Abstract
Ghrelin, produced mainly by gastric X/A-like cells, triggers a hunger signal to the central nervous system to stimulate appetite. It remains unclear whether X/A-like cells sense gastric distention and thus regulate ghrelin production. Here we show that PIEZO1 expression in X/A-like cells decreases in patients with obesity when compared to controls, whereas it increases after sleeve gastrectomy. Male and female mice with specific loss of Piezo1 in X/A-like cells exhibit hyperghrelinaemia and hyperphagia and are more susceptible to overweight. These phenotypes are associated with impairment of the gastric CaMKKII/CaMKIV–mTOR signalling pathway. Activation of PIEZO1 by Yoda1 or gastric bead implantation inhibits ghrelin production, decreases energy intake and induces weight loss in mice. Inhibition of ghrelin production by Piezo1 through the CaMKKII/CaMKIV–mTOR pathway can be recapitulated in a ghrelin-producing cell line mHypoE-42. Our study reveals a mechanical regulation of ghrelin production and appetite by PIEZO1 of X/A-like cells, which suggests a promising target for anti-obesity therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and Supplementary Information. Source data for Figs. 1–8 and Extended Data Figs. 1–10 are provided with the online version of the paper. Source data are provided with this paper.
References
Hall, K. D. et al. Quantification of the effect of energy imbalance on body weight. Lancet 378, 826–837 (2011).
Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
De, C. et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719 (2001).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Cummings, D. E. et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat. Med. 8, 643–644 (2002).
Muscogiuri, G. et al. Prader–Willi syndrome: an uptodate on endocrine and metabolic complications. Rev. Endocr. Metab. Disord. 20, 239–250 (2019).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Wren, A. M. et al. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001).
Kobelt, P. et al. Anti-ghrelin Spiegelmer NOX-B11 inhibits neurostimulatory and orexigenic effects of peripheral ghrelin in rats. Gut 55, 788–792 (2006).
Altabas, V. & Zjačić-Rotkvić, V. Anti-ghrelin antibodies in appetite suppression: recent advances in obesity pharmacotherapy. ImmunoTargets Ther. 4, 123–130 (2015).
Kojima, M. & Kangawa, K. Ghrelin: structure and function. Physiol. Rev. 85, 495–522 (2005).
Müller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Xu, G. et al. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology 150, 3637–3644 (2009).
Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 148, 1219–1233 (2015).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Wu, J., Lewis, A. H. & Grandl, J. Touch, tension, and transduction: the function and regulation of piezo ion channels. Trends Biochem. Sci. 42, 57–71 (2017).
Murthy, S. E., Dubin, A. E. & Patapoutian, A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18, 771–783 (2017).
Min, S. et al. Control of feeding by Piezo-mediated gut mechanosensation in Drosophila. eLife 10, e63049 (2021).
Hughes, K. et al. Distinct mechanoreceptor pezo-1 isoforms modulate food intake in the nematode Caenorhabditis elegans. G3 Bethesda Md 12, jkab429 (2022).
Ye, Y. et al. A critical role of the mechanosensor PIEZO1 in glucose-induced insulin secretion in pancreatic β-cells. Nat. Commun. 13, 4237 (2022).
Sugisawa, E. et al. RNA sensing by gut Piezo1 is essential for systemic serotonin synthesis. Cell 182, 609–624 (2020).
Li, Z. et al. mTOR signaling in X/A-like cells contributes to lipid homeostasis in mice. Hepatology 69, 860–875 (2019).
Ellefsen, K. L. et al. Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers. Commun. Biol. 2, 298 (2019).
Marcelo, K. L., Means, A. R. & York, B. The Ca(2+)/calmodulin/CaMKK2 axis: nature’s metabolic CaMshaft. Trends Endocrinol. Metab. 27, 706–718 (2016).
Tokumitsu, H. & Sakagami, H. Molecular mechanisms underlying Ca2+/calmodulin-dependent protein kinase kinase signal transduction. Int. J. Mol. Sci. 23, 11025 (2022).
Lin, F. et al. The camKK2/camKIV relay is an essential regulator of hepatic cancer. Hepatology 62, 505–520 (2015).
Berthoud, H. R. & Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 59, 55–92 (2008).
