Abstract
Different 2D and quasi-2D perovskite materials have demonstrated significant improvements in the device stability compared to 3D perovskites due to their increased hydrophobicity and suppressed ion migration. However, fundamental investigations of these materials have been scarce and consequently detailed understanding of the processes responsible for experimental phenomena are often lacking despite huge interest in these materials. Even more importantly, there have been a limited number of structure-property studies for different material compositions, and research is generally by trial and error rather than by design. Here we discuss different stability issues in these materials and identify questions which need to be answered to design materials with further stability improvements.
Similar content being viewed by others
Introduction
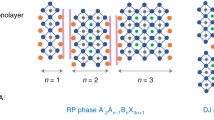
2D and quasi-2D perovskite materials have been attracting increasing attention due to their higher stability in ambient compared to 3D perovskite materials1,2,3, resulting in increased stability of perovskite solar cells (PSCs) and light-emitting diodes (LEDs) using these materials. These materials can be visualized by cutting the typical perovskite 3D crystal consisting of corner-sharing lead halide octahedra along (100), (110), and (111) directions and inserting bulky organic spacer cations, as shown in Fig. 12, where 2D perovskites are obtained when one octahedral layer is separated by spacer cations (n = 1), while quasi-2D perovskites are obtained when n octahedral layers (n = 2, 3,…) are separated by organic cations. Spin-coated films typically contain domains with different n, i.e., they contain multiple phases, and careful optimization of the deposition process is needed to improve phase purity. It should be noted that these materials are based on corner-sharing [MX6] octahedra, where M2+ is metal cation, commonly Pb, and X− is halide anion, which represents a perovskite network4. Other types of low dimensional hybrid organic–inorganic metal halide materials also exist, typically comprising of edge-sharing and/or face-sharing connectivity, which does not represent a perovskite network. Various names exist in the literature for different types of low dimensional hybrid metal halide materials, including perovskite, perovskite-like, perovskitoid, and halometalate hybrid materials with perovskite sub-networks4. Combined with existence of hexagonal polymorphs (with proposed label “perovskite-related hexagonal polytypes”)4, there is some confusion concerning nomenclature. Perhaps a possible compromise between brevity and accuracy would be to refer to all these materials as perovskite-related, with dimensionality and connectivity stated in bracket, such as perovskite-related (2D corner-sharing), perovskite-related (1D, face-sharing), etc. Among various 2D/quasi-2D halide perovskite materials, (100)-2D perovskites are the most common2. There are three types of (100) 2D/quasi-2D perovskite materials, namely Ruddlesden-Popper (RP), Dion-Jacobson (DJ) perovskites, and alternating cations in interlayer space (ACI) perovskites1,2,3. RP perovskites remain the most commonly studied, and they have a general formula C2An − 1MnX3n + 1, where C is the monovalent bulky spacer cation, A is a small monovalent organic cation or Cs+, M is a divalent metal cation (Pb2+ or Sn2+), X is a halide anion, and n is the number of octahedral layers. The octahedral layers in RP phase have staggered configuration with (1/2, 1/2) shift in ab-plane1,3. In DJ perovskites, with the formula DAn-1MnX3n+1 where D is divalent spacer cation, the octahedral layers are typically perfectly aligned with no relative shift ((0,0) shift)1,3, while in ACI perovskites there is (1/2, 0) shift3. ACI perovskites are not common among halide perovskites and have not been reported in oxide perovskites, and involve guanidinium (GA) spacer cations alternating with a small cation in the interlayer space3. In oxide perovskites, RP materials have two interlayer cations per unit cell and exhibit staggered configuration with (1/2,1/2) shift in ab-plane, while DJ perovskites have one interlayer cation per unit cell and exhibit either (0, 0) shift (interlayer cations adopt cubic-type coordination) or (1/2, 0) shift (interlayer cations adopt trigonal prismatic coordination), while interlayer cations in both cases can be monovalent or divalent4. In 2D metal halide perovskites, there are examples of different octahedral layer offsets in structures proposed to be classified as RP or DJ perovskites4,5, and the deviation of observed offsets from the “norm” derived from the appropriation of classification from oxide perovskites has been attributed to the conformational flexibility and variations in possible cation-cation and cation-inorganic octahedral interactions5. Obviously, there are significant differences between oxide and halide perovskites, and it has been stated that halide “DJ perovskites” should not be considered as such, due to differences between organic diammonium cations in halides and M2+ cations in oxides4. Nevertheless, the use of “RP” and “DJ” for halide perovskites with monovalent and divalent spacer cations, respectively, has become relatively common. It should be noted, however, that while the classification of materials with monovalent spacer cations as RP and those with divalent spacer cations as DJ does not in all cases fit the expected octahedral layer offsets commonly associated with those classifications, it is nevertheless important to have separate categories (regardless of their nomenclature) for materials with monovalent and divalent spacer cations. The presence of spacer cation bilayer in materials commonly classified as RP perovskites results in distinct set of properties– easy exfoliation of RP crystals, larger interlayer spacing compared to DJ materials, as well as differences in charge transport properties and material stability. In addition, the reported variations in material properties as a function of material composition further highlight the need for systematic structure-property investigations of perovskite materials with different spacer cations3. A number of materials with different spacer cations, such as n-butylammonium (BA), phenylethylammonium (PEA), 5-ammoniumvaleric acid (5-AVA) etc. for RP and 1,4-butyldiammonium (BDA), 4-aminomethylpiperidinium (4-AMP), etc. for DJ has been reported to date2,3 (see ref. 3. for a comprehensive review), but there are many as yet unexplored materials and the relationships between the choice of spacer cation and the resulting material properties remain poorly understood. Furthermore, it is necessary to systematically investigate the effects of perovskite composition, since bromides have been less studied compared to iodides6. In addition, the properties (including stability) are dependent on the preparation method3, likely because the preparation method affects the presence of defects. As a result, contradictory results could be reported in the literature arising from different sample preparation methods; for example, it was reported that the excess of the 2D precursor contrary to expectations does not necessarily improve the stability of 3D/2D films7.
Organic spacer cations can separate the n layers of corner-sharing lead halide octahedra (n = 1 for 2D, n > 1 for quasi-2D) along (100), (110), and (111) directions. For the most common case of (100) direction, RP, DJ, and ACI denote Ruddlesden-Popper, Dion-Jacobson, and alternating cations in interlayer space perovskites, respectively.
