Abstract
The coexistence of brown adipocytes with low and high thermogenic activity is a fundamental feature of brown adipose tissue heterogeneity and plasticity. However, the mechanisms that govern thermogenic adipocyte heterogeneity and its significance in obesity and metabolic disease remain poorly understood. Here we show that in male mice, a population of transcription factor jun-B (JunB)-enriched (JunB+) adipocytes within the brown adipose tissue exhibits lower thermogenic capacity compared to high-thermogenic adipocytes. The JunB+ adipocyte population expands in obesity. Depletion of JunB in adipocytes increases the fraction of adipocytes exhibiting high thermogenic capacity, leading to enhanced basal and cold-induced energy expenditure and protection against diet-induced obesity and insulin resistance. Mechanistically, JunB antagonizes the stimulatory effects of PPARγ coactivator-1α on high-thermogenic adipocyte formation by directly binding to the promoter of oestrogen-related receptor alpha, a PPARγ coactivator-1α downstream effector. Taken together, our study uncovers that JunB shapes thermogenic adipocyte heterogeneity, serving a critical role in maintaining systemic metabolic health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated during the current study are shared with researchers. The snRNA-seq datasets have been deposited in the Gene Expression Omnibus and are publicly available under accession https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE244239. Source data are provided with this paper.
Code availability
No custom code was used.
References
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517 (2009).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 123, 3404–3408 (2013).
Hanssen, M. J. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 863–865 (2015).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 123, 215–223 (2013).
Kajimura, S., Spiegelman, B. M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Chondronikola, M. et al. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab. 23, 1200–1206 (2016).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Spaethling, J. M. et al. Single-cell transcriptomics and functional target validation of brown adipocytes show their complex roles in metabolic homeostasis. FASEB J. 30, 81–92 (2016).
Oguri, Y. & Kajimura, S. Cellular heterogeneity in brown adipose tissue. J. Clin. Investig. 130, 65–67 (2020).
Cinti, S. et al. CL316,243 and cold stress induce heterogeneous expression of UCP1 mRNA and protein in rodent brown adipocytes. J. Histochem Cytochem. 50, 21–31 (2002).
Song, A. et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Investig. 130, 247–257 (2020).
Sun, W. et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102 (2020).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Kajimura, S. et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 460, 1154–1158 (2009).
Wang, L. et al. Adiponectin restrains ILC2 activation by AMPK-mediated feedback inhibition of IL-33 signaling. J. Exp. Med. 218, e20191054 (2021).
Alcivar, A. A., Hake, L. E., Hardy, M. P. & Hecht, N. B. Increased levels of junB and c-jun mRNAs in male germ cells following testicular cell dissociation. Maximal stimulation in prepuberal animals. J. Biol. Chem. 265, 20160–20165 (1990).
Schorpp-Kistner, M., Wang, Z. Q., Angel, P. & Wagner, E. F. JunB is essential for mammalian placentation. EMBO J. 18, 934–948 (1999).
Passegue, E., Jochum, W., Schorpp-Kistner, M., Mohle-Steinlein, U. & Wagner, E. F. Chronic myeloid leukemia with increased granulocyte progenitors in mice lacking junB expression in the myeloid lineage. Cell 104, 21–32 (2001).
Zenz, R. et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437, 369–375 (2005).
Hasan, Z. et al. JunB is essential for IL-23-dependent pathogenicity of Th17 cells. Nat. Commun. 8, 15628 (2017).
Carr, T. M., Wheaton, J. D., Houtz, G. M. & Ciofani, M. JunB promotes Th17 cell identity and restrains alternative CD4+ T-cell programs during inflammation. Nat. Commun. 8, 301 (2017).
Deng, T. & Karin, M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 7, 479–490 (1993).
Shaulian, E. & Karin, M. AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 (2001).
Meijer, C. A. et al. Activator protein-1 (AP-1) signalling in human atherosclerosis: results of a systematic evaluation and intervention study. Clin. Sci. 122, 421–428 (2012).
Thomsen, M. K. et al. JUNB/AP-1 controls IFN-gamma during inflammatory liver disease. J. Clin. Investig. 123, 5258–5268 (2013).
Hyakusoku, H. et al. JunB promotes cell invasion, migration and distant metastasis of head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 35, 6 (2016).
Yoshitomi, Y. et al. JunB regulates angiogenesis and neurovascular parallel alignment in mouse embryonic skin. J. Cell Sci. 130, 916–926 (2017).
