Abstract
Metformin is a widely prescribed anti-diabetic medicine that also reduces body weight. There is ongoing debate about the mechanisms that mediate metformin’s effects on energy balance. Here, we show that metformin is a powerful pharmacological inducer of the anorexigenic metabolite N-lactoyl-phenylalanine (Lac-Phe) in cells, in mice and two independent human cohorts. Metformin drives Lac-Phe biosynthesis through the inhibition of complex I, increased glycolytic flux and intracellular lactate mass action. Intestinal epithelial CNDP2+ cells, not macrophages, are the principal in vivo source of basal and metformin-inducible Lac-Phe. Genetic ablation of Lac-Phe biosynthesis in male mice renders animals resistant to the effects of metformin on food intake and body weight. Lastly, mediation analyses support a role for Lac-Phe as a downstream effector of metformin’s effects on body mass index in participants of a large population-based observational cohort, the Multi-Ethnic Study of Atherosclerosis. Together, these data establish Lac-Phe as a critical mediator of the body weight-lowering effects of metformin.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available within the main and Extended Data files. Source data are provided with this paper.
References
LaMoia, T. E. & Shulman, G. I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 42, 77–96 (2021).
Foretz, M., Guigas, B., Bertrand, L., Pollak, M. & Viollet, B. Metformin: from mechanisms of action to therapies. Cell Metab. 20, 953–966 (2014).
Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35, 731–737 (2012).
Knell, G., Li, Q., Pettee Gabriel, K. & Shuval, K. Long-term weight loss and metabolic health in adults concerned with maintaining or losing weight: findings from NHANES. Mayo Clin. Proc. 93, 1611–1616 (2018).
Apolzan, J. W. et al. Long-term weight loss with metformin or lifestyle intervention in the Diabetes Prevention Program Outcomes Study. Ann. Intern. Med. 170, 682–690 (2019).
Yerevanian, A. & Soukas, A. A. Metformin: mechanisms in human obesity and weight loss. Curr. Obes. Rep. 8, 156–164 (2019).
Lee, A. & Morley, J. E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes. Res. 6, 47–53 (1998).
Day, E. A. et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 1, 1202–1208 (2019).
Coll, A. P. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020).
Zhang, S.-Y. et al. Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight. Cell Metab. 35, 875–886.e5 (2023).
Klein, A. B. et al. The GDF15-GFRAL pathway is dispensable for the effects of metformin on energy balance. Cell Rep. 40, 111258 (2022).
Klein, A. B., Kleinert, M., Richter, E. A. & Clemmensen, C. GDF15 in appetite and exercise: Essential player or coincidental bystander? Endocrinology 163, bqab242 (2022).
Li, V. L. et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 606, 785–790 (2022).
Hoene, M. et al. Exercise-induced N-lactoylphenylalanine predicts adipose tissue loss during endurance training in overweight and obese humans. Metabolites 13, 15 (2022).
Abbasi, F. et al. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism 53, 159–164 (2004).
Bild, D. E. et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am. J. Epidemiol. 156, 871–881 (2002).
Lévy, J. et al. Intestinal inhibition of Atg7 prevents tumour initiation through a microbiome-influenced immune response and suppresses tumour growth. Nat. Cell Biol. 17, 1062–1073 (2015).
He, L. et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 (2009).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014).
Chandel, N. S. et al. Are metformin doses used in murine cancer models clinically relevant? Cell Metab. 23, 569–570 (2016).
Howell, J. J. et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 25, 463–471 (2017).
Jansen, R. S. et al. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl Acad. Sci. USA 112, 6601–6606 (2015).
LaMoia, T. E. et al. Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc. Natl Acad. Sci. USA 119, e2122287119 (2022).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
Madison, B. B. et al. cis Elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002).
Yao, Y. et al. Mucus sialylation determines intestinal host–commensal homeostasis. Cell 185, 1172–1188.e28 (2022).
Servin-Vences, M. R. et al. PIEZO2 in somatosensory neurons controls gastrointestinal transit. Cell 186, 3386–3399.e15 (2023).
