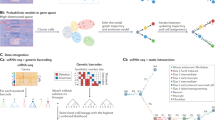
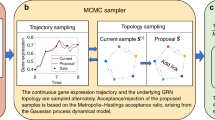
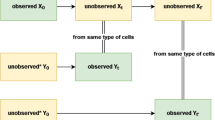
Abstract
Understanding the temporal dynamics of gene expression is crucial for developmental biology, tumour biology and biogerontology. However, some timepoints remain challenging to measure in the laboratory, particularly during very early or very late stages of a biological process. Here we propose Sagittarius, a transformer-based model that can accurately simulate gene expression profiles at timepoints outside the range of times measured in the laboratory. The key idea behind Sagittarius is to learn a shared reference space for time-series measurements, thereby explicitly modelling unaligned timepoints and conditional batch effects between time series, and making the model widely applicable to diverse biological settings. We show Sagittarius’s promising performance when extrapolating mammalian developmental gene expression, simulating drug-induced expression at unmeasured dose and treatment times, and augmenting datasets to accurately predict drug sensitivity. We also used Sagittarius to extrapolate mutation profiles for early-stage cancer patients, which enabled us to discover a gene set connected to the Hedgehog signalling pathway that may be related to tumorigenesis in sarcoma patients, including PTCH1, ARID2 and MYCBP2. By augmenting experimental temporal datasets with crucial but difficult-to-measure extrapolated datapoints, Sagittarius enables deeper insights into the temporal dynamics of heterogeneous transcriptomic processes and can be broadly applied to biological time-series extrapolation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data necessary for reproducing the paper are available in the figshare repository at https://figshare.com/projects/Sagittarius/144771. We provide a more detailed note describing the datasets provided in the figshare repository. Namely, the main experiments can be run with the preprocessed data files provided, and we also include data for the cross-dataset analyses such as the application in drug repurposing. Finally, we provide large-scale simulated data from Sagittarius for the Evo-devo and TCGA datasets.
Code availability
A Python repository including the Sagittarius implementation and code to reproduce the results in this paper is available at https://github.com/addiewc/Sagittarius113, with additional details for reproducing results provided in the repository (https://doi.org/10.5281/zenodo.7879454)114. We ran experiments on Linux 8.7 with RTX A4000 GPU. We used Python 3.9.7, PyTorch115 1.9.1, anndata116 0.8.0, cudatoolkit 11.1.74, matplotlib117 3.4.3, NumPy118 1.21.2, pandas119 1.3.3, pip 21.3.1, pybiomart90 0.2.0, python-louvain95 0.15, Scanpy120 1.8.2, SciPy121 1.7.1, seaborn122 0.11.2, sklearn93 0.0, statsmodels123 0.13.0, tqdm124 4.62.3, umap-learn37 0.5.1, yaml 0.2.5, lifelines125 0.26.5, BioRender Student Plan and Adobe Illustrator 25.2.3.
References
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Arbeitman, M. N. et al. Gene expression during the life cycle of Drosophila melanogaster. Science 297, 2270–2275 (2002).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Lee, J. S. et al. Single-cell transcriptome of bronchoalveolar lavage fluid reveals sequential change of macrophages during SARS-CoV-2 infection in ferrets. Nat. Commun. 12, 4567 (2021).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353 (2018).
Douglass, E. F. Jr et al. A community challenge for a pancancer drug mechanism of action inference from perturbational profile data. Cell Rep. Med. 3, 100492 (2022).
Kohonen, P. et al. A transcriptomics data-driven gene space accurately predicts liver cytopathology and drug-induced liver injury. Nat. Commun. 8, 15932 (2017).
Almogy, G. et al. Cost-efficient whole genome-sequencing using novel mostly natural sequencing-by-synthesis chemistry and open fluidics platform. Preprint at bioRxiv https://doi.org/10.1101/2022.05.29.493900 (2022).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Ramsköld, D. et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Cardoso-Moreira, M. et al. Gene expression across mammalian organ development. Nature 571, 505–509 (2019).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Cao, J., Zhou, W., Steemers, F., Trapnell, C. & Shendure, J. Sci-fate characterizes the dynamics of gene expression in single cells. Nat. Biotechnol. 38, 980–988 (2020).
Subramanian, A. et al. A next generation Connectivity Map: L1000 platform and the first 1,000,000 profiles. Cell 171, 1437–1452.e17 (2017).
Tabula Muris Consortium. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Wang, W. et al. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 26, 1644–1653 (2020).
Sunkin, S. M. et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 41, D996–D1008 (2013).