Sartipy, P. & Loskutoff, D. J. Expression profiling identifies genes that continue to respond to insulin in adipocytes made insulin-resistant by treatment with tumor necrosis factor-alpha. J. Biol. Chem. 278, 52298–52306 (2003).
Nakajima, K. & Wall, R. Interleukin-6 signals activating junB and TIS11 gene transcription in a B-cell hybridoma. Mol. Cell. Biol. 11, 1409–1418 (1991).
Pinent, M. et al. Adipose triglyceride lipase and hormone-sensitive lipase are involved in fat loss in JunB-deficient mice. Endocrinology 152, 2678–2689 (2011).
Polak, P. et al. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 8, 399–410 (2008).
Liu, M. et al. Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab. 19, 967–980 (2014).
Liu, D. et al. Activation of mTORC1 is essential for beta-adrenergic stimulation of adipose browning. J. Clin. Investig. 126, 1704–1716 (2016).
Zhang, X. et al. Adipose mTORC1 suppresses prostaglandin signaling and beige adipogenesis via the CRTC2–COX-2 pathway. Cell Rep. 24, 3180–3193 (2018).
Wada, S. et al. The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 30, 2551–2564 (2016).
Tran, C. M. et al. Rapamycin blocks induction of the thermogenic program in white adipose tissue. Diabetes 65, 927–941 (2016).
Lee, P. L., Tang, Y., Li, H. & Guertin, D. A. Raptor/mTORC1 loss in adipocytes causes progressive lipodystrophy and fatty liver disease. Mol. Metab. 5, 422–432 (2016).
Xiao, H. et al. Architecture of the outbred brown fat proteome defines regulators of metabolic physiology. Cell 185, 4654–4673 (2022).
Bakiri, L., Lallemand, D., Bossy-Wetzel, E. & Yaniv, M. Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J. 19, 2056–2068 (2000).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Cartharius, K. et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21, 2933–2942 (2005).
Huss, J. M., Kopp, R. P. & Kelly, D. P. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J. Biol. Chem. 277, 40265–40274 (2002).
Schreiber, S. N. et al. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 101, 6472–6477 (2004).
Berbee, J. F. et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 6, 6356 (2015).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 121, 96–105 (2011).
Bostrom, P. et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Nakajima, K. et al. Identification of a novel interleukin-6 response element containing an Ets-binding site and a CRE-like site in the junB promoter. Mol. Cell. Biol. 13, 3027–3041 (1993).
Fan, F. et al. JunB is a key regulator of multiple myeloma bone marrow angiogenesis. Leukemia 35, 3509–3525 (2021).
Kobierski, L. A., Chu, H. M., Tan, Y. & Comb, M. J. cAMP-dependent regulation of proenkephalin by JunD and JunB: positive and negative effects of AP-1 proteins. Proc. Natl. Acad. Sci. USA 88, 10222–10226 (1991).
Hsu, J. C., Cressman, D. E. & Taub, R. Promoter-specific trans-activation and inhibition mediated by JunB. Cancer Res. 53, 3789–3794 (1993).
Lord, K. A., Abdollahi, A., Hoffman-Liebermann, B. & Liebermann, D. A. Proto-oncogenes of the fos/jun family of transcription factors are positive regulators of myeloid differentiation. Mol. Cell. Biol. 13, 841–851 (1993).
Petrovic, N. et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 (2010).
Rodeheffer, M. S., Birsoy, K. & Friedman, J. M. Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 (2008).
Berry, R. & Rodeheffer, M. S. Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol. 15, 302–308 (2013).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 (2019).
Jespersen, N. Z. et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 (2013).
Plummer, N. W., Ungewitter, E. K., Smith, K. G., Yao, H. H. & Jensen, P. A new mouse line for cell ablation by diphtheria toxin subunit A controlled by a Cre-dependent FLEx switch. Genesis 55, e23067 (2017).
Quiros, P. M., Goyal, A., Jha, P. & Auwerx, J. Analysis of mtDNA/nDNA ratio in mice. Curr. Protoc. Mouse Biol. 7, 47–54 (2017).
Luo, Y. et al. Myeloid adrenergic signaling via CaMKII forms a feedforward loop of catecholamine biosynthesis. J. Mol. Cell Biol. 9, 422–434 (2017).
Wang, C. et al. Adipocyte-derived PGE2 is required for intermittent fasting-induced Treg proliferation and improvement of insulin sensitivity. JCI Insight 7, e153755 (2022).
Mina, A. I. et al. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab. 28, 656–666 (2018).