Hayashi, M. et al. Enteroendocrine cell lineages that differentially control feeding and gut motility. eLife 12, e78512 (2023).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Gerstein, H. C. et al. Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care 40, 280–283 (2017).
Mannucci, E. et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care 24, 489–494 (2001).
Sun, E. W. et al. Metformin triggers PYY secretion in human gut mucosa. J. Clin. Endocrinol. Metab. 104, 2668–2674 (2019).
Lau, J. et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 58, 7370–7380 (2015).
Emmerson, P. J. et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 23, 1215–1219 (2017).
Scott, B. et al. Metformin and feeding increase levels of the appetite-suppressing metabolite Lac-Phe in humans. Nat. Metab. https://doi.org/10.1038/s42255-024-01018-7 (2024).
Gonçalves, R. and Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 111, 14.1.1–14.1.16 (2015).
Jung, Y., Zhao, M. & Svensson, K. J. Isolation, culture, and functional analysis of hepatocytes from mice with fatty liver disease. STAR Protoc. 1, 100222 (2020).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Kim, J. T., Li, V. L., Terrell, S. M., Fischer, C. R. & Long, J. Z. Family-wide annotation of enzymatic pathways by parallel in vivo metabolomics. Cell Chem. Biol. 26, 1623–1629.e3 (2019).
Tahir, U. A. et al. Metabolomic profiles and heart failure risk in black adults: insights from the Jackson Heart Study. Circ. Heart Fail. 14, e007275 (2021).
Tahir, U. A. et al. Whole genome association study of the plasma metabolome identifies metabolites linked to cardiometabolic disease in black individuals. Nat. Commun. 13, 4923 (2022).
Katz, D. H. et al. Whole genome sequence analysis of the plasma proteome in black adults provides novel insights into cardiovascular disease. Circulation 145, 357–370 (2022).
Baron, R. M. & Kenny, D. A. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 51, 1173–1182 (1986).
James, L. R. & Brett, J. M. Mediators, moderators, and tests for mediation. J. Appl. Psychol. 69, 307–321 (1984).
Judd, C. M. & Kenny, D. A. Process analysis: estimating mediation in treatment evaluations. Eval. Rev. 5, 602–619 (1981).
Bollen, K. A. & Stine, R. Direct and indirect effects: classical and bootstrap estimates of variability. Sociol. Methodol. 20, 115–140 (1990).
Shrout, P. E. & Bolger, N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods 7, 422–445 (2002).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R package for causal mediation analysis. J. Stat. Soft. 59, 1–38 (2014).
Acknowledgements
We thank members of the Long and Svensson lab for helpful discussions. We thank T. Wyss-Coray for sharing the BV-2 microglial cell line. We thank P. J. Emmerson for sharing the GFRAL neutralizing antibody and control IgG. We thank L. Liu from the Wu lab for helping with Seahorse assays. S.X. is supported by the Stanford School of Medicine Dean’s Fellowship and a Postdoctoral Fellowship from the American Heart Association (24POST1200064). V.L.L. is supported by the NIH (GM113854) and a Bio-X SIGF Graduate Student Fellowship. This work was supported by the NIH (K08HL145095 to M.B., DK124265 and DK136526 to J.Z.L.). This study was also supported in part by the National Heart, Lung and Blood Institute (NHLBI) TOPMed MESA Multi-Omics (HHSN2682015000031/HSN26800004). MESA projects are conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1TR001881, DK063491 and R01HL105756. We thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutes can be found at http://www.mesa-nhlbi.org. Supported in part by the NIH, NHLBI contract 1R01HL151855 and the National Institute of Diabetes and Digestive and Kidney Diseases contract UM1DK078616.
