Abstract
The post-translational modification lysine succinylation is implicated in the regulation of various metabolic pathways. However, its biological relevance remains uncertain due to methodological difficulties in determining high-impact succinylation sites. Here, using stable isotope labelling and data-independent acquisition mass spectrometry, we quantified lysine succinylation stoichiometries in mouse livers. Despite the low overall stoichiometry of lysine succinylation, several high-stoichiometry sites were identified, especially upon deletion of the desuccinylase SIRT5. In particular, multiple high-stoichiometry lysine sites identified in argininosuccinate synthase (ASS1), a key enzyme in the urea cycle, are regulated by SIRT5. Mutation of the high-stoichiometry lysine in ASS1 to succinyl-mimetic glutamic acid significantly decreased its enzymatic activity. Metabolomics profiling confirms that SIRT5 deficiency decreases urea cycle activity in liver. Importantly, SIRT5 deficiency compromises ammonia tolerance, which can be reversed by the overexpression of wild-type, but not succinyl-mimetic, ASS1. Therefore, lysine succinylation is functionally important in ammonia metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The mass spectrometric raw data and complete MS datasets have been uploaded to the Center for Computational Mass Spectrometry, the MassIVE repository at UCSD, and can be downloaded using the following link: ftp://MSV000089726@massive.ucsd.edu. The mass spectrometric raw data are deposited with the MassIVE ID number MSV000089726; it is also available at ProteomeXchange with the ID PXD034880. Data were searched against the M. musculus proteome with 58,430 protein entries (UniProtKB-TrEMBL). Metabolomics data were deposited at Metabolomics Workbench with Project ID PR001365 (Project https://doi.org/10.21228/M88Q5H). All other data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Du, J. et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011).
Zhang, Z. et al. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 7, 58–63 (2011).
Park, J. et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 50, 919–930 (2013).
Rardin, M. J. et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 18, 920–933 (2013).
Weinert, B. T. et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 4, 842–851 (2013).
Colak, G. et al. Identification of lysine succinylation substrates and the succinylation regulatory enzyme CobB in Escherichia coli. Mol. Cell Proteom. 12, 3509–3520 (2013).
Gut, P. et al. SUCLA2 mutations cause global protein succinylation contributing to the pathomechanism of a hereditary mitochondrial disease. Nat. Commun. 11, 5927 (2020).
Li, F. et al. NADP+-IDH mutations promote hypersuccinylation that impairs mitochondria respiration and induces apoptosis resistance. Mol. Cell 60, 661–675 (2015).
Wang, Y. et al. KAT2A coupled with the alpha-KGDH complex acts as a histone H3 succinyltransferase. Nature 552, 273–277 (2017).
Kurmi, K. et al. Carnitine palmitoyltransferase 1A has a lysine succinyltransferase activity. Cell Rep. 22, 1365–1373 (2018).
Wagner, G. R. et al. A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab. 25, 823–837 (2017).
Wagner, G. R. & Hirschey, M. D. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell 54, 5–16 (2014).
Peng, C. et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteom. 10, M111.012658 (2011).
Weinert, B. T. et al. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 10, 716 (2014).
Weinert, B. T., Moustafa, T., Iesmantavicius, V., Zechner, R. & Choudhary, C. Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 34, 2620–2632 (2015).
Hansen, B. K. et al. Analysis of human acetylation stoichiometry defines mechanistic constraints on protein regulation. Nat. Commun. 10, 1055 (2019).
Nakayasu, E. S. et al. A method to determine lysine acetylation stoichiometries. Int J. Proteom. 2014, 730725 (2014).
Zhou, T., Chung, Y. H., Chen, J. & Chen, Y. Site-specific identification of lysine acetylation stoichiometries in mammalian cells. J. Proteome Res 15, 1103–1113 (2016).
Baeza, J. et al. Stoichiometry of site-specific lysine acetylation in an entire proteome. J. Biol. Chem. 289, 21326–21338 (2014).
Meyer, J. G. et al. Quantification of lysine acetylation and succinylation stoichiometry in proteins using mass spectrometric data-independent acquisitions (SWATH). J. Am. Soc. Mass. Spectrom. 27, 1758–1771 (2016).