Radovic, A., He, J., Ramanan, J., Brubaker, M. A. & Lehrmann, A. M. Agent forecasting at flexible horizons using ODE flows. In ICML Workshop on Invertible Neural Networks, Normalizing Flows, and Explicit Likelihood Models (2021).
Peng, G., Cui, G., Ke, J. & Jing, N. Using single-cell and spatial transcriptomes to understand stem cell lineage specification during early embryo development. Annu. Rev. Genomics Hum. Genet. 21, 163–181 (2020).
Haniffa, M. et al. A roadmap for the Human Developmental Cell Atlas. Nature 597, 196–205 (2021).
Sohn, K., Lee, H. & Yan, X. Learning Structured Output Representation using Deep Conditional Generative Models. in Advances in Neural Information Processing Systems (eds. Cortes, C., Lawrence, N., Lee, D., Sugiyama, M. & Garnett, R.) vol. 28 3483–3491 (Curran Associates, Inc., 2015).
Lotfollahi, M. et al. Predicting cellular responses to complex perturbations in high-throughput screens. Mol. Syst. Biol. e11517 (2023).
Cho, K. et al. Learning Phrase Representations using RNN Encoder–Decoder for Statistical Machine Translation. in Proceedings of the 2014 Conference on Empirical Methods in Natural Language Processing (EMNLP) (eds. Moschitti, A., Pang, B. & Daelemans, W.) 1724–1734 (Association for Computational Linguistics, 2014).
Chen, R. T. Q., Rubanova, Y., Bettencourt, J. & Duvenaud, D. K. Neural Ordinary Differential Equations. in Advances in Neural Information Processing Systems (eds. Bengio, S. et al.) vol. 31 6571–6583 (Curran Associates, Inc., 2018).
Shukla, S. N. & Marlin, B. Multi-time attention networks for irregularly sampled time series. In International Conference on Learning Representations (ICLR, 2021).
Chen, R. T. Q., Amos, B. & Nickel, M. Learning neural event functions for ordinary differential equations. International Conference on Learning Representations (ICLR, 2021).
Vaswani, A. et al. Attention is All you Need. in Advances in Neural Information Processing Systems (eds. Guyon, I. et al.) vol. 30 5998–6008 (Curran Associates, Inc., 2017).
Rahaman, N. et al. On the spectral bias of neural networks. Proc. Mach. Learning Res. 97, 5301–5310 (2019).
Cancer Genome Atlas Research Networket al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 45, 1113–1120 (2013).
Yeo, G. H. T., Saksena, S. D. & Gifford, D. K. Generative modeling of single-cell time series with PRESCIENT enables prediction of cell trajectories with interventions. Nat. Commun. 12, 3222 (2021).
Tam, P. P. & Behringer, R. R. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 68, 3–25 (1997).
Pijuan-Sala, B. et al. A single-cell molecular map of mouse gastrulation and early organogenesis. Nature 566, 490–495 (2019).
Qiu, C. et al. Systematic reconstruction of cellular trajectories across mouse embryogenesis. Nat. Genet. 54, 328–341 (2022).
Briggs, J. A. et al. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science 360, eaar5780 (2018).
McInnes, L., Healy, J., Saul, N. & Grossberger, L. UMAP: Uniform Manifold Approximation and Projection. The Journal of Open Source Software 3, 861 (2018).
Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 24, 417–441 (1933).
Viegas, J. O. et al. RNA degradation eliminates developmental transcripts during murine embryonic stem cell differentiation via CAPRIN1-XRN2. Dev. Cell 57, 2731–2744.e5 (2022).
Tomecki, R., Sikorski, P. J. & Zakrzewska-Placzek, M. Comparison of preribosomal RNA processing pathways in yeast, plant and human cells—focus on coordinated action of endo- and exoribonucleases. FEBS Lett. 591, 1801–1850 (2017).
Watada, E. et al. Age-dependent ribosomal DNA variations in mice. Mol. Cell. Biol. 40, e00368-20 (2020).
Nimura, K. et al. Regulation of alternative polyadenylation by Nkx2-5 and Xrn2 during mouse heart development. eLife 5, e16030 (2016).
Chatterjee, S. & Grosshans, H. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature 461, 546–549 (2009).
Chatterjee, S., Fasler, M., Büssing, I. & Grosshans, H. Target-mediated protection of endogenous microRNAs in C. elegans. Dev. Cell 20, 388–396 (2011).
