci. Rep. 10, 10115 (2020).
Li, X. et al. Nanotransducers for wireless neuromodulation. Matter 4, 1484–1510 (2021).
Gowers, S. A. N. et al. Clinical translation of microfluidic sensor devices: focus on calibration and analytical robustness. Lab Chip 19, 2537–2548 (2019).
Yuan, M. et al. Superparamagnetic iron oxide-enclosed hollow gold nanostructure with tunable surface plasmon resonances to promote near-infrared photothermal conversion. Adv. Compos. Hybrid Mater. 5, 2387–2398 (2022).
Nadort, A., Zhao, J. & Goldys, E. M. Lanthanide upconversion luminescence at the nanoscale: fundamentals and optical properties. Nanoscale 8, 13099–13130 (2016).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Li, M., Cushing, S. K. & Wu, N. Plasmon-enhanced optical sensors: a review. Analyst 140, 386–406 (2015).
Author information
Authors and Affiliations
Contributions
Conceptualization, N.S.S.M.; Methodology, N.S.S.M. and Y.-AkS.; Investigation, N.S.S.M., K.B.R., Y.-AkS., S.K.; Writing-original draft preparation, N.S.S.M.; Writing-review and editing, N.S.S.M., K.B.R., Y.-AkS., S.K.; Supervision, Y.-AkS. and S.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Shreyas Shah, J. R. Mejía-Salazar, Seyedeh Mehri Hamidi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jet-Sing Lee. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mousavi, N.S.S., Ramadi, K.B., Song, YA. et al. Plasmonics for neuroengineering. Commun Mater 4, 101 (2023). https://doi.org/10.1038/s43246-023-00429-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-023-00429-5