Abstract
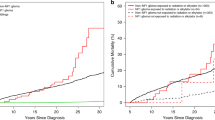
Pediatric glioma therapy has evolved to delay or eliminate radiation for low-grade tumors. This study examined these temporal changes in therapy with long-term outcomes in adult survivors of childhood glioma. Among 2,501 5-year survivors of glioma in the Childhood Cancer Survivor Study diagnosed 1970–1999, exposure to radiation decreased over time. Survivors from more recent eras were at lower risk of late mortality (≥5 years from diagnosis), severe/disabling/life-threatening chronic health conditions (CHCs) and subsequent neoplasms (SNs). Adjusting for treatment exposure (surgery only, chemotherapy, or any cranial radiation) attenuated this risk (for example, CHCs (1990s versus 1970s), relative risk (95% confidence interval), 0.63 (0.49–0.80) without adjustment versus 0.93 (0.72–1.20) with adjustment). Compared to surgery alone, radiation was associated with greater than four times the risk of late mortality, CHCs and SNs. Evolving therapy, particularly avoidance of cranial radiation, has improved late outcomes for childhood glioma survivors without increased risk for late recurrence.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The CCSS is a US National Cancer Institute-funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. CCSS data are publicly available on dbGaP at https://www.ncbi.nlm.nih.gov/gap/ through its accession number phs001327.v2.p1. and on the St. Jude Survivorship Portal within the St. Jude Cloud at https://survivorship.stjude.cloud/. In addition, utilization of the CCSS data that leverages the expertise of CCSS Statistical and Survivorship research and resources will be considered on a case-by case basis. For this utilization, a research Application Of Intent followed by an Analysis Concept Proposal must be submitted for evaluation by the CCSS Publications Committee. Users interested in utilizing this resource are encouraged to visit http://ccss.stjude.org. Full analytical data sets associated with CCSS publications since January of 2023 are also available on the St. Jude Survivorship Portal at https://viz.stjude.cloud/community/cancer-survivorship-community~4/publications. Source data are provided with this paper.
References
Ostrom, Q. T. et al. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-Oncol. 16, x1–x36 (2015).
Bandopadhayay, P. et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat. Genet. 48, 273–282 (2016).
Ryall, S. et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 37, 569–583 (2020).
Shi, W. et al. Mechanism and protection of radiotherapy induced sensorineural hearing loss for head and neck cancer. BioMed Res. Int. 2021, 3548706 (2021).
Armstrong, G. T. et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J. Natl Cancer Inst. 101, 946–958 (2009).
Merchant, T. E., Conklin, H. M., Wu, S., Lustig, R. H. & Xiong, X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 27, 3691–3697 (2009).
Ullrich, N. J. et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology 68, 932–938 (2007).
Pierce, S. M., Barnes, P. D., Loeffler, J. S., McGinn, C. & Tarbell, N. J. Definitive radiation therapy in the management of symptomatic patients with optic glioma. Survival and long-term effects. Cancer 65, 45–52 (1990).
Hauptmann, M. et al. Brain cancer after radiation exposure from CT examinations of children and young adults: results from the EPI-CT cohort study. Lancet Oncol. 24, 45–53 (2023).
Meadows, A. T. et al. Second malignant neoplasms in children: an update from the Late Effects Study Group. J. Clin. Oncol. 3, 532–538 (1985).
Meadows, A. T. et al. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet 2, 1015–1018 (1981).
Hancock, S. L., Cox, R. S. & McDougall, I. R. Thyroid diseases after treatment of Hodgkin’s disease. N. Engl. J. Med. 325, 599–605 (1991).
Constine, L. S. et al. Thyroid dysfunction after radiotherapy in children with Hodgkin’s disease. Cancer 53, 878–883 (1984).
Wohl, M. E., Griscom, N. T., Traggis, D. G. & Jaffe, N. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics 55, 507–516 (1975).
Benoist, M. R. et al. Effects of pulmonary function of whole lung irradiation for Wilm’s tumour in children. Thorax 37, 175–180 (1982).
Krishnatry, R. et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer 122, 1261–1269 (2016).
Rosenstock, J. G. et al. Chiasmatic optic glioma treated with chemotherapy. A preliminary report. J. Neurosurg. 63, 862–866 (1985).
Sumer, T. et al. Chemotherapy in recurrent noncystic low-grade astrocytomas of the cerebrum in children. J. Surg. Oncol. 10, 45–54 (1978).
Friedman, H. S. et al. Treatment of children with progressive or recurrent brain tumors with carboplatin or iproplatin: a Pediatric Oncology Group randomized phase II study. J. Clin. Oncol. 10, 249–256 (1992).
Ater, J. L. et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J. Clin. Oncol. 30, 2641–2647 (2012).
Ater, J. L. et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer 122, 1928–1936 (2016).
de Blank, P. M., Berman, J. I. & Fisher, M. J. Systemic chemotherapy and white matter integrity in tracts associated with cognition among children with neurofibromatosis type 1. Pediatr. Blood Cancer 63, 818–824 (2016).