Nevertheless, highly promising improvements achieved in the stability of solar cells1,8,9,10,11,12,13,14,15 and LEDs16,17,18,19,20,21 have stimulated the interest in 2D and quasi-2D perovskite materials. LEDs typically include quasi-2D perovskite emitting layers19,20,21 or the use of 2D passivating or surface layers16,17,18, and similarly solar cell applications of these materials include the use of quasi-2D absorber layer, as well as the use of 3D/2D solar cells1. Here quasi-2D refers to devices where perovskite layer has been prepared from a solution with stoichiometry corresponding to a certain n, while in 3D/2D devices commonly an organic ammonium halide salt is spin-coated on top of the 3D perovskite layer, resulting in the formation of 3D/2D structure. 2D and low n quasi-2D perovskites have higher exciton binding energies and poor charge transport in the c axis (out-of-plane) direction, which limits their solar cell efficiency1,22. While the conductivity in in-plane direction is significantly higher than out-of-plane conductivity for n = 1 2D materials, it is still lower than that of 3D materials22. Both in-plane and out-of-plane conductivities increase while the anisotropy in two directions decreases with increasing n22. While pure phase perovskites with n > 1 are also crystallographically layered materials, due to changes in their optical and electronic properties, i.e., reduced degree of quantum and dielectric confinement as n increases they are referred to as quasi-2D materials. There is no common definition of “low n” in the literature, though one could generally define n ≤ 5 as low n quasi-2D, while higher n (n > 5) materials are sometimes referred to as quasi-3D based on the optoelectronic properties (exciton binding energies, bandgaps which are closer to 3D than 2D materials). Among low n samples, it is also worth mentioning that n = 1, 2 materials lack rotational degrees of freedom, which is important for enabling surface relaxation in n > 2 quasi-2D materials that facilitates exciton dissociation at surfaces favorable for solar cell applications. Consequently, compositions with n = 3–5 are common in quasi-2D perovskite solar cell applications1,8,12. In recent years, the progress has been made in improving the performance of both quasi-2D and 3D/2D-based PSCs. The best reported efficiencies for quasi-2D devices exceed 18%8,12, while the use of 3D/2D architecture results in efficiencies routinely exceeding 20%12,13,23. While quasi-2D perovskites can exhibit improved thermal and environmental stability compared to 3D perovskites12, the use of 3D/2D architecture offers a possibility of achieving an excellent compromise between high absorption and efficiency of 3D perovskites and superior ambient stability of 2D perovskites1,13. However, it should be noted that the stability of 3D/2D structures depends not only on the 2D perovskite used, but also on the 3D perovskite, with mixed cation films exhibiting improved stability compared to methylammonium lead iodide24.
The use of 2D and quasi-2D perovskite materials is a very promising approach for improving the device stability. However, improved understanding of their degradation pathways, in particular the stability dependence on the perovskite composition, is essential for targeted design of materials facilitating further stability improvements. Furthermore, while these materials are commonly more stable than 3D perovskites, all organic–inorganic halide perovskites have some degree of inherent instability upon exposure to ambient atmosphere, elevated temperature, illumination, and/or bias voltage. It is likely that multifaceted approaches, such as employing additives, grain boundary passivation, interfacial layers, and optimized device architecture in addition to the replacement of 3D perovskite with quasi-2D or 3D/2D perovskite will be needed to obtain lifetimes relevant for practical applications.
Material stability under different conditions
While the degradation mechanisms in 3D perovskites have been extensively studied, pathways in 2D and quasi-2D perovskites are far less commonly studied even though their understanding is essential for improving the device stability3. The scarcity of fundamental investigation of degradation mechanisms applies to all types of 2D and quasi-2D perovskites, with RP materials investigated more comprehensively compared to DJ and even more so ACI perovskites.
Comparison between different types of 2D/quasi-2D perovskites
Different types of 2D and quasi-2D materials have been reported for applications in solar cells and LEDs. RP9,10,13 and DJ8,10,11 perovskites, as well as DJ:RP perovskites14, were reported to result in improved solar cell stability compared to 3D perovskites, and the direct comparisons between devices with RP- and DJ-based perovskites resulted in significant stability advantage for DJ perovskite based devices10,15 under exposure to humidity10,15, thermal stress10,15, and illumination10,25. In addition, solar cells with DJ and ACI perovskites exhibited increased efficiency under illumination, different from those based on RP perovskites25. Similar to solar cells, in LEDs the use of both DJ20,21 and RP perovskites16,17,19 have been reported to result in improved stability compared to 3D perovskites16,17,18,20, and direct comparison between RP- and DJ-based LEDs revealed improved stability of DJ-based devices21. The device stability will be discussed in more detail in section “Device Stability Issues”.
Generally, DJ and ACI materials are considered to be more stable compared to RP materials due to the absence (in DJ) or reduction (in ACI) of weak van der Waals forces (between the spacer cations in the spacer cation bilayer in RP perovskites) and shorter interlayer distance8, as well as intrinsic stabilization of the layered structure due to hydrogen bonding of the spacer to inorganic slabs on both sides10. Shorter interlayer distance affects the dielectric screening and mitigates quantum confinement of the charge carriers8, and results in improved charge transport of DJ perovskites compared to RP counterparts and consequently better solar cell performance23. It was also proposed that additional out-of-plane lattice contraction occurs under illumination in DJ and ACI perovskites due to enhanced I-I interaction across the organic barrier, resulting in increased carrier mobility and consequently improved photovoltaic performance25. However, the presence of both mono- and di-ammonium cations within the perovskite structure was found to be able to either improve or worsen the stability compared to a pure DJ perovskite, depending on the cation used, which was attributed to defect passivation and film morphology and crystallinity improvement14. In addition, it has been reported that quasi-2D RP materials exhibit better ambient and thermal (100 °C) stability compared to DJ materials, while they exhibited comparable photostability (under 1 sun illumination in ambient)26. This was attributed to increased hydrophobicity and higher thermodynamic stability of quasi-2D RP films26. However, opposing claims can also be found in the literature, with DJ perovskites claimed to be more stable under high humidity, elevated temperature and illumination1. The difference in stability between RP and DJ perovskites was attributed to the presence of weak interaction between perovskite layers due to weak van der Waals forces between spacer cations in the bilayer1. Therefore, further study is needed to reconcile the findings of decreased stability of quasi-2D DJ perovskite films in comparison with quasi-2D RP films26 with other literature claims1, as well as the consistent findings of improved device stability for DJ perovskites in comparison with RP perovskites, including ambient atmosphere, heat, and/or illumination stability10,15,25. Such experiments should be performed under standardized conditions to facilitate easy comparisons, since it is possible that the results of thermal stress in inert atmosphere would yield different results from thermal stress in ambient air.