Author information
Authors and Affiliations
Contributions
S.X., M.D.B. and J.Z.L. conceived and designed the study. S.X., V.L.L., X.L., X.C., W.W., F.A., J.W.K., A.S.-H.T., S.D., G.T., X.S., S.Z., L.F., Z.-Z.C., K.D.T., X.G., M.O.G., A.C.W., Y.-D.I.C., L.A.L., S.S.R., J.I.R., C.B.C., U.A.T., R.E.G., M.D.B. and J.Z.L. collected data. S.X., V.L.L., X.L., X.C., W.W., F.A., J.W.K., A.S.-H.T., S.D., G.T., X.S., S.Z., L.F., Z.-Z.C., K.D.T., X.G., M.O.G., A.C.W., Y.-D.I.C., L.A.L., S.S.R., J.I.R., C.B.C., U.A.T., R.E.G., M.D.B. and J.Z.L analyzed and interpreted the data. S.X., M.D.B. and J.Z.L. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Tara TeSlaa, Gary Churchill and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
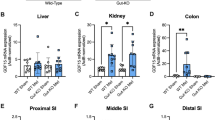
Extended Data Fig. 1 Additional characterization of metabolites after metformin treatment.
(a) Retention time of metformin-induced Lac-Phe (red trace) and an authentic Lac-Phe standard (blue trace) with a 30-min LC method. (b) MS2 profile of metformin-induced Lac-Phe with 10 eV collision energy. Note a characteristic transition from 236.1 to 88.0 characteristic of the lactamide daughter ion of Lac-Phe. (c) Correlation of plasma metformin and Lac-Phe levels in the Stanford Cohort (N = 21). Pearson r = 0.1435, R2 = 0.0206, p = 0.5350 (two-sided). Error bands represent 95% confidence intervals. (d) Plasma levels of lactate pre- and post- 3 months of metformin treatment in the Stanford cohort (n = 21). (e) Plasma levels of lactate in MESA participants on metformin (n = 179) compared to participants not on metformin (n = 3477). Dashed lines indicate medians and quartiles. (f) Metformin levels in the plasma of 12–14 week-old C57BL/6J DIO male mice treated with metformin at indicated doses, PO (n = 5, except for 100 mg/kg and 300 mg/kg at 24 h where n = 4). 100 mg/kg, 300 mg/kg, and 600 mg/kg corresponds to 0.60 × 106nmol/kg, 1.81 × 106nmol/kg, 3.62 × 106nmol/kg, respectively. (g) Metformin levels in tissues of 14–15 week-old C57BL/6J DIO male mice treated with metformin (300 mg/kg, PO) (n = 4). (h) Fold changes of lactate and Lac-Phe in plasma of 12–14 week-old C57BL/6J DIO male mice treated with metformin (300 mg/kg, PO) (n = 5, except for 100 mg/kg and 300 mg/kg at 24 h where n = 4). (i) Fold changes of Lac-Phe in plasma of 14–15 week-old C57BL/6J DIO male mice 4 h and 24 h after fasting and 1 h and 4h after refeeding. (n = 5, except for the 4h fasting time point where n = 4). P values in (a-b), and (f) were calculated with two-sided paired t tests. P value in (c) was generated using linear regression models adjusting for age, sex, fasting plasma glucose, total cholesterol, and hypertension status. All error bars in (f-i) are SEM.
Extended Data Fig. 2 Metformin inhibits complex I to drive Lac-Phe production in primary macrophages.
(a) Cellular respiration following treatment of primary macrophages with the indicated concentrations of metformin (n = 5/concentration). (b) Cellular respiration of primary macrophages following treatment with the indicated concentrations of biguanides overnight (n = 5/concentration for biguanides, N = 6/concentration for control). (c) Fold-change in media Lac-Phe levels in primary macrophages following overnight treatment with the indicated inhibitor of oxidative phosphorylation at the indicated concentration (n = 3/concentration) (d) Fold-change in media Lac-Phe levels or 13C-labeled Lac-Phe levels in primary macrophage (n=3/condition) using 13C-labeled lactate. P values in (c) were calculated using two-sided one sample t test. *p < 0.05, **p < 0.01. The exact p values in (c) are: 0.051, 0.005, 0.011, 0.014, 0.002, 0.008, 0.084, 0.018. N values from (a-d) represent biological independent samples. All error bars are SEM.
Extended Data Fig. 3 Metformin inhibits complex I to drive Lac-Phe production in Caco-2 cells.