Rardin, M. J. et al. MS1 peptide ion intensity chromatograms in MS2 (SWATH) data independent acquisitions. improving post acquisition analysis of proteomic experiments. Mol. Cell Proteom. 14, 2405–2419 (2015).
Diez-Fernandez, C. et al. Kinetic mutations in argininosuccinate synthetase deficiency: characterisation and in vitro correction by substrate supplementation. J. Med. Genet. 53, 710–719 (2016).
Soria, L. R. et al. Enhancement of hepatic autophagy increases ureagenesis and protects against hyperammonemia. Proc. Natl Acad. Sci. USA 115, 391–396 (2018).
Azorin, I., Minana, M. D., Felipo, V. & Grisolia, S. A simple animal model of hyperammonemia. Hepatology 10, 311–314 (1989).
Wijdicks, E. F. Hepatic encephalopathy. N. Engl. J. Med. 375, 1660–1670 (2016).
Monfort, P. et al. Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy: therapeutical implications. Neurochem. Int. 55, 106–112 (2009).
Karlberg, T. et al. Structure of human argininosuccinate synthetase. Acta Crystallogr. D 64, 279–286 (2008).
Wu, R. et al. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat. Methods 8, 677–683 (2011).
Olsen, J. V. et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal 3, ra3 (2010).
Nakagawa, T., Lomb, D. J., Haigis, M. C. & Guarente, L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 (2009).
Tan, M. et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 19, 605–617 (2014).
Yu, J. et al. Metabolic characterization of a Sirt5 deficient mouse model. Sci. Rep. 3, 2806 (2013).
Peng, M. et al. Battery of behavioral tests in mice to study postoperative delirium. Sci. Rep. 6, 29874 (2016).
Guerreiro, J. R. et al. Argininosuccinate synthetase is a functional target for a snake venom anti-hypertensive peptide: role in arginine and nitric oxide production. J. Biol. Chem. 284, 20022–20033 (2009).
Zhang, R. et al. Histone malonylation is regulated by SIRT5 and KAT2A. iScience 26, 106193 (2023).
Xie, X. et al. Simultaneous affinity enrichment of two post-translational modifications for quantification and site localization. J. Vis. Exp. https://doi.org/10.3791/60780 (2020).
Christensen, D. G. et al. Identification of novel protein lysine acetyltransferases in Escherichia coli. mBio 9, e01905–e01918 (2018).
Schilling, B., Gibson, B. W. & Hunter, C. L. Generation of high-quality SWATH((R)) acquisition data for label-free quantitative proteomics studies using TripleTOF((R)) mass spectrometers. Methods Mol. Biol. 1550, 223–233 (2017).
Collins, B. C. et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 8, 291 (2017).
Skyline. University of Washington (2018). http://proteome.gs.washington.edu/software/skyline
Wei, L., Meyer, J. G. & Schilling, B. Quantification of site-specific protein lysine acetylation and succinylation stoichiometry using data-independent acquisition mass spectrometry. J. Vis. Exp. https://doi.org/10.3791/57209 (2018).
Schilling, B. et al. Platform-independent and label-free quantitation of proteomic data using MS1 extracted ion chromatograms in skyline: application to protein acetylation and phosphorylation. Mol. Cell Proteom. 11, 202–214 (2012).
Tsou, C. C. et al. DIA-Umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat. Methods 12, 258–264 (2015).
Perkins, D. N., Pappin, D. J., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Kim, S. & Pevzner, P. A. MS-GF+ makes progress towards a universal database search tool for proteomics. Nat. Commun. 5, 5277 (2014).
Keller, A., Nesvizhskii, A. I., Kolker, E. & Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 (2002).
Shteynberg, D. et al. iProphet: multi-level integrative analysis of shotgun proteomic data improves peptide and protein identification rates and error estimates. Mol. Cell Proteom. 10, M111.007690 (2011).
MacLean, B. et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010).
Meyer, J. G. StoichCalc. GitHub https://github.com/jgmeyerucsd/StoichCalc (2016).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Liu, X., Ser, Z. & Locasale, J. W. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal. Chem. 86, 2175–2184 (2014).