Chowdhury, T., Samajdar, A., Sardar, M. & Chatterjee, S. Dauer quiescence as well as continuity of the life cycle after dauer-exit in Caenorhabditis elegans are dependent on the endoribonuclease activity of XRN-2. Preprint at bioRxiv https://doi.org/10.1101/2022.05.02.489690 (2022).
Kato, M., de Lencastre, A., Pincus, Z. & Slack, F. J. Dynamic expression of small non-coding RNAs, including novel microRNAs and piRNAs/21U-RNAs, during Caenorhabditis elegans development. Genome Biol. 10, R54 (2009).
Qiao, G.-J., Chen, L., Wu, J.-C. & Li, Z.-R. Identification of an eight-gene signature for survival prediction for patients with hepatocellular carcinoma based on integrated bioinformatics analysis. PeerJ 7, e6548 (2019).
Takada, H. & Kurisaki, A. Emerging roles of nucleolar and ribosomal proteins in cancer, development, and aging. Cell. Mol. Life Sci. 72, 4015–4025 (2015).
Loganathan, T., Ramachandran, S., Shankaran, P., Nagarajan, D. & Mohan S, S. Host transcriptome-guided drug repurposing for COVID-19 treatment: a meta-analysis based approach. PeerJ 8, e9357 (2020).
Belyaeva, A. et al. Causal network models of SARS-CoV-2 expression and aging to identify candidates for drug repurposing. Nat. Commun. 12, 1024 (2021).
Minamiyama, M. et al. Naratriptan mitigates CGRP1-associated motor neuron degeneration caused by an expanded polyglutamine repeat tract. Nat. Med. 18, 1531–1538 (2012).
Iorio, F. et al. A landscape of pharmacogenomic interactions in cancer. Cell 166, 740–754 (2016).
Yang, C. et al. A survey of optimal strategy for signature-based drug repositioning and an application to liver cancer. eLife 11, e71880 (2022).
Cheng, X. et al. Drug repurposing for cancer treatment through global propagation with a greedy algorithm in a multilayer network. Cancer Biol. Med. 19, 74–89 (2022).
Folkes, A. J. et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 51, 5522–5532 (2008).
Roth, G. J. et al. Nintedanib: from discovery to the clinic. J. Med. Chem. 58, 1053–1063 (2015).
Suzuki, N., Nakagawa, F., Matsuoka, K. & Takechi, T. Effect of a novel oral chemotherapeutic agent containing a combination of trifluridine, tipiracil and the novel triple angiokinase inhibitor nintedanib, on human colorectal cancer xenografts. Oncol. Rep. 36, 3123–3130 (2016).
Seashore-Ludlow, B. et al. Harnessing connectivity in a large-scale small-molecule sensitivity dataset. Cancer Discov. 5, 1210–1223 (2015).
Menden, M. P. et al. Community assessment to advance computational prediction of cancer drug combinations in a pharmacogenomic screen. Nat. Commun. 10, 2674 (2019).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e16 (2017).
Meyers, R. M. et al. Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nat. Genet. 49, 1779–1784 (2017).
Chen, X. et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 11, 3475 (2020).
Bozic, I. et al. Accumulation of driver and passenger mutations during tumor progression. Proc. Natl Acad. Sci. USA 107, 18545–18550 (2010).
Arazo, E., Ortego, D., Paul, A., O’Connor, N. E. & McGuinness, K. Unsupervised label noise modeling and loss correction. Proc. Mach. Learning Res. 97, 312–321 (2019).
Li, J., Socher, R. & Hoi, S. C. H. DivideMix: Learning with noisy labels as semi-supervised learning. In International Conference on Learning Representations (2020).
Brown, L. C. et al. LRP1B mutations are associated with favorable outcomes to immune checkpoint inhibitors across multiple cancer types. J. Immunother. Cancer 9, e001792 (2021).
Arang, N. & Gutkind, J. S. G protein-coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett. 594, 4201–4232 (2020).
Ichikawa, D. et al. Integrated diagnosis based on transcriptome analysis in suspected pediatric sarcomas. NPJ Genom. Med. 6, 49 (2021).
Pietrobono, S., Gagliardi, S. & Stecca, B. Non-canonical Hedgehog signaling pathway in cancer: activation of GLI transcription factors beyond Smoothened. Front. Genet. 10, 556 (2019).
Lo, W. W., Pinnaduwage, D., Gokgoz, N., Wunder, J. S. & Andrulis, I. L. Aberrant hedgehog signaling and clinical outcome in osteosarcoma. Sarcoma 2014, 261804 (2014).