Ambient stability
Ambient stability testing commonly involves testing the sample in ambient air, which contains ~20% oxygen, room temperature, and varying percentages of ambient humidity, with humidity considered a key factor contributing to degradation27,28. The exact oxygen concentration and ambient temperature are often not specified, and no attempt is often made to control it. Another important detail which is commonly not specified is whether the samples are in the dark or exposed to ambient illumination. It would be expected that general trends in relative comparisons between different perovskite materials would be valid over a range of ambient exposure conditions, although it should be noted that the humidity-induced degradation in perovskites tends to accelerate for RH > 50%28. Despite expected validity of general trends over a range of relative humidities, it would be highly useful and desirable to standardize ambient stability testing conditions for perovskite materials and/or experimentally verify whether the same trends are applicable over a wide range of relative humidity conditions. It has been stated 2D and quasi-2D perovskites typically exhibit improved ambient stability compared to 3D perovskites, which is commonly attributed to the presence of bulky hydrophobic spacer cations3,9, and hydrophobicity can be further improved by using fluorinated spacer cations11. It has been shown that 2D perovskites maintain good stability with ambient dark storage over a period of 5 months (at 16% RH)6, which is definitely encouraging for the achievement of improved ambient stability in perovskite devices. Nevertheless, evidence exists that the moisture ingress into the 3D/2D or quasi-2D perovskite films still occurs. It has been reported that quasi-2D and 3D/2D perovskites in the presence of moisture (high humidity 78%28 to 90%27 RH) exhibit disproportionation, i.e., change in the presence of different n phases in the film, which results in the inhibition of further ingress of moisture into the film27,28. In addition, the presence of the capping 2D layer in 3D/2D structure was reported to result in reduced loss of volatile MAI, slowing down the degradation27. The disproportionation process is illustrated in Fig. 2a, b. However, it has also been reported that quasi-2D n = 2 and n = 3 RP perovskites degraded in the presence of moisture into n = 1 phase and PbI229. The differences among literature reports can likely be attributed to the differences in film preparation and composition, as well as variations in stability testing protocols. In contrast to RP perovskites, DJ perovskites were reported to form a 1D hydrated form upon exposure to humidity, as illustrated in Fig. 2c, d, and this hydration process is partly reversible by annealing (n = 1 exhibits higher reversibility compared to n = 2)30, illustrating that these materials may be highly promising due to possibility to regain performance losses in 3D/2D architectures.
Model of MAPbI3/quasi-2D heterojunctions in contact with water (a) schematic of 3D MAPbI3 film covered with PEA-based quasi-2D perovskite (n = 5) (b) illustration of disproportionation of n = 5 PEA-based perovskite into 3D perovskite MAPbI3 and more stable n = 3 or n = 2 quasi-2D perovskites, with the loss of I− ions indicated by violet circles and the loss of MA+ ions indicated by green circles illustrating the irreversible degradation of the 3D perovskite to volatile compounds and PbI2. Reprinted with permission from ref. 27. Copyright 2019 American Chemical Society; Schematic representations of structural changes in Dion-Jacobson perovskite structure in the presence of water (c) the (PDMA)PbI4 layered structure and d hydrated 1D (PDMA)Pb2I6·2H2O structure. Reprinted with permission from ref. 30. Copyright 2021 American Chemical Society.
In addition to the chemical composition of the 2D/quasi-2D perovskite, the sample quality and the role of defects should also be taken into account. For example, it has been reported that the degradation of BA2PbI4 crystals was dependent on the interaction between water molecules and uncoordinated defects, and that the defect passivation (which also resulted in increased hydrophobicity due to passivating chemical selected) could significantly improve ambient stability31.
Thermal stability
Thermal stability investigations for material stability have also not been standardized, with some studies investigating thermal stability using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) measurements6, while some examined thermal stability of perovskite films in inert atmosphere (at 100 °C)32, and others examined thermal stability (100 °C) in ambient26. As with other stability tests, the thermal stability of 2D/quasi-2D perovskites was dependent on the choice of spacer cation6. Heating was also found to induce a change in the crystal orientation in the film6. Thermal stability of 2D and quasi-2D perovskites in inert atmospheres was found to be improved compared to 3D perovskites, but there was a non-monotonic stability trend with the number of layers n32. However, thermal stress, as well as illumination or the combination of illumination and thermal stress, can lead to the diffusion of the spacer cation in 3D/2D perovskites into the bulk of the 3D layer, resulting in the formation of quasi-2D perovskite near the substrate24. The reorganization of 3D/2D film structure induced by heating or illumination can thus have significant effects on the device efficiency (changes at interfaces affecting charge collection) and stability (increased sensitivity to moisture after 2D capping layer is degraded).
Photostability
Photostability of different 2D/quasi-2D perovskites has been studied. Similar to other stability tests, the illumination conditions vary between different literature reports. For example, light sources used include 1 sun simulated solar illumination26, 450 nm blue LED (100 mW/cm2)32, and even laser pulses with subband gap excitation33. The use of different excitation sources, different testing environments, and different types of samples (single crystals, thin films) is expected to result in contradictions in observed stability trends. Even for the same type of sample, such as thin film, sample preparation may affect the results when degradation processes involve native defects. For the purpose of obtaining relevant information for solar cell applications, obviously the tests under 1 Sun simulated solar illumination (preferably including UV component of the spectrum) would be most relevant. Among different materials, it was reported that 2D bromides exhibited worse photostability compared to methylammonium lead bromide, while the opposite was the case for iodides, and the stability of different 2D perovskites was dependent on the spacer cation6. The stability for the same spacer cation was dependent on the number of octahedral layers n32.
The instability of these materials under illumination can be attributed to photooxidation32. Similar to 3D perovskites, 2D and quasi-2D perovskites are susceptible to photooxidation, which can be mitigated by edge-state passivation using triphenylphosphine oxide and 3-methyl-1-phenyl-2-phospholene-1-oxide19. It was also found that the degradation of single crystals was initiated from the edges, and it was hypothesized that distortion of lead halide octahedra under illumination leads to the loss of volatile spacer cations and that photostability is improved by encapsulation34. While encapsulation can indeed prevent the loss of volatile degradation products, it also prevents ingress of oxygen, so the suppression of photodegradation is consistent with photooxidation. In addition to degradation under illumination in ambient, changes have also been observed under inert atmosphere due to diffusion of cations and resulting reorganization of 3D/2D into quasi-2D structure24. Finally, it was also proposed that illumination contributes to the degradation of ammonium lead halides under illumination into Pb0 involving reactions with PbII–amide complex as an intermediate product35. Interestingly, DJ perovskites have been reported to exhibit inferior photostability under 1 sun illumination in ambient compared to RP perovskites26, but this observation is in contradiction with improved stability of DJ-based solar cells compared to RP-based ones10,25. Further investigation, preferably involving de-coupling different stressors as well as combining them under standardized testing conditions, would therefore be of interest. Such investigation should also involve different types of spacer cations, since it is possible that the comparisons between different classes of materials may not necessarily hold for all the possible spacer cation choices.
Additional consideration for photostability is phase segregation in mixed halide films. The use of spacer cations has been reported to reduce both illumination-induced phase separation in mixed halide solar cells, as well as electric field induced phase segregation in LEDs compared to 3D perovskites (including those with mixed small cations)18. However, photoinduced phase segregation was found to be strongly dependent on the spacer cation used36. Stiffness of the spacer cation was identified as a possible reason for suppressed halide segregation, but further investigation is necessary to understand how the choice of spacer cation affects the halide segregation. Photoinduced segregation in DJ perovskites has been less studied compared to RP materials37.