Liu, X. et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175, 502–513 (2018).
Liu, X. et al. High-resolution metabolomics with Acyl-CoA profiling reveals widespread remodeling in response to diet. Mol. Cell Proteom. 14, 1489–1500 (2015).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Supek, F., Bosnjak, M., Skunca, N. & Smuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6, e21800 (2011).
Chen, J., Bardes, E. E., Aronow, B. J. & Jegga, A. G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 37, W305–W311 (2009).
Xia, J. & Wishart, D. S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 6, 743–760 (2011).
Acknowledgements
This project was supported by NIH grant R24DK085610 (E.V. and B.S.), Gladstone Institutes intramural funds (E.V.) and Buck Institute intramural funds (E.V. and B.S.). R.Z. was supported by a postdoctoral fellowship from the Glenn Foundation for Medical Research and the National Natural Science Foundation of China (No. 82301607). X.X. was supported by the NIH (T32GM8806). C.C. was supported by the by a postdoctoral fellowship from the Larry L. Hillblom Foundation. J.G.M. was supported by the NIH (T32 AG000266). B.S. was supported by NIH grant U01AG060906 and NIH Shared Instrumentation Grant 1S10OD016281 (Buck Institute). We thank S. Galkina for assisting with grip strength tests and tail vein mRNA injection; J. Morales, G. Vega Hormazabal and A. Roberts for assisting with mouse ammonia tolerance tests and tissue collection; C. R. Kahn, C. B. Newgard and M. Hirschey for helpful discussions; J. Carroll for graphic design; and G. Howard for language editing.
Author information
Authors and Affiliations
Contributions
R.Z., J.F., C.C., B.S. and E.V. conceived the study. R.Z., J.F., X.X., C.C., B.S. and E.V. contributed to the design of the study and wrote the manuscript with help of the other co-authors. X.X., J.G.M., L.W., J.B. and J.R. performed the proteomics analyses, and B.S. supervised proteomics analyses. C.C., X.X., R.Z. and J.B. contributed to the analyses and visualization of proteomics. R.Z. constructed and purified recombinant proteins, and measured protein thermal stability and enzymatic activity kinetics. Y.N. prepared samples for metabolomics analyses, X.L. performed metabolomics experiments, and J.W.L. supervised metabolomics analyses. R.Z. contributed to the analyses and visualization of metabolomics datasets. P.A.K. performed targeted citrulline measurement. R.Z., R.K., R.R. and W.H. contributed to mouse maintenance and breeding, performing behavioural tests, and collected mouse tissues and blood. J.F., R.R. and Y.Z. performed mouse ammonia tolerance test. J.F. and Y.Z. performed experiments using cell culture, and measured ammonia levels. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
E.V. is a scientific co-founder of Napa Therapeutics and serves on the scientific advisory board of Seneque. E.V. receives research support from BaReCia. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Peng Jiang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Gimenez-Cassina in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
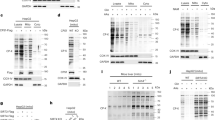
Extended Data Fig. 1 Identification of lysine succinylated proteins and peptides.
Venn diagrams showing the overlap of identified succinylated proteins and peptides in WT and Sirt5-/- mouse liver.
Extended Data Fig. 2 Comparison of immunoenriched succinylomes.
(a) Comparison of immunoenriched succinylation proteomes performed in this and previous studies (Rardin et al., Cell Metab. 2013; Park et al., Mol Cell. 2013)3,4. (b-c) Venn diagrams showing The numbers of succinylated proteins by SIRT5 (b) and lysine sites targeted by SIRT5 (c) (> twofold increase and p < 0.01 using two-sided unpaired Student’s t test) identified in this study (yellow circle, Sample sizes: WT, n = 4; S5KO, n = 4) or in our previous study (Rardin et al., Cell Metab. 2013.) (blue circle, Sample sizes: WT, n = 5; S5KO, n = 5), or in both studies (green section) were counted. (b) KEGG pathway analysis of succinylated proteins identified in this study (yellow bars) or in Rardin et al. (blue bars) or in both studies (green bars) that were targeted by SIRT5 (> twofold increase and p < 0.01 using two-sided unpaired Student’s t test. This study: WT, n = 4; S5KO, n = 4. Rardin et al.: WT, n = 5; S5KO, n = 5) using ToppFun (p values were generated using probability density function)56. Top 12 most-enriched pathways are shown here.