(a-b) Cellular respiration of Caco-2 cells following treatment with the indicated concentrations of metformin (a, n = 5/concentration) or biguanide (b, n = 6/concentration) overnight. (c-d) Percent inhibition of basal respiration (c, n = 5/concentration for metformin; n = 6/concentration for buformin and phenformin) and Lac-Phe concentration in media (d, n = 3/concentration) following overnight treatment of Caco-2 cells with the indicated biguanide at the indicated concentrations. (e) Fold-change in media Lac-Phe levels in Caco-2 cells following overnight treatment with the indicated oxidative phosphorylation inhibitor at the indicated concentration (n = 3/concentration) (f-g) Fold-change in media Lac-Phe levels or 13C-labeled Lac-Phe levels in Caco-2 cells (n = 3/condition) using either 13C-labeled glucose (f) or 13C-labeled lactate (g). (h) Cell viability after treated with Lac-Phe at indicated concentrations overnight as revealed by CCK-8 assay (n = 5/concentration). P values in (e) were calculated using two-sided one sample t test. *p < 0.05, **p < 0.01, *** p < 0.001. The exact p values in (e) are: 0.0050, 0.0144, 0.3965, 0.9973, 0.0232, 0.0004, 0.0064, 0.0007. N numbers in (a-h) represent biological independent samples. All error bars are SEM.
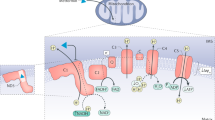
Extended Data Fig. 4 Additional characterization of cells and mice with genetic ablation of CNDP2.
(a) Western blotting using anti-CNDP2 and anti-β-tubulin antibodies of WT and CNDP2-KO primary macrophages, Caco-2 cells, and BV-2 cells. (b) Relative plasma lactate levels in 12–14 week-old male WT or global CNDP2-KO DIO mice in the basal state or 1 h after treatment with metformin (300 mg/kg, PO) (n = 6 for WT; n = 7 for CNDP2-KO mice). Error bars are SEM. (c) Schematic illustration of conditional knockout of Cndp2 in macrophage or gut. (d) Western blotting using an anti-β-tubulin antibody in peritoneal macrophages, gut, or kidney tissues isolated from the indicated genotypes. (e) Western blotting using anti-CNDP2 and anti-β-tubulin antibodies in heart, lung, liver tissues isolated from the indicated genotypes. P values in (b) were calculated with two-sided multiple paired t tests with Holm-Šídák corrections. Western Blot experiments in (a), (d), and (e) have been done twice, similar results obtained.
Extended Data Fig. 5 Additional control analyses for the mediation effect of Lac-Phe on metformin-associated BMI reduction.
(a) Schematic of the two subgroups of MESA in the post-hoc subgroup analysis stratified by ΔBMI. The change in BMI of the complete MESA sample prior to subgroup analysis was 0.24 ± 0.04 kg/m2, n = 3645. Participants with increased BMI are shown in red; participants with decreased BMI are shown in blue. (b-d) The mediation model in Fig. 3b was reordered to test if ΔBMI partially mediates the relationship between metformin use (exposure) and Lac-Phe levels (outcome). (b) Among MESA participants with decreased BMI, the total effect of metformin use on Lac-Phe levels was assessed in an ‘unmediated model’ using an age- and sex-adjusted linear regression model. (c) To construct mediation models, the individual associations of metformin use, lac-phe, lactate, and ΔBMI were assessed using linear regression models as described in Methods. The direct effect of metformin use on Lac-Phe levels was then assessed using an age- and sex-adjusted linear regression model adjusted for ΔBMI. No reduction in the direct effect of metformin on Lac-Phe levels was appreciated compared to the total effect of metformin on Lac-Phe in the unmediated model suggesting no meditation. (d) The mediation effect of ΔBMI derived from the mediation models in (c), n = 1184. Values are median with 95% confidence intervals. Statistical significance were calculated using nonparametric bootstrapping with the percentile method. (e-g) The mediation effects of Lac-Phe and lactate on the effect of metformin-associated BMI increase. (e) Among MESA participants with increased BMI, the total effect of metformin use on ΔBMI was assessed in an ‘unmediated model’ using an age- and sex-adjusted linear