Peterson, R. K. et al. Predictors of neuropsychological late effects and white matter correlates in children treated for a brain tumor without radiation therapy. Pediatr. Blood Cancer 66, e27924 (2019).
Armstrong, G. T. et al. Reduction in late mortality among 5-year survivors of childhood cancer. N. Engl. J. Med. 374, 833–842 (2016).
Gibson, T. M. et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 19, 1590–1601 (2018).
Smith, M. A., Freidlin, B., Ries, L. A. & Simon, R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J. Natl Cancer Inst. 90, 1269–1277 (1998).
Silber, J. H. et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J. Clin. Oncol. 10, 1390–1396 (1992).
Fouladi, M. et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J. Clin. Oncol. 23, 7152–7160 (2005).
Lahteenmaki, P. M. et al. Scholastic achievements of children with brain tumors at the end of comprehensive education: a nationwide, register-based study. Neurology 69, 296–305 (2007).
Bandopadhayay, P. et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood Cancer 61, 1173–1179 (2014).
Fangusaro, J. et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 20, 1011–1022 (2019).
Bouffet, E. et al. LGG-46. trametinib therapy in pediatric patients with low-grade gliomas (lgg) with BRAF gene fusion: a disease-specific cohort in the first pediatric testing of trametinib. Neuro-Oncol. 20, i114 (2018).
Kondyli, M. et al. Trametinib for progressive pediatric low-grade gliomas. J. Neuro-Oncol. 140, 435–444 (2018).
Robison, N. et al. LGG-44. A phase I dose escalation trial of the MEK1/2 inhibitor MEK162 (binimetinib) in children with low-grade gliomas and other RAS/RAF pathway-activated tumors. Neuro-Oncol. 20, i114 (2018).
Bouffet, E. et al. Primary analysis of a phase II trial of dabrafenib plus trametinib (dab + tram) in BRAF V600–mutant pediatric low-grade glioma (pLGG). J. Clin. Oncol. 40, LBA2002–LBA2002 (2022).
Robison, L. L. et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J. Clin. Oncol. 27, 2308–2318 (2009).
Leisenring, W. M. et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J. Clin. Oncol. 27, 2319–2327 (2009).
Fangusaro, J. Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front. Oncol. 2, 105 (2012).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
Neglia, J. P. et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J. Natl Cancer Inst. 93, 618–629 (2001).
de Blank, P. M. et al. Impact of vision loss among survivors of childhood central nervous system astroglial tumors. Cancer 122, 730–739 (2016).
Packer, R. J. et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J. Clin. Oncol. 21, 3255–3261 (2003).
de Blank, P. et al. Late morbidity and mortality in adult survivors of childhood glioma with neurofibromatosis type 1: report from the Childhood Cancer Survivor Study. Genet. Med. 22, 1794–1802 (2020).
Effinger, K. E. et al. Long-term health and social function in adult survivors of paediatric astrocytoma: a report from the Childhood Cancer Survivor Study. Eur. J. Cancer 106, 171–180 (2019).
Bhatia, S. et al. Subsequent neoplasms after a primary tumor in individuals with neurofibromatosis type 1. J. Clin. Oncol. 37, 3050–3058 (2019).
Howlader, N. et al. (eds). SEER Cancer Statistics Review, 1975–2018. National Cancer Institute https://seer.cancer.gov/csr/1975_2018 (2021).
Green, D. M. et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 61, 53–67 (2014).
Acknowledgements
This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Author information
Authors and Affiliations
Contributions
P.M.K.d.B., K.R.L., M.F.O. and D.C.B. conceptualized and designed the study. All authors contributed to the collection and assembly of data. Data analysis was provided by M.X., S.M.S. and D.S. P.M.K.d.B., K.R.L., M.F.O., D.C.B., M.X., S.M.S., D.S. and G.T.A. provided data interpretation. P.M.K.d.B. wrote the first draft of the manuscript, and all authors contributed to manuscript editing and revision. All authors approved the final manuscript and are responsible for the final work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Rodrigue Allodji, Anna Berghoff, Ryota Tamura and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Supplementary information
Source data
Source Data Fig. 1
Individual level source data on each cohort member.
Source Data Figs. 2–6
Sheet: Fig. 2: number of patients with treatment exposures by decade. Sheet: Fig. 3a,h: cumulative incidence rate by year from diagnosis. Sheet: Fig. 4a,f: cumulative incidence rate by year from diagnosis. Sheet: Fig. 5a,b: cumulative incidence rate by year from diagnosis. Sheet: Fig. 6a,b: cumulative incidence rate by year from diagnosis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Blank, P.M.K., Lange, K.R., Xing, M. et al. Temporal changes in treatment and late mortality and morbidity in adult survivors of childhood glioma: a report from the Childhood Cancer Survivor Study. Nat Cancer 5, 590–600 (2024). https://doi.org/10.1038/s43018-024-00733-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-024-00733-0
This article is cited by
-
Improving long-term outcomes in pediatric low-grade glioma
Nature Cancer (2024)