Extended Data Fig. 3 MS/MS of peptide GAK-(succinyl)-YVSHGATGKGNDQVRFE.
MS/MS of peptide GAK-(succinyl)-YVSHGATGKGNDQVRFE with Succinyl(K)@112 of protein argininosuccinate synthase (ASS1, SwissProt accession number P16460) with the precursor ion at m/z 556.02, z = 4. The succinylation site localization is confirmed with succinylation on K112 as we observe a strong b3-ion containing the succinylation group, while as expected, y-ions y9 to y17 do not comprise the succinylation site. The y18 ion then contains the succinylation group as it contains the K-succinyl moiety (on K3 of the peptide and K112 of the protein). This confirms that the succinylation site is located in position K3 of the succinylated peptide shown here which corresponds to position K112 of the ASS1 protein.
Extended Data Fig. 4 The two succinylation sites K112 and K121 of ASS1 are differentiated by mass spectrometric analysis and confirmed by heavy standards.
(a and d) Extracted ion chromatograms (XICs) of the endogenous succinylated peptide isomers GA112KsuccYVSHGATGKGNDQVRFE (a-c) and GAKYVSHGATG121KsuccGNDQVRFE (d-f). Both with precursor ions: m/z = 556.02; with charge z = 4. PTM-site differentiating ions are highlighted in red. (b and e) The corresponding heavy stable isotope-labeled succinylated peptide (heavy valine, V114; 13C5, 15N1) XICs. Both with precursor ions: m/z = 557.52; charge z = 4. PTM-site differentiating ions are highlighted in red. (c and f) MS/MS spectra of the heavy stable isotope-labeled succinylated peptide isomers (heavy valine, V114; 13C5, 15N1). Both with precursor ion: m/z = 557.52; charge z = 4. PTM-site differentiating ions are highlighted in red.
Extended Data Fig. 5 Thermal stability and enzymatic activity of recombinant ASS1 proteins.
(a) The integrity of purified recombinant His-tagged ASS1 proteins was examined through SDS-PAGE electrophoresis and Coomassie staining. MW: molecular weight. (b) Thermal stability of recombinant ASS1 proteins was examined by differential scanning fluorimetry. (c) Changes in melting temperature (Tm) values compared with that of wild-type ASS1 for each purified ASS1 proteins. Error bars depict S.E.M. from four measures. ns, not significant, ***p < 10-3, ****p < 10-4, using two-sided unpaired Student’s t test. (d) SIRT5 knockdown efficiency in wild-type (WT), and dCas9-KRAB-MeCP2 and sgRNA (scramble sgRNA negative control (sgNC), or two different sgRNAs targeting SIRT5 promoter) stably expressing CRISPRi 293 T cells was examined by western blotting (WB). Lysine succinylation level of purified His-tagged ASS1 was examined by WB. Protein purity was examined through coomassie staining. MW: molecular weight. (e) Thermal stability of recombinant human ASS1 proteins purified from WT, sgNC- or sgSIRT5-expressing 293 T cells was examined by differential scanning fluorimetry. (f) Changes in melting temperature (Tm) values compared with that of the ASS1 purified from WT 293 T cells for each purified ASS1 proteins. Error bars depict S.E.M. from three measures. ****p < 10-4, using two-sided unpaired Student’s t test. (g-i) Steady-state kinetic analysis of the enzymatic activities of ASS1 proteins purified from WT, sgNC- or sgSIRT5-expressing 293 T cells were measured by the production of pyrophosphate from increasing concentrations of citrulline (g) and aspartate (h). n = 4 measurements/sample, mean ± S.D. Values are shown for average Km and Vmax ± S.E.M. (i).
Extended Data Fig. 6 Measurement of urea cycle intermediate metabolites and key enzymes in WT and SIRT5 KO mouse liver tissues.