Banerjee, S. et al. Loss of the PTCH1 tumor suppressor defines a new subset of plexiform fibromyxoma. J. Transl. Med. 17, 246 (2019).
Martinez, M. F. et al. Nevoid basal cell carcinoma syndrome: PTCH1 mutation profile and expression of genes involved in the Hedgehog pathway in Argentinian patients. Cells 8, 144 (2019).
Ge, Z. et al. Clinical significance of high c-MYC and low MYCBP2 expression and their association with Ikaros dysfunction in adult acute lymphoblastic leukemia. Oncotarget 6, 42300–42311 (2015).
Vatapalli, R. et al. Histone methyltransferase DOT1L coordinates AR and MYC stability in prostate cancer. Nat. Commun. 11, 4153 (2020).
Yoon, J. W. et al. Noncanonical regulation of the Hedgehog mediator GLI1 by c-MYC in Burkitt lymphoma. Mol. Cancer Res. 11, 604–615 (2013).
Tazzari, M. et al. Molecular determinants of soft tissue sarcoma immunity: targets for immune intervention. Int. J. Mol. Sci. 22, 7518 (2021).
Wang, X., Haswell, J. R. & Roberts, C. W. M. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer—mechanisms and potential therapeutic insights. Clin. Cancer Res. 20, 21–27 (2014).
Fan, X. et al. The association between methylation patterns of DNAH17 and clinicopathological factors in hepatocellular carcinoma. Cancer Med. 8, 337–350 (2019).
Hassounah, N. B., Bunch, T. A. & McDermott, K. M. Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling. Clin. Cancer Res. 18, 2429–2435 (2012).
Stecca, B. & Ruiz i Altaba, A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J. Mol. Cell. Biol. 2, 84–95 (2010).
Brechbiel, J., Miller-Moslin, K. & Adjei, A. A. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat. Rev. 40, 750–759 (2014).
Chen, J., Zhang, J., Hong, L. & Zhou, Y. EGFLAM correlates with cell proliferation, migration, invasion and poor prognosis in glioblastoma. Cancer Biomark. 24, 343–350 (2019).
Yu, Q. et al. Upregulated NLGN1 predicts poor survival in colorectal cancer. BMC Cancer 21, 884 (2021).
Ren, Y.-M. et al. Exploring the key genes and pathways of side population cells in human osteosarcoma using gene expression array analysis. J. Orthop. Surg. Res. 13, 153 (2018).
Cutcliffe, C. et al. Clear cell sarcoma of the kidney: up-regulation of neural markers with activation of the Sonic hedgehog and Akt pathways. Clin. Cancer Res. 11, 7986–7994 (2005).
Wald, Y., Feder, A., Greenfeld, D. & Shalit, U. On Calibration and Out-of-Domain Generalization. in Advances in Neural Information Processing Systems (eds. Ranzato, M., Beygelzimer, A., Dauphin, Y., Liang, P. S. & Vaughan, J. W.) vol. 34 2215–2227 (Curran Associates, Inc., 2021).
Setty, M. et al. Characterization of cell fate probabilities in single-cell data with Palantir. Nat. Biotechnol. 37, 451–460 (2019).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genom. 19, 477 (2018).
Fischer, D. S. et al. Inferring population dynamics from single-cell RNA-sequencing time series data. Nat. Biotechnol. 37, 461–468 (2019).
de Ruiter, J. pybiomart: a simple Pythonic interface to BioMart. GitHub https://github.com/jrderuiter/pybiomart (2018).
Joshi, C. J., Ke, W., Drangowska-Way, A., O’Rourke, E. J. & Lewis, N. E. What are housekeeping genes? PLoS Comput. Biol. 18, e1010295 (2022).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
Aynaud, T. python-louvain 0.15: Louvain algorithm for community detection. GitHub https://github.com/taynaud/python-louvain (2020).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Berry, L. M. & Zhao, Z. An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab. Lett. 2, 51–59 (2008).
Corsello, S. M. et al. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat. Med. 23, 405–408 (2017).
Kingma, D. P. & Ba, J. Adam: A Method for Stochastic Optimization. in 3rd International Conference on Learning Representations, ICLR 2015, San Diego, CA, USA, May 7–9, 2015, Conference Track Proceedings (eds. Bengio, Y. & LeCun, Y.) (2015).
Mariani, O. et al. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell 11, 361–374 (2007).
Bae, J. Y. et al. Evaluation of immune-biomarker expression in high-grade soft-tissue sarcoma: HLA-DQA1 expression as a prognostic marker. Exp. Ther. Med. 20, 107 (2020).