Other stability issues
Among other factors affecting the stability of perovskite devices is ion migration during operation, which contributes to ultimate device failure due to reactions between migrating halide anions and the electrodes. Different from 3D perovskites, ion migration in 2D and quasi-2D perovskites is proposed to be confined to two dimensions3. Additional factor contributing to the reduction of ion migration in 2D/quasi-2D perovskites is increased difficulty in the formation of vacancy-type defects in these materials, which consequently reduces ion migration38. Ion migration is reported to be reduced in solar cells with 3D/2D active layers9,12, as well as LEDs with 3D/2D emitting layers16,17 and quasi-2D emitting layers20 where migration of negatively charged defects (cation vacancies) also needs to be considered16. The halide ion mobility was found to be dependent on the number of octahedral layers n, with increasing activation energy and decreasing diffusion coefficients with decreasing number of layers39, in agreement with the expectation of ion migration reduction in 2D perovskites22,39,40. In addition, the reduction of ion migration is also dependent on the choice of spacer cation22,40, with more bulky π-conjugated cations or longer chain aliphatic cations more effective in reducing ion migration compared to short chain aliphatic spacer cations22,40.
The reduced ion migration in 3D/2D-based LEDs was attributed to increased migration barrier across 2D capping layer and improved film morphology17. Both RP9,12,16,17 and DJ perovskites20 contributed to mitigation of the ion migration during operation of the devices. However, it should be noted that ion migration in the presence of 2D perovskites still occurs13,36, but it can be further suppressed by insertion of additional interfacial layers13. In addition, the edges of quasi-2D perovskites can exhibit low stability, with the formation of 3D perovskite at the edges, which was attributed to the substitution of the spacer cation by the methylammonium cation, although exact process was not fully clear41. The substitution of the spacer cation by methylammonium cation can also be achieved by immersion in solution42.
Other challenges include retaining the phase purity and crystal orientation, as these affect the device performance12. While efforts have been made to control phase purity and crystal orientation during deposition12, it has been shown that elevated temperature can result in crystal reorientation3, while the changes in phase composition can occur with ambient exposure27,28,42 or illumination24. However, it was also found that the choice of the spacer cation had significant effect on the stability of 2D/3D interface, as illustrated in Fig. 3. The cation which formed pure phase 2D capping layer resulted in stable 3D/2D interface under ambient exposure and elevated temperature, which could possibly be related to the differences in packing of organic cations (exact mechanism requires further research)43. The packing and/or hydrogen bonding of the organic cations can also significantly affect charge transport and solar cell performance23.
PL spectra (450 nm excitation) of fresh and aged (4 months) 2D/3D perovskite films for a 2-TMAI, b 3-TMAI, and c 2-TEAI–based 2D perovskite; PL spectra upon thermal stress (heating at 50 °C) of 2D/3D perovskite films for d 2-TMAI, e 3-TMAI, and f 2-TEAI–based 2D perovskite; g Illustration of the proposed interfacial mechanism. Reprinted with permission from ref. 43. Copyright 2020 Royal Society of Chemistry.
Finally, similar to 3D perovskites, 2D perovskites are also sensitive to exposure to vacuum, which results in the loss of volatile organic components and the formation of metallic lead44. This has important implications on the accuracy of characterization measurements on these materials which are performed in vacuum44.
It should also be noted that the field of studying the stability of 2D and quasi-2D perovskites would generally benefit from transfer of characterization methods and good practices in characterization as well as reporting which are more common in 3D perovskites. In particular, comprehensive investigation of the material stability including detailed structural characterization under conditions relevant for device operation is expected to play a key role in developing accurate understanding of the degradation processes involved. For example, studies of ion migration would benefit from employing consistent methodologies and utilizing different measurement techniques, such as comprehensive impedance characterization (AC/DC conductivity)45 and transient ion drift measurements46. Transient ion drift measurements in particular would be interesting, especially for examining the effects of film preparation conditions on the small cation diffusion in quasi-2D materials, as the activation energy of MA+ was shown to be dependent on film preparation in 3D perovskite46. It should be noted that the interpretation of the measured data is dependent on the adopted models, which can result in significant variation in mobile ion densities depending on the model used47. The validity of the models used in 3D materials needs to be examined for 2D and quasi-2D materials, including taking into account significant anisotropy of in-plane and out-of-plane properties.
Device stability issues
Unlike the studies of material stability, protocols have been proposed for investigation of the stability of PSCs48 and perovskite LEDs49,50. Thus, it would be useful to align material stability studies to device stability, for example to use temperatures relevant for modified ISOS protocols proposed for PSCs48, namely 65 and 85 °C. Out of these, 85 °C would be more relevant, as the test temperature of 65 °C has been originally introduced into ISOS protocols due to limitations in thermal stability of some common organic charge transport layers, such as 2,2′,7,7′-Tetrakis-(N,N-di-4-methoxyphenylamino)-9,9′-spirobifluorene (Spiro-OMeTAD). For humidity there is a wider range of relevant values, but in all cases humidity must be monitored and reported, while light source should also be clearly specified (type, intensity, spectrum, calibration, presence of filters)48. While the protocols for stability testing of LEDs at present do not make clear specifications on environmental conditions, reporting exact conditions during tests would be useful. Making an estimate on the temperature increase of the perovskite material during bias would also be very useful to establish needed material stability testing protocols relevant for LEDs.