Tremblay, A. & Bellisle, F. Nutrients, satiety, and control of energy intake. Appl. Physiol. Nutr. Metab. Physiol. 40, 971–979 (2015).
Wren, A. M. & Bloom, S. R. Gut hormones and appetite control. Gastroenterology 132, 2116–2130 (2007).
Buhmann, H., le Roux, C. W. & Bueter, M. The gut–brain axis in obesity. Best. Pract. Res. Clin. Gastroenterol. 28, 559–571 (2014).
Andrews, Z. B. et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454, 846–851 (2008).
Yin, X., Li, Y., Xu, G., An, W. & Zhang, W. Ghrelin fluctuation, what determines its production? Acta Biochim. Biophys. Sin. 41, 188–197 (2009).
Wang, P., Jia, Y., Liu, T., Jan, Y.-N. & Zhang, W. Visceral mechano-sensing neurons control Drosophila feeding by using Piezo as a sensor. Neuron 108, 640–650 (2020).
Gaur, S., Levy, S., Mathus-Vliegen, L. & Chuttani, R. Balancing risk and reward: a critical review of the intragastric balloon for weight loss. Gastrointest. Endosc. 81, 1330–1336 (2015).
Anderson, K. A. et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 (2008).
Xu, G. et al. Ghrelin contributes to derangements of glucose metabolism induced by rapamycin in mice. Diabetologia 55, 1813–1823 (2012).
Acknowledgements
We thank J. Guo (The Chinese University of Hong Kong) for providing the Piezo1 plasmid and J. Lyu from Jinan University for providing CaMKKII and CaMKIV plasmids.
This work was supported by grants from the National Natural Science Foundation of China (82170818 and 81770794) and the Fundamental Research Funds for the Central Universities (21620423) to G.X.
Author information
Authors and Affiliations
Contributions
G.X. and W.Z. designed the research. Y.Z., Y.L., T.T., J.Z., W.G., H.D., M.H., H.M., X.T., S.L., J.Y., H.Z., Q.W., H.C. and Z.H. conducted the study. Y.Z., H.C. and G.X. analysed data. G.X., H.C. and Y.Z. wrote and edited the paper. All authors contributed to the discussion, revised the paper and approved the final versions of the paper. G.X. is responsible for the integrity of the work as a whole.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Melanie Kaelberer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
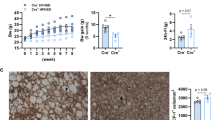
Extended Data Fig. 1 Body weight of pubs of control and Ghrl-Piezo1-/- mice.
a, A representative photograph showing 1-week-old Ghrl-Cre, Piezo1fl/fl, and Ghrl-Piezo1-/- mice. b, Genotyping PCR results for control and Ghrl-Piezo1-/- mice. Representative images of three replicates. c, Body weight of control and Ghrl-Piezo1-/- mice at one- and two- week after born (both male and females, n = 6 mice per group). Data are shown as mean values ± SEM, and are representative of six biological replicates, P values were calculated by one-way ANOVA with Tukey post-hoc test.
Extended Data Fig. 2 Glucose metabolism in male control and Ghrl-Piezo1-/- mice.
a, Intraperitoneal Glucose Tolerance Test (IPGTT) and associated area under the curve (AUC) values of Piezo1fl/fl and Ghrl-Piezo1-/- mice under NCD (n = 5 mice per group). b, Insulin tolerance tests (ITT) and AUC of Piezo1fl/fl and Ghrl-Piezo1-/- mice under NCD (n = 5 mice per group). Data are shown as mean values ± SEM, and are representative of five biological replicates, P values were calculated by two-sided t-tests. *p < 0.05, **p < 0.01, ***p < 0.001. c, IPGTT and AUC values of Piezo1fl/fl and Ghrl-Piezo1-/- mice under HFD (n = 6 mice per group). d, Insulin tolerance tests (ITT) and AUC values of Piezo1fl/fl and Ghrl-Piezo1-/- mice under HFD. Date are expressed as mean values ± SEM. Student’s t-test was used for comparison between two groups (n = 6 mice per group). Data are shown as mean values ± SEM, and are representative of six biological replicates, P values were calculated by two-sided t-tests.