Wang, H. et al. HER4 promotes cell survival and chemoresistance in osteosarcoma via interaction with NDRG1. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1839–1849 (2018).
Yan, X., Chua, M.-S., Sun, H. & So, S. N-Myc down-regulated gene 1 mediates proliferation, invasion, and apoptosis of hepatocellular carcinoma cells. Cancer Lett. 262, 133–142 (2008).
Cheng, J. et al. NDRG1 as a biomarker for metastasis, recurrence and of poor prognosis in hepatocellular carcinoma. Cancer Lett. 310, 35–45 (2011).
Hua, Y. et al. Plasma membrane proteomic analysis of human osteosarcoma and osteoblastic cells: revealing NDRG1 as a marker for osteosarcoma. Tumour Biol. 32, 1013–1021 (2011).
Graf, S. A. et al. The myelin protein PMP2 is regulated by SOX10 and drives melanoma cell invasion. Pigment Cell Melanoma Res. 32, 424–434 (2019).
Cheng, L. et al. Integration of genomic copy number variations and chemotherapy-response biomarkers in pediatric sarcoma. BMC Med. Genom. 12, 23 (2019).
Guo, Q., Sun, H., Zheng, K., Yin, S. & Niu, J. Long non-coding RNA DLX6-AS1/miR-141-3p axis regulates osteosarcoma proliferation, migration and invasion through regulating Rab10. RSC Adv. 9, 33823–33833 (2019).
International Cancer Genome Consortiumet al. International network of cancer genome projects. Nature 464, 993–998 (2010).
Mito, J. K. et al. Cross species genomic analysis identifies a mouse model as undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma. PLoS ONE 4, e8075 (2009).
Capra, M. et al. Frequent alterations in the expression of serine/threonine kinases in human cancers. Cancer Res. 66, 8147–8154 (2006).
Pandey, P. et al. Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget 7, 19430–19444 (2016).
Woicik, A. addiewc/Sagittarius: Sagittarius. Zenodo https://doi.org/10.5281/zenodo.7879454 (2023).
Woicik, A. Simulated EvoDevo dataset. figshare https://doi.org/10.6084/m9.figshare.20425572 (2022).
Paszke, A. et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. in Advances in Neural Information Processing Systems (eds. Wallach, H. et al.) vol. 32 8026–8037 (Curran Associates, Inc., 2019).
Virshup, I., Rybakov, S., Theis, F. J., Angerer, P. & Wolf, F. A. anndata: annotated data. Preprint at bioRxiv https://doi.org/10.1101/2021.12.16.473007 (2021).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
The Pandas Development Team. pandas-dev/pandas: pandas. Zenodo https://doi.org/10.5281/zenodo.7857418 (2023).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Waskom, M. L. seaborn: statistical data visualization. J. Open Source Softw. 6, 3021 (2021).
Seabold, S. & Perktold, J. Statsmodels: econometric and statistical modeling with Python. In Proc. 9th Python in Science Conference (eds van der Walt, S. & Millman, J.) 92–96 https://doi.org/10.25080/majora-92bf1922-011 (SciPy, 2010).
da Costa-Luis, C. et al. tqdm: a fast, extensible progress bar for Python and CLI. Zenodo https://doi.org/10.5281/zenodo.7697295 (2023).
Davidson-Pilon, C. lifelines: survival analysis in Python. J. Open Source Softw. 4, 1317 (2019).
Acknowledgements
S.W. is supported by the Sony Research Award. Figures 1, 4a,b and 6a,f were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
A.W. and S.W. conceptualized the work and designed the method. A.W., S.W. and J.M. designed the experiments. A.W. and M.Z. ran the experiments, and A.W., M.Z. and J.C. developed computational tools for Sagittarius. A.W. and S.W. wrote the manuscript and designed the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Machine Intelligence thanks Chenling Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Note 1, Tables 1 and 2 and Figs. 1–28.
Supplementary Data 1
Enriched processes for gene sets that Sagittarius extrapolates accurately. Gene Ontology terms enriched by the genes that Sagittarius predicts with at least 0.4 test Pearson correlation comparing timepoints, along with the number of species for which the term is enriched and the enrichment analysis’s two-sided Fisher exact test P value (with Bonferroni correction for multiple hypothesis testing) for each time series.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woicik, A., Zhang, M., Chan, J. et al. Extrapolating heterogeneous time-series gene expression data using Sagittarius. Nat Mach Intell 5, 699–713 (2023). https://doi.org/10.1038/s42256-023-00679-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42256-023-00679-5