It should also be noted that other components in the device, namely electrodes, charge transport layers, and interfaces are all contributing to the device degradation51. Many materials commonly used as charge transport layers (CTLs) are unstable under illumination, and also it is necessary to pay attention to the interfaces in the devices to ensure favorable energy level alignment for efficient charge collection and avoiding accumulation of charges51. The passivation of defects at interfaces is generally beneficial for device performance, but it should be noted that interface passivation which involves charge transfer can result in changes in energy level alignment not only at that particular interface but also other interfaces in the device52. This can be beneficial for inverted solar cells, where unfavorable energy level alignment for electron collection can hinder employing 2D surface modifications at the top surface, which regardless of energy level alignment, increase device resistance to exposure to humidity52. While the use of quasi-2D and 3D/2D perovskite active layers generally results in stability improvements compared to 3D perovskites8,9,10,11,12,13,14,53,54,55,56,57, and in case of 3D/2D often also improved efficiency, it is not the only possible solution to the problem, nor is it a replacement of the need for passivation of defects and interfaces in the PSCs. For example, it has been shown that the use of additives in precursor solutions improves the performance of both 3D and 3D/2D devices, with the 3D/2D without the additive having higher efficiency than 3D with the additive and overall best PCE achieved by 3D/2D device with additive53. In 3D/2D architecture, 2D layer can function as top interface modification, but further interface optimization to additionally suppress ion migration and reduce recombination losses has been shown to benefit both efficiency and stability13. A variety of spacer cations has been demonstrated to offer stability benefits57,58,59,60, and attempts to classify them and offer guidance on spacer cation selection have been made60, although there is clearly need for better understanding of properties of these materials and their application in devices57. In addition to conventional 3D/2D structures, the use of same small cation halides for bulk and surface passivation55 and the use of mixed organic ammonium halides to achieve 1D/2D surface passivation56 should be mentioned. Although it has been claimed that quasi-2D and 3D/2D perovskites could likely replace 3D perovskites as active layers57, it is worthwhile to also optimize other device components carefully (with particular attention paid to defect passivation and interface modifications) to obtain high performance devices both in terms of efficiency and stability. Different approaches, such as the use of additives57,61,62,63, interface modifications/interfacial layers at different interfaces64,65,66 and minimization of ion migration in general (such as extrinsic ion migration due to electrode choices)67 are all important contributors to the development of stable PSCs. While interface modifications play a key role in the improvement of efficiency and stability of PSCs, it should be noted that energy level alignment at the interfaces is really critical not only for device efficiency but also for device stability66. Charge accumulation due to unfavorable energy level alignment across the interface can result in increased ion migration resulting in reduced stability67.
Similar to solar cells, other device components (charge transport layers, electrodes) also contribute to device degradation in LEDs, and interfaces play a key role in device stability. However, there are also differences – the lack of simulated solar illumination, need to have balanced charge injection, different compositions of perovskite materials (in particular for shorter wavelength spectral ranges), as well as different bias conditions. As the LEDs operate at higher voltages than solar cells, ion migration is obviously exacerbated in these devices. The use of 2D materials in LEDs reduces intergrain ion migration, although devices are still susceptible to ion migration50, and overall results in improved stability15,16,17,18,19,20. Similar to solar cells, the performance of these devices can be enhanced by using various additives68,69,70,71, which can passivate defects as well as modify the crystallization of the quasi-2D perovskite layer, as well as interface modifications, where the use of amine-terminated carbon nanodots has been recently shown to yield dramatic improvements in efficiency, brightness, and stability compared to devices without carbon nanodots72. However, the stability of LEDs is still lagging significantly behind that of solar cells, and the advantages of the use of quasi-2D materials are less significant compared to solar cells, with some quasi-2D-based devices even with passivated interface exhibiting T80 of the order of tens of minutes72. The problem of balancing the charge injection, reducing Joule heating and further suppressing ion migration are key issues for improving the LED stability.
Outlook
One of the biggest challenges which still remains is improving our understanding of the relationship between the structure of 2D and quasi-2D perovskites, in particular the choice of the spacer cation, and their properties3. It is known that this has a significant effect on the stability, for example stability under illumination6,11,43, thermal stability11, and ambient exposure43. Some relative comparisons, such as that of alkyl chain spacers of different length, have been made73, but alkyl chain spacers represent just one out of many different groups of spacer cations classified by their chemical composition, namely linear and branched alkyl chains, conjugated spacers, aromatic spacers, aromatic heterocyclic spacers, and hetero-atom containing spacers (S, O, F, Cl, Br, I)60. It should also be noted that not all spacer cations lead to the formation of layered 2D structures74,75, and further studies are needed to improve our understanding of the effect of spacer cation interactions and ordering on the possibility of formation of layered 2D structures. The requirement of good spacer cation solubility is also important for the formation of good quality 2D perovskite, while high dielectric constant is desirable to reduce quantum and dielectric confinement60. In addition, although structure-property relationships are generally not well understood, one of the consistent findings is that halogenated spacers may offer advantages due to increased hydrophobicity and non-covalent interactions in the crystal lattice that improve stability11. Although increased hydrophobicity can be achieved by, for example, adjusting alkyl chain length73, fluorinated spacers57,76,77 are of particular interest, since they can not only offer increased hydrophobicity but also enable improved charge transport properties57. It should be noted that while interactions among spacer cations can be beneficial and stabilize the structure, excessive interactions can result in lattice distortions which are detrimental60. Nevertheless, non-covalent interactions can play a significant role in stabilizing halide perovskites, among which halogen bonding has been recently recognized to play an important role in improving stability against moisture and reducing halide migration78. Halogen bonding (XB) occurs between XB donor, commonly fluorinated moiety, and XB acceptor (another halogen, N or O)78, and this effect can be another contributing factor to improved stability observed with fluorinated spacers. However, further study is needed to understand the role of XB in 2D perovskites containing halogenated spacers78. In general, further studies are needed to fully understand the effects of spacer cation choice, since even a relatively small difference, such as one additional carbon atom in 2-thiopheneethylammonium iodide compared to 2-thiophenemethylammonium iodide, can have significant effect on the stability of 3D/2D interfaces43. Another area of interest is the development of layered perovskites which incorporate semiconducting organic moieties to improve their electronic properties79,80. In addition, theoretical studies are expected to make a significant contribution to elucidation of the relationships between the choice of spacer cation, including intermolecular interactions between spacer cations, and the properties of resulting perovskite, but in this Perspective we are primarily concerned with experimental investigations.
In addition to this major area requiring further study, significant area of interest is the clarification of the structural reorganization of these materials (transformations of 3D/2D into quasi-2D perovskite, changes in phase composition of quasi-2D perovskites, etc.) upon exposure to ambient environment, illumination, and/or elevated temperature. This has important implications on both device efficiency as well as device stability43. Yet, it is still not understood how exactly does this process occur nor why some spacer cations show greater resistance to such transformations. Nevertheless, due to significant advantage of 3D/2D solar cells in terms of efficiency and their significantly improved stability compared to those with 3D active layers, it is necessary to study stability and transformations of 3D/2D structure in more detail to develop strategies leading to improved stability of 3D/2D interface. This will likely include grain boundary passivation in 3D layer, and suitable selection of spacer cations (or a mixture of cations) to optimize the 2D layer. It should be noted that while papers comparing their performance generally show improved efficiency and/or stability of 3D/2D devices compared to 3D control devices, due to less stringent test conditions (for example, in solar cells dark storage remains the most commonly reported due to impressive times achieved, but this is also the least meaningful testing condition since illumination plays a critical role in device stability) and variations in perovskite compositions, device architectures, and encapsulation it is difficult to compare the results of these tests to other published work. For commercialization, it is critically important to demonstrate stability under more stringent testing conditions, such as damp heat and outdoor testing for example, where the devices need to exhibit stability at high temperature, high humidity (damp heat) and under illumination with varying ambient conditions (outdoor testing). In Table 1 we have summarized some reports of these two testing conditions for different 3D and 3D/2D devices selected either on the basis of high efficiency or good stability13,81,82,83,84,85,86,87,88,89,90,91,92. It can be clearly observed that more stability testing under stringent conditions (outdoor testing, IEC 61215 tests (damp heat, thermal cycling)) is needed for 3D/2D devices as well as quasi-2D devices, since these tests are needed to conclusively demonstrate stability improvements and commercialization potential. It should be noted that appropriate encapsulation92,93 should be employed for such tests to be successful, due to more harsh testing conditions which can cause encapsulation failure. Standardization of encapsulation protocols would be very helpful to facilitate further development, since at present variations prevent direct comparisons of reported results under practically relevant accelerated aging conditions.