Extended Data Fig. 3 Double immunofluorescence staining of pCaMKIV and GHRELIN in the mouse gastric mucosa of Piezo1fl/fl and Ghrl-Piezo1-/- mice under NCD.
a, Co-localization of pCaMKIV(red) and GHRELIN (green) in the mouse gastric mucosa of Piezo1fl/fl and Ghrl-Piezo1-/- mice under NCD (n = 6 mice per group). b, Quantitative analysis of pCaMKIV-positive X/A-like cells in the stomach of male mice. 294 Piezo1-positive cells and 177 Ghrelin-positive cells from six control mice, 241 pCaMKIV-positive cells and 295 Ghrelin-positive cells from six Ghrl-Piezo1-/- mice were quantified (n = 6 mice per group). c, Quantitative analysis of ghrelin intensity. Three typical images from each mice (six Piezo1fl/fl and six Ghrl-Piezo1-/- mice) were chose for quantitative analysis of ghrelin intensity (n = 6 mice per group). Data are shown as mean values ± SEM, and are representative of six biological replicates, P values were calculated by two-sided t-tests, *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
Extended Data Fig. 4 Quantitative analysis of Piezo1 and ghrelin in the gastric mucosa of Piezo1fl/fl and Ghrl-Piezo1-/- mice under NCD in Fig. 2k,l.
a, Quantitative analysis of Piezo1-positive X/A-like cells in the stomach of male mice under NCD. 342 Piezo1-positive cells and 153 ghrelin-positive cells from 6 Piezo1fl/fl mice, 305 Piezo1-positive cells and 271 Ghrelin-positive cells from 6 Ghrl-Piezo1-/- mice were quantified for double-labelling immunofluorescence (n = 6 mice per group). 325 Piezo1-positive cells and 145 Ghrelin-positive cells from six control mice, 297 Piezo1-positive cells and 245 Ghrelin-positive cells from six Ghrl-Piezo1-/- mice were quantified for in situ hybridization. b, Quantitative analysis of ghrelin intensity in the stomach of mice of indicated genotypes fed NCD. Three view from each mice (six Piezo1fl/fl and six Ghrl-Piezo1-/- mice) were chose for quantitative analysis of ghrelin intensity (n = 6 mice per group). Data are shown as mean values ± SEM, and are representative of six biological replicates, P values were calculated by two-sided t-tests, *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
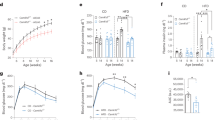
Extended Data Fig. 5 Female Ghrl-Piezo1-/- mice presented hyperghrelinmia and body weight gain.
a, Female Piezo1fl/fl and Ghrl-Piezo1-/- mice were fed an NCD and then placed into metabolic cages. Food intake was monitored in 24 h (n = 5 mice per group). b, Food consumption in light/dark cycle relative to body weight (g/g BW) (n = 5 mice per group). c, Body weight curves of female Piezo1fl/fl and Ghrl-Piezo1-/- mice (n = 5 mice per group). d, Respiratory exchange rate (n = 5 mice per group). e, Energy expenditure were monitored (n = 5 mice per group). f, Intraperitoneal Glucose Tolerance Test (IPGTT) and associated area under the curve (AUC) values of female Piezo1fl/fl and Ghrl-Piezo1-/- mice (n = 6 mice per group). g, Insulin tolerance tests (ITT) and AUC (n = 6 mice per group). h, Piezo1, Piezo2 and Ghrl mRNA levels in gastric corpus mucosa (n = 6 mice per group). i, Plasma des-acyl Ghrelin and acylated Ghrelin of female Piezo1fl/fl and Ghrl-Piezo1-/- mice (n = 6 mice per group). j, Representative Western blots for PIEZO1, CaMKKII, pCaMKIV, CaMKIV, pmTOR, mTOR, pS6K, S6K, pS6, S6, Pro-GHRELIN and β-ACTIN (n = 6 mice per group). Data are shown as mean values ± SEM, and are representative of five or six biological replicates, P values were calculated by two-sided t-tests. *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
Extended Data Fig. 6 Quantification for double-labelling immunofluorescence in male Piezo1fl/fl and Ghrl-Piezo1-/-mice under HFD in Fig. 3i.