(a-c) Argininosuccinate (a), arginine (b), and ornithine (c) in the liver tissues from 6-month old WT and SIRT5 KO male mice were identified and quantified through metabolomics profiling. n = 8. Error bars indicate mean ± S.D. P values were generated using two-sided unpaired Student’s t test. (d) Liver citrulline levels in 12-14 months old WT and SIRT5 KO female mice were measured by mass spectrometry. n = 5. Error bars indicate mean ± S.D. P values were generated using two-sided unpaired Student’s t test. (e) Volcano plot showing the differential expression of the quantified proteins through MS1 filtering using peptides identified from whole cell lysates of WT and SIRT5 KO mouse livers. Peptides in urea cycle enzymes are highlighted in red. Fold-change > 1.5 or < 0.67, and p < 0.05 were considered as differentially expressed using two-sided unpaired Student’s t test. (f) Bar graph showing the identified peptides from each of the urea cycle enzymes in WT and SIRT5 KO mouse liver samples.
Extended Data Fig. 7 SIRT5 deficiency prevents the increase of locomotor activity upon high-ammonium-diet feeding in male mice.
(a) Food intake was recorded weekly per cage and then divided by the number of mice in the cage to calculate the food intake per mouse. Sample sizes: Control, n = 6; HAD, n = 4. Error bars indicate mean ± S.E.M. (b) Body weight was monitored weekly. Sample sizes: WT_Control, n = 12; S5KO_Control, n = 10; WT_HAD, n = 12; S5KO_HAD, n = 9. Error bars indicate mean ± S.E.M. (c-d) Grip strength was measured on Days 0, 14, and 28, respectively. Grip strength values were normalized with body weights at the corresponding time points (c). Grip strength improvement was calculated as ∆GripStrength (Day 27–Day 0). Sample sizes: WT_Control, n = 12; S5KO_Control, n = 10; WT_HAD, n = 12; S5KO_HAD, n = 9. Error bars indicate mean ± S.E.M. P values were generated with two-sided unpaired Student’s t test. (e-f) Open-field test of WT and Sirt5-/- male mice was performed after 4 weeks of control diet or HAD feeding. Ambulatory movement time (e), and stereotypic episode (f) were calculated. Sample sizes: WT_Control, n = 12; S5KO_Control, n = 10; WT_HAD, n = 12; S5KO_HAD, n = 9. Error bars indicate mean ± S.E.M. P values were generated with two-sided unpaired Student’s t test.
Extended Data Fig. 8 High-ammonium-diet feeding does not affect motor coordination or locomotor activity in female mice.
(a) Ammonia levels measured in the plasma from 4-month-old WT and Sirt5-/- (S5KO) female mice after being fed on a chow diet (control) or high-ammonium diet (HAD) for 4 weeks without fasting. Sample sizes: WT_Control, n = 12; S5KO_Control, n = 9; WT_HAD, n = 10; S5KO_HAD, n = 13. Error bars indicate mean ± S.E.M. (b) Body weight was monitored weekly. Sample sizes: WT_Control, n = 12; S5KO_Control, n = 9; WT_HAD, n = 10; S5KO_HAD, n = 13. Error bars indicate mean ± S.E.M. (c) Food intake was recorded weekly per cage and then divided by the number of mice in the cage to calculate the food intake per mouse. Sample sizes: Control, n = 5; HAD, n = 5. Error bars indicate mean ± S.E.M. (d) Grip strength improvement was calculated as ∆GripStrength (Day 27–Day 0). Grip strength values were normalized with body weights at the corresponding time points. Sample sizes: WT_Control, n = 12; S5KO_Control, n = 9; WT_HAD, n = 10; S5KO_HAD, n = 13. Error bars indicate mean ± S.E.M. P values were generated with two-sided unpaired Student’s t test. (e) Per-mouse change in rotarod assay performance (time on rotarod on Day 28 – time on rotarod on Day 0). Sample sizes: WT_Control, n = 12; S5KO_Control, n = 9; WT_HAD, n = 10; S5KO_HAD, n = 13. Error bars indicate mean ± S.E.M. P values were generated with two-sided unpaired student t test. (f-l) Open-field testing of WT and S5KO female mice was performed after 4 weeks of control diet or HAD feeding. Representative tracks of paths are shown in (f). Movement time (g), total distance (h), ambulatory movement time (i), stereotypic episode (j), and center time (k) and center distance (l) were calculated. Sample sizes: WT_Control, n = 12; S5KO_Control, n = 9; WT_HAD, n = 10; S5KO_HAD, n = 13. Error bars indicate mean ± S.E.M. P values were generated with two-sided unpaired Student’s t test.