Finally, halide perovskite studies often report contradictory observations primarily due to differences in material/device preparation, as well as different testing conditions. Thus, this area of research would also benefit from (a) more comprehensive reporting of the experimental details, including small details on sample preparation (b) more comprehensive characterization of the samples, since some factors such as grain size can have significant effect on the properties and (c) more standardized testing conditions for material stability (preferably closely matching humidity and temperature levels employed in device testing). An example of a detailed reporting of synthesis procedures94 illustrates how even small experimental details affect the perovskite properties. Quasi-2D materials in our experience are even more sensitive to the synthesis details, and thus it is critical to improve the level of detail on sample synthesis to reduce contradictory reports in the literature.
References
Gangadharan, D. T. & Ma, D. L. Searching for stability at lower dimensions: current trends and future prospects of layered perovskite solar cells. Energy Environ. Sci. 12, 2860–2889 (2019).
Liu, P., Yu, S. & Xiao, S. Research progress on two-dimensional (2D) halide organic–inorganic hybrid perovskites. Sustain. Energy Fuels 5, 3950–3978 (2021).
Li, X., Hoffman, J. M. & Kanatzidis, M. G. The 2D halide perovskite rulebook: how the spacer influences everything from the structure to optoelectronic device efficiency. Chem. Rev. 121, 2230–2291 (2021).
Mercier, N. Hybrid halide perovskites: discussions on terminology and materials. Angew. Chem. Int. Ed. 58, 17912–17917 (2019).
Tremblay, M. H. et al. Structures of (4-Y‑C6H4CH2NH3)2PbI4 {Y = H, F, Cl, Br, I}: tuning of hybrid organic inorganic perovskite structures from Ruddlesden–Popper to Dion–Jacobson Limits. Chem. Mater. 31, 6145–6153 (2019).
Vasileiadou, E. S. et al. Shedding light on the stability and structure–property relationships of two-dimensional hybrid lead bromide perovskites. Chem. Mater. 33, 5085–5107 (2019).
Lei, N., Pan, L., Ye, T., Chen, S. & Wang, X. Whether addition of phenethylammonium ion is always beneficial to stability enhancement of MAPbI3 perovskite film? Adv. Mater. Interfaces 7, 2000197 (2020).
Gong, J., Hao, M. W., Zhang, Y. L., Liu, M. Z. & Zhou, Y. Y. Layered 2D halide perovskites beyond the ruddlesden–popper phase: tailored interlayer chemistries for high-performance solar cells. Angew. Chem. Int. Ed. 61, e202112022 (2022).
Chen, P. et al. In situ growth of 2D perovskite capping layer for stable and efficient perovskite solar cells. Adv. Funct. Mater. 28, 1706923 (2018).
Ahmad, S. et al. Dion-Jacobson phase 2D layered perovskites for solar cells with ultrahigh stability. Joule 3, 794–806 (2019).
Lv, G. W. et al. Multiple-noncovalent-interaction-stabilized layered Dion–Jacobson perovskite for efficient solar cells. Nano Lett 21, 5788–5797 (2021).
Zhao, X. M., Liu, T. R. & Loo, Y. L. Advancing 2D perovskites for efficient and stable solar cells: challenges and opportunities. Adv. Mater. 34, 2105849 (2022).
Chen, W. et al. Interfacial stabilization for inverted perovskite solar cells with long-term stability. Sci. Bull. 66, 991–1002 (2021).
Fu, P. et al. Dion–Jacobson and Ruddlesden–Popper double-phase 2D perovskites for solar cells. Nano Energy 88, 106249 (2021).
Ma, C. Q., Shen, D., Ng, T. W., Lo, M. F. & Lee, C. S. 2D perovskites with short interlayer distance for high-performance solar cell application. Adv. Mater. 30, 1800710 (2018).
Han, T. H. et al. Surface-2D/Bulk-3D heterophased perovskite nanograins for long-term-stable light-emitting diodes. Adv. Mater. 32, 1905674 (2020).
Kim, H. et al. Proton-transfer-induced 3D/2D hybrid perovskites suppress ion migration and reduce luminance overshoot. Nat. Commun. 11, 3378 (2020).
Xiao, Z. G. et al. Mixed-halide perovskites with stabilized bandgaps. Nano Lett 17, 6863–6869 (2017).
Quan, L. N. et al. Edge stabilization in reduced-dimensional perovskites. Nat. Commun. 11, 170 (2020).
Ngai, K. H. et al. Enhanced electrochemical stability by alkyldiammonium in Dion–Jacobson perovskite toward ultrastable light-emitting diodes. Adv. Opt. Mater. 9, 2100243 (2021).
Shang, Y. Q. et al. Highly stable hybrid perovskite light-emitting diodes based on Dion–Jacobson structure. Sci. Adv. 5, eaaw8072 (2019).
Sheng, X., Li, Y. H., Xi, M. & Shi, E. Z. Quasi-2D halide perovskite crystals and their optoelectronic applications, J. Mater. Chem. A https://doi.org/10.1039/d2ta02219b (2022).
Zhang, F. et al. Metastable Dion-Jacobson 2D structure enables efficient and stable perovskite solar cells. Science 375, 71–76 (2022).
Fiorentino, F., Albaqami, M. D., Poli, I. & Petrozza, A. Thermal- and light-induced evolution of the 2D/3D interface in lead-halide perovskite films. ACS Appl. Mater. Interfaces, https://doi.org/10.1021/acsami.1c09695 (2022).
Li, W. B. et al. Light-activated interlayer contraction in two-dimensional perovskites for high-efficiency solar cells. Nature Nanotechnology 17, 45–52 (2022).
Vasileiadou, E. S. et al. Insight on the stability of thick layers in 2D Ruddlesden–Popper and Dion–Jacobson lead iodide perovskites. J. Am. Chem. Soc. 143, 2523–2536 (2021).
Schlipf, J. et al. Shedding light on the moisture stability of 3D/2D hybrid perovskite heterojunction thin films. ACS Appl. Energy Mater 2, 1011–1018 (2019).
Wygant, B. R. et al. Probing the degradation chemistry and enhanced stability of 2D organolead halide perovskites. J. Am. Chem. Soc. 141, 18170–18181 (2019).