a, Quantitative analysis of Piezo1-positive ghrelin cells in the stomach of male Piezo1fl/fl and Ghrl-Piezo1-/-mice fed HFD (n = 6/group). 358 Piezo1-positive cells and 190 Ghrelin-positive cells from six control mice, 280 Piezo1-positive cells and 229 Ghrelin-positive cells from six Ghrl-Piezo1-/- mice under HFD were quantified (n = 6 mice per group). b, Quantitative analysis of Ghrelin intensity of male mice of indicated genotypes fed HFD (n = 6 mice per group). Three typical images from each mice were chosen for quantitative analysis of ghrelin intensity. c, Quantitative analysis of pCaMKIV-positive X/A-like cells in the stomach of male mice. 356 pCaMKIV-positive cells and 176 Ghrelin-positive cells from six control mice, 181 pCaMKIV-positive cells and 189 Ghrelin-positive cells from six Ghrl-Piezo1-/- mice under HFD were quantified for double-labelling immunofluorescence (n = 6 mice per group). d, Quantitative analysis of Ghrelin intensity in the stomach male mice of indicated genotypes fed HFD. Three typical images from each mice (n = 6 mice per group) were chosen for quantitative analysis of ghrelin intensity. Data are shown as mean values ± SEM, and are representative of six biological replicates, P values were calculated by two-sided t-tests.*p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
Extended Data Fig. 7 Double immunofluorescence staining of chromogranin A (ChrA) and Ghrelin in the gastric mucosa of mice treated with yoda1 and/or GsMTx4.
a, Co-localization of chromogranin A (red) and Ghrelin (green) in the mouse gastric mucosa. b, Quantitative analysis of Ghrelin intensity. Three typical images from each mice were chosen for quantitative analysis of ghrelin intensity (n = 6 mice per group). Data are shown as mean values ± SEM, and are representative of six biological replicates, P values were calculated by two-sided t-tests.*p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
Extended Data Fig. 8 Evaluation of mouse comfort.
a, Assessment of abdominal mechanical sensitivity. Mechanical sensitivity of the abdomen was assessed with calibrated (0.07, 0.16, and 1 g) von Frey flaments (VFFs) in sham and bead implanted mice (n = 4 mice per group). b and c, Bars show the time spent in the centre and the total distance moved in sham and bead implanted mice (n = 4 mice per group). Data are shown as mean values ± SEM, and are representative of four biological replicates, P values were calculated by two-sided t-tests.
Extended Data Fig. 9 Quantitative analysis of Ghrelin intensity of male mice receiving sham or bead operation.
a, Quantitative analysis of Ghrelin intensity of male mice receiving sham or bead operation under NCD (n = 5 mice per group).Three images from each mice (five sham and five bead implanted mice) were chose for quantitative analysis of ghrelin intensity. b, Quantitative analysis of ghrelin intensity o of male mice receiving sham or bead operation under HFD (n = 6 mice per group). Three images from each mice (six sham and six bead implanted mice) were chose for quantitative analysis of ghrelin intensity. Data are shown as mean values ± SEM, and are representative of five or six biological replicates, P values were calculated by two-sided t-tests. *p < 0.05, **p < 0.01, ***p < 0.001,****p < 0.0001.
Extended Data Fig. 10 Gastric bead implantation in Ghrl-Piezo1-/- mice.
a, Food intake(n = 5 mice per group). b, Body weight(n = 5 mice per group). c, Western blot analysis of gastric corpus mucosal extracts with the indicated antibodies. d, Ghrl mRNA levels(n = 5 mice per group). e, Plasma des-acyl ghrelin. f, plasma acylated ghrelin levels of male Ghrl-Piezo1-/- mice receiving sham or intra-gastric bead implantation(n = 5 mice per group). Data are shown as mean values ± SEM, and are representative of five or six biological replicates, P values were calculated by two-sided t-tests.
Supplementary information
Supplementary Information
Supplementary Methods.
Supplementary Tables
Supplementary Tables 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Liu, Y., Tao, T. et al. Gastric mechanosensitive channel Piezo1 regulates ghrelin production and food intake. Nat Metab 6, 458–472 (2024). https://doi.org/10.1038/s42255-024-00995-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-00995-z
This article is cited by
-
The pressure not to eat
Nature Metabolism (2024)