Extended Data Fig. 9 WT ASS1, but not K121E mutant ASS1, reversed ammonia intolerance caused by SIRT5 deficiency in mice.
(a) Schematic diagram of workflow of mRNA delivery followed by i.p. ammonia tolerance test. 12-week old WT and SIRT5 KO mice were administered 10 μg mRNA expressing firefly luciferase, WT mouse ASS1, or K121E mutant mouse ASS1, through tail vein injection. Mice were fasted at 22 h after mRNA injection for 4 h. Then, the mice underwent i.p. ammonia tolerance test. Ammonium acetate was injected at 4 mmol/kg body weight, and blood was collected at 0, 20, 60, and 90 min via submandibular bleeding for plasma ammonia measurement. (b) Representative bioluminescence image of WT mice 24 h after tail vein injection of jetRNA transfection reagent without (vehicle control) or with 10 μg firefly luciferase mRNA. 4.5 mg D-luciferin was i.p. injected per mouse 10 min before imaging. (c) Western blotting examination of ASS1 expression levels in SIRT5 KO mouse liver tissues 24 h after luciferase or ASS1 mRNA tail vein injection. This experiment was repeated two times independently with similar results. MW: molecular weight. (d) Immunoprecipitation of succinyllysine in WT and SIRT5 KO mouse liver tissues 24 h after WT ASS1 mRNA injection, lysine succinylation and ASS1 were examined by western blotting. This experiment was repeated two times independently with similar results. MW: molecular weight. (e) Western blotting examination of ASS1 expression levels in the brain, liver, and kidney tissues of SIRT5 KO mice. Tissues from a luciferease mRNA-injected mouse and two WT ASS1 mRNA-injected mice are shown. This experiment was repeated two times independently with similar results. MW: molecular weight. (f-p) Plasma ammonia levels at different time point of blood collection after ammonium acetate injection in male mice (f-h, WT_Luciferase, n = 9; S5KO_Luciferase, n = 5; WT_ASS1_KE, n = 9; S5KO_ASS1_WT, n = 10), female mice (i-k, WT_Luciferase, n = 10; S5KO_Luciferase, n = 7; WT_ASS1_KE, n = 7; S5KO_ASS1_WT, n = 9), and male and female mice combined (m-p, WT_Luciferase, n = 19; S5KO_Luciferase, n = 12; WT_ASS1_KE, n = 16; S5KO_ASS1_WT, n = 19) mice. Error bars indicate mean ± S.E.M. P values were generated using Dunnett’s multiple comparisons test post one-way ANOVA. (l) The curve of plasma ammonia levels across 90 min after ammonium acetate i.p. injection in WT and SIRT5 KO mice received firefly luciferase, WT, or K121E mutant mouse ASS1 mRNA i.v. administration (male and female combined). Error bars indicate mean ± S.E.M.
Supplementary information
Supplementary Data 1
SIRT5 mouse liver succinylation enrichment proteome.
Supplementary Data 2
SIRT5 mouse liver succinylation stoichiometry proteome.
Supplementary Data 3
SIRT5 mouse liver metabolomics.
Source data
Source Data Fig. 6
Unprocessed western blots and/or gels for Fig. 6.
Source Data Extended Data Fig./Table 5
Unprocessed western blots and/or gels for Extended Data Fig. 5.
Source Data Extended Data Fig./Table 9
Unprocessed western blots and/or gels for Extended Data Fig. 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, R., Fang, J., Xie, X. et al. Regulation of urea cycle by reversible high-stoichiometry lysine succinylation. Nat Metab 6, 550–566 (2024). https://doi.org/10.1038/s42255-024-01005-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-01005-y