Tang, J. B. et al. Imaging the moisture-induced degradation process of 2D organolead halide perovskites. ACS Omega https://doi.org/10.1021/acsomega.1c06989 (2022).
Dučinskas, A., Milić, J. V., Maier, J. & Grätzel, M. Unravelling the behavior of dion−jacobson layered hybrid perovskites in humid environments. ACS Energy Lett. 6, 337–344 (2021).
Zha, Y. F. et al. Structural characterizations on the degradation of 2D organic–inorganic hybrid perovskites and its enlightenment to improved stability. Nanotechnology 33, 285702 (2022).
Udalova, N. N. et al. Nonmonotonic photostability of BA2MAn−1PbnI3n+1 homologous layered perovskites. ACS Appl. Mater. Interfaces 14, 961–970 (2022).
Aharon, S. et al. 2D Pb-halide perovskites can self-heal photodamage better than 3D ones. Adv. Funct. Mater. 32, 2113354 (2022).
Fang, H. H. et al. Unravelling light-induced degradation of layered perovskite crystals and design of efficient encapsulation for improved photostability. Adv. Funct. Mater. 28, 1800305 (2018).
Hu, J. N., Kerner, R. A., Pelczer, I., Rand, B. P. & Schwartz, J. Organoammonium-Ion-based perovskites can degrade to Pb0 via Amine–Pb(II) coordination. ACS Energy Lett. 6, 2262–2267 (2021).
Mathew, P. S., DuBose, J. T., Cho, J. S. & Kamat, P. V. Spacer cations dictate photoinduced phase segregation in 2D mixed halide perovskites. ACS Energy Lett. 6, 2499–2501 (2021).
Wang, Y. R. et al. Photo de-mixing in Dion–Jacobson two-dimensional mixed halide perovskites. Adv. Energy Mater. 12, 2200768 (2022).
Xiao, X. et al. Suppressed ion migration along the in-plane direction in layered perovskites. ACS Energy Lett 3, 684–688 (2018).
Cho, J., DuBose, J. T., Le, A. N. T., Kamat, P. V. Suppressed halide ion migration in 2D lead halide perovskites. ACS Mater. Lett. 2, 565–570 (2020).
Wygant, B. R., Ye, A. Z., Dolocan, A. & Mullins, C. B. Effects of alkylammonium choice on stability and performance of quasi-2D organolead halide perovskites. J. Phys. Chem. C 124, 10887–10897 (2020).
Qin, Z. J. et al. Spontaneous formation of 2D/3D heterostructures on the edges of 2D Ruddlesden–Popper hybrid perovskite crystals. Chem. Mater. 32, 5009–5015 (2020).
Zhao, C. Y., Tian, W. M., Leng, J., Zhao, Y. & Jin, S. Y. Controlling the property of edges in layered 2D perovskite single crystals. J. Phys. Chem. Lett. 10, 3950–3954 (2019).
Sutanto, A. A. et al. Dynamical evolution of the 2D/3D interface: a hidden driver behind perovskite solar cell instability. J. Mater. Chem. A 8, 2343–2348 (2020).
Hofstetter, Y. J. et al. Vacuum-induced degradation of 2D perovskites. Front. Chem. 8, 66 (2020).
Pellet, N. et al. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. 53, 3151–3157 (2014).
Futscher, M. H. et al. Quantification of ion migration in CH3NH3PbI3 perovskite solar cells by transient capacitance measurements. Mater. Horizons 6, 1497–1503 (2019).
Futscher, M. H. & Milić, J. V. Mixed conductivity of hybrid halide perovskites: emerging opportunities and challenges. Front. Energy Res. 9, 629074 (2021).
Khenkin, M. V. et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 5, 35–49 (2020).
Anaya, M. et al. Best practices for measuring emerging light-emitting diode technologies. Nat. Photon. 13, 818–821 (2019).
Woo, S.-J., Kim, J. S. & Lee, T.-W. Characterization of stability and challenges to improve lifetime in perovskite LEDs. Nat. Photon. 15, 630–634 (2021).
Wei, J. et al. Mechanisms and suppression of photoinduced degradation in perovskite solar cells. Adv. Energy Mater. 11, 2002326 (2021).
Wang, Y. et al. Improvement in the performance of inverted 3D/2D perovskite solar cells by ambient exposure. Solar RRL 6, 2200224 (2022).
Kareem, S. H., Elewi, M. H., Naji, A. M., Ahmed, D. S. & Mohammed, M. K. Efficient and stable pure α-phase FAPbI3 perovskite solar cells with a dual engineering strategy: additive and dimensional engineering approaches. Chem. Eng. J. 443, 136469 (2022).
Wang, C. et al. Minimizing voltage deficit in methylammonium-free perovskite solar cells via surface reconstruction. Chem. Eng. J. 444, 136622 (2022).
Zhang, X. et al. Dual optimization of bulk and surface via guanidine halide for efficient and stable 2D/3D hybrid perovskite solar cells. Adv. Energy Mater. 12, 2201105 (2022).
Mozaffari, N. et al. Above 23% efficiency by binary surface passivation of perovskite solar cells using guanidinium and octylammonium spacer cations. Solar RRL 6, 2200355 (2022).
Kim, E.-B., Akhtar, M. S., Shin, H.-S., Ameen, S. & Nazeeruddin, M. K. A review on two-dimensional (2D) and 2D-3D multidimensional perovskite solar cells: perovskites structures, stability, and photovoltaic performances. J. Photochem. Photobiol. C Photochem. Rev. 48, 100405 (2021).
Wu, G. et al. Surface passivation using 2D perovskites toward efficient and stable perovskite solar cells. Adv. Mater. 34, 2105635 (2022).
Malouangou, M. D. et al. Recent progress in perovskite materials using diammonium organic cations toward stable and efficient solar cell devices: Dion–Jacobson. Energy Technol. 10, 2101155 (2022).
Cao, Q. et al. Two-dimensional perovskites: impacts of species, components, and properties of organic spacers on solar cells. Nano Today 43, 101394 (2022).
Xiong, S. et al. Direct observation on p-to n-type transformation of perovskite surface region during defect passivation driving high photovoltaic efficiency. Joule 5, 467–480 (2021).
Fu, L. et al. Defect passivation strategies in perovskites for an enhanced photovoltaic performance. Energy Environ. Sci. 13, 4017–4056 (2020).
Tang, S. et al. Simultaneous bulk and surface defect passivation for efficient inverted perovskite solar cells. J. Phys. Chem. Lett. 13, 5116–5122 (2022).
Gao, Z. W., Wang, Y. & Choy, W. C. Buried interface modification in perovskite solar cells: a materials perspective. Adv. Energy Mater. 12, 2104030 (2022).
Li, Z. et al. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 376, 416–420 (2022).
Tan, S. et al. Stability-limiting heterointerfaces of perovskite photovoltaics. Nature 605, 268–273 (2022).