regression model. (f) To construct mediation models, the individual associations of metformin use, Lac-Phe, lactate, and ΔBMI were assessed using linear regression models as described in Methods. The direct effect of metformin use on ΔBMI was then assessed using an age- and sex-adjusted linear regression model adjusted for either Lac-Phe (left) or lactate (right). No reduction in the direct effects of metformin on ΔBMI compared to the total effect of metformin on ΔBMI in the unmediated model suggested no meditation effect of either Lac-Phe or lactate. (g) The mediation effects of Lac-Phe and lactate. derived from the mediation models in (f), n = 1460. Values are plotted as mean with 95% confidence intervals. Confidence intervals and statistical significance (unadjusted p values) were calculated using nonparametric bootstrapping with the percentile method, as described in Methods. (h) The mediation effects of additional 136 metabolites measured in MESA participants with decreased BMI using the amide negative LC-MS method. Seven out of the 136 tested metabolites were found to have a predicted mediation effect with unadjusted p-value ≤ 0.05 (right). Values are median values with 95% confidence intervals. The confidence intervals and statistical significance of the predicted mediation effects for these seven metabolites were calculated using nonparametric bootstrapping with the percentile method (n = 1619), as described in Methods.
Extended Data Fig. 6 Physiological effects of metformin and Lac-Phe treatments in DIO mice.
(a) Glucose levels of WT and CNDP2-KO DIO mice (12–14 week-old, male) during GTT after receiving metformin (300 mg/kg, PO) 30 minute before receiving glucose (1 g/kg, i.p.). N = 5 per group. (b) Circulating PYY levels in WT and CNDP2-KO DIO mice (12–14 week-old, male) after metformin treatment (300 mg/kg, PO). N = 5 per group. (c) Glucose levels of C57BL/6J DIO mice (14-15 week-old, male) during GTT after receiving Lac-Phe (50 mg/kg, i.p.) or vehicle control 30 minute before receiving glucose (1 g/kg, i.p.). N = 6 per group. (d) Glucose levels of C57BL/6J DIO mice (14-15 week-old, male) during ITT after receiving Lac-Phe (50 mg/kg, i.p.) or vehicle control 30 minute before receiving insulin (1 U/kg, i.p.). N = 6 per group. (e) Glucose levels of C57BL/6J DIO mice (14-15 week-old, male) during PTT after receiving Lac-Phe (50 mg/kg, i.p.) or vehicle control 30 minute before receiving sodium pyruvate (2.5 g/kg, i.p.). N = 7 per group. (f) Effect of GDF15 on body weight in C57BL/6J DIO mice (12–14 week-old, male) after receiving anti-GFRAL antibody or control IgG. N = 5 per group. P values in (a) and (c-e) were measured with two-sided two-way ANOVA. P values in (b) and (f) were calculated with two-sided Welch t tests. All error bars are SEM.
Supplementary information
Supplementary Tables 1 & 2
Table 1. Baseline characteristics of MESA participants with available plasma profiling of Lac-Phe, lactate, and metformin levels by LC–MS. Values are mean (s.d.), unless otherwise specified. Table 2. Baseline characteristics from the post-hoc subgroup analysis of MESA participants who gained weight and participants who lost weight during the study period from exams one to five. Values are mean (s.d.), unless otherwise specified. P values were generated using unpaired two-sample t-tests.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Unprocessed Western Blots.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Unprocessed Western Blots.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, S., Li, V.L., Lyu, X. et al. Lac-Phe mediates the effects of metformin on food intake and body weight. Nat Metab 6, 659–669 (2024). https://doi.org/10.1038/s42255-024-00999-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-00999-9
This article is cited by
-
Metformin induces a Lac-Phe gut–brain signalling axis
Nature Metabolism (2024)
-
Metformin and feeding increase levels of the appetite-suppressing metabolite Lac-Phe in humans
Nature Metabolism (2024)
-
Metformin acts through appetite-suppressing metabolite: Lac-Phe
Nature Reviews Endocrinology (2024)