Yan, X. et al. Ion migration in hybrid perovskites: classification, identification, and manipulation. Nano Today 44, 101503 (2022).
Liu, C. et al. Highly efficient quasi-2D green perovskite light‐emitting diodes with bifunctional amino acid. Adv. Opt. Mater. 10, 2200276 (2022).
Xiang, T. et al. 12-Crown-4 ether assisted in-situ grown perovskite crystals for ambient stable light emitting diodes. Nano Energy 95, 107000 (2022).
Mishra, J. K. et al. Defect passivation using a phosphonic acid surface modifier for efficient RP perovskite blue-light-emitting diodes. ACS Appl. Mater. Interfaces 14, 34238–34246 (2022).
Ma, J., Yang, L., Zhang, Y., Kuang, Y. & Shao, M. Rearranging the phase distribution of quasi-2D perovskite for efficient and narrow emission perovskite light-emitting diodes. J. Phys. Chem. Lett. 13, 4739–4746 (2022).
Dong, W. et al. Amine-terminated carbon dots linking hole transport layer and vertically oriented quasi-2D perovskites through hydrogen bonds enable efficient LEDs. ACS Nano 16, 9679–9690 (2022).
Kim, H. et al. Optimal interfacial engineering with different length of alkylammonium halide for efficient and stable perovskite solar cells. Adv. Energy Mater. 9, 1902740 (2019).
Kamminga, M. E. et al. Confinement effects in low-dimensional lead iodide perovskite hybrids. Chem. Mater. 28, 4554–4562 (2016).
Ovčar, J. et al. Mixed halide ordering as a tool for the stabilization of Ruddlesden–Popper Structures. Chem. Mater. 34, 4286–4297 (2022).
Hope, M. A. et al. Nanoscale phase segregation in supramolecular π-templating for hybrid perovskite photovoltaics from NMR crystallography. J. Am. Chem. Soc. 143, 1529–1538 (2021).
Liu, Y. et al. Ultrahydrophobic 3D/2D fluoroarene bilayer-based water-resistant perovskite solar cells with efficiencies exceeding 22%. Sci. Adv. 5, eaaw2543 (2019).
Ball, M. L., Milić, J. V. & Loo, Y.-L. The Emerging Role of Halogen Bonding in Hybrid Perovskite Photovoltaics. Chem. Mater. 34, 2495–2502 (2022).
Milić, J. V. Multifunctional layered hybrid perovskites. J. Mater. Chem. C 9, 11428–11443 (2021).
Gao, Y., Wei, Z. T., Hsu, S. N., Boudouris, B. W. & Dou, L. T. Two-dimensional halide perovskites featuring semiconducting organic building blocks. Mater. Chem. Front. 4, 3400–3418 (2020).
Azmi, R. et al. Damp heat–stable perovskite solar cells withtailored-dimensionality 2D/3D heterojunctions. Science 376, 73–77 (2022).
Jang, Y. W. et al. Intact 2D/3D halide junction perovskite solar cells via solid-phase in-plane growth. Nat. Energy 6, 63–71 (2021).
Cheng, F. et al. 85 °C/85%-Stable n-i-p perovskite photovoltaics with NiOx hole transport layers promoted by perovskite quantum dots. Adv. Sci. https://doi.org/10.1002/advs.202201573 (2022).
Mohammadi, M. et al. Encapsulation strategies for highly stable perovskite solar cells under severe stress testing: damp heat, freezing, and outdoor illumination conditions. ACS Appl. Mater. Interfaces 13, 45455–45464 (2021).
Peng, J. et al. Nanoscale localized contacts for high fill factors in polymer-passivated perovskite solar cells. Science 371, 390–395 (2021).
Song, S. et al. Selective defect passivation and topographical control of 4-dimethylaminopyridine at grain boundary for efficient and stable planar perovskite solar cells. Adv. Energy Mater. 11, 2003382 (2021).
Kim, Y. et al. Methoxy-functionalized triarylamine-based hole-transporting polymers for highly efficient and stable perovskite solar cells. ACS Energy Lett 5, 3304–3313 (2020).
Lv, Y., Zhang, H., Liu, R., Sun, Y. & Huang, W. Composite encapsulation enabled superior comprehensive stability of perovskite solar cells. ACS Appl. Mater. Interfaces 12, 27277–27285 (2020).
Jošt, M. et al. Perovskite solar cells go outdoors: field testing and temperature effects on energy yield. Adv. Energy Mater. 10, 2000454 (2020).
Matsui, T. et al. Compositional engineering for thermally stable, highly efficient perovskite solar cells exceeding 20% power conversion efficiency with 85 °C/85% 1000 h stability. Adv. Mater. 31, 1806823 (2019).
Bella, F. et al. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science 354, 203–206 (2016).
Emery, Q. et al. Encapsulation and outdoor testing of perovskite solar cells: comparing industrially relevant process with a simplified lab procedure. ACS Appl. Mater. Interfaces 14, 5159–5167 (2022).
Wang, Y. T. et al. Djurišić, encapsulation and stability testing of perovskite solar cells for real life applications. ACS Mater. Au 2, 215–236 (2022).
Bai, S. et al. Reproducible planar heterojunction solar cells based on one-step solution-processed methylammonium lead halide perovskites. Chem. Mater. 29, 462–473 (2017).
Acknowledgements
This work was supported by the Seed Funding for Basic Research and Seed Funding for Strategic Interdisciplinary Research Scheme of the University of Hong Kong, RGC GRF projects 17301520, RGC CRF projects 7018-20G and 7035-20G, NSFC project 6207032617, project PZS-2019-02-2068 financed by the “Research Cooperability” Program of the Croatian Science Foundation funded by the European Union from the European Social Fund under the Operational Program Efficient Human Resources 2014–2020.
Author information
Authors and Affiliations
Contributions
A.B.D. prepared the first draft of the paper, A.B.D., T.L.L., J. P., and A.M.C.N. wrote the paper, T.L.L., J.P., I.A., and A.A.S. prepared figures and table.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Jovana Milic and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary handling editor: John Plummer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leung, T.L., Ahmad, I., Syed, A.A. et al. Stability of 2D and quasi-2D perovskite materials and devices. Commun Mater 3, 63 (2022). https://doi.org/10.1038/s43246-022-00285-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-022-00285-9
This article is cited by
-
Solution-processed memristors: performance and reliability
Nature Reviews Materials (2024)
-
Quasi-two dimensional Ruddlesden-Popper halide perovskites for laser applications
Frontiers of Physics (2024)
-
Potential and perspectives of halide perovskites in light emitting devices
Nano Convergence (2023)
-
Ammonium cations with high pKa in perovskite solar cells for improved high-temperature photostability
Nature Energy (2023)
-
Structural, electronic, and optical properties of lower-dimensional hybrid perovskite lead-iodide frameworks + SOC via density functional theory
Emergent Materials (2023)