Acknowledgements
This work is supported by R01 Awards (DK132643 and DK110439 to M.L. from NIDDK, HL148337 to X.Y. from NHLBI, and CA163890 and CA194496 to E.P. from NCI); Innovative Basic Science Award (1-17-IBS-261 to M.L.) from the American Diabetes Association; Grant in Aid (15GRNT24940018 to M.L.) and Postdoc Fellowship Awards (20POST35120020 to X.Z.) from American Heart Association; P20 Award (GM121176, Project Director: V. Deretic, to Mentored Principal Investigators, M.L.); CoBRE Pilot Award associated with P30 (P30GM103400 principal investigator, J. Liu; to M.L.), CVMD Pilot Award (to M.L.), CTSC pilot Award associated with grant (UL1TR001449) (principal investigators, M. Campen and N. Pandhi; to M.L.) and UNMCCC pilot Award associated with P30 (CA118100) (principal investigator, Y. Sanchez; to M.L.) at the University of New Mexico Health Sciences Center (UNMHSC). This project was supported in part by the Dedicated Health Research Funds from the University of New Mexico School of Medicine. We thank the Autophagy, Inflammation and Metabolism Center at UNMHSC for providing the Cellomics HCS scanner for our present study and technical support. We want to thank UNM Comprehensive Cancer Center (UNMCCC) for the core support on snRNA-seq (completed in the ATG Core), bioinformatic analysis (performed by K. Brayer at the Cancer Research Facility) and flow cytometry.
Author information
Authors and Affiliations
Contributions
M.L. and X.Z. designed the project. X.Z., X.D., C.W., Q.L., D.W., A.S., G.H., L.L., Y.L., X.Y., A.E.G. and C.Q. conducted the experiments, and X.Z., X.D., C.W., Q.L., D.W., G.H., L.L., Y.L., X.Y. and A.E.G. analysed the results. M.S., N.B., F.S. and L.E.F. completed the collection of human tissue samples. S.P.D. provided Cellomic and Seahorse technical support. Q.W. and A.S. provided the adipose tissue slides of AdipoChaser-GFP mice and completed the immunostaining of adipose tissue. J.Z. contributed to the transcriptional activity assays. X.O.Y. contributed to the design of JunB animal models and transcriptional activity assays. E.P. financially supported this project and edited the manuscript. X.Z. organized the data, and X.Z. and M.L. wrote the manuscript. L.E.F. edited the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Camilla Scheele and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Isabella Samuelson and Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
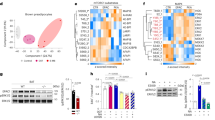
Extended Data Fig. 1 The mRNA levels of JunB were upregulated by inhibition of mTORC1 and by obesity in BAT, related to Fig. 1.
a. The layout of fold changes (rapamycin: control) of 96 genes that are cAMP responsive in PCR array analysis. The reverse transcription was performed with total RNA extracted from BAT in mice treated with or without rapamycin (n = 3/group), and then the synthesized cDNAs was used for PCR array analysis. b. RNA-sequencing gene expression signatures of iWAT from 10-week-old male Raptor KO and control mice. n = 2–3/group. c. The protein levels of JunB were upregulated by HFD feeding in adipose tissue. d. Quantification of JunB expression in Extended Data Figure c. e. mRNA levels of JunB were induced in WAT of HFD-fed mice (6 mice/group). f. mRNA levels of Ucp1 and Pgc-1α in human deep neck fat were negatively correlated with BMI. g. The expression pattern of JunB in adipocyte subsets and APCs in human BAT. h. Quantification the protein level in Fig. 1k. i. JunB is expressed in Plin1-enriched primary differentiated adipocytes. j. JunB was highly enriched in primary preadipocytes which declined during differentiation (n = 3, independent experiments). Statistical analysis was performed using Pearson’s correlation coefficient in Extended Data Fig. 1f. Extended Data Fig. 1d, e, h, j were analyzed using unpaired two-sided T-Test.
Extended Data Fig. 2 The JunB Chaser mouse model was generated to show the presence of JunB-expressing adipocytes in fat, related to Fig. 2.
a. The generation strategy for JunBCreERT2 mice as described in the genomic structure. An internal ribosome entry site (IRES) fused to a CreERT2 fusion gene was inserted downstream of the internal stop codon of Junb gene. b. Representative tissue distribution of iCre and Junb mRNA in adult JunBCreERT2 transgenic mice as determined by qPCR. c. Immunofluorescence staining of YFP+ cells in liver and pancreas of JunBCreERT2 mice. Differentiated YFP+ brown adipocytes were present during primary adipogenesis by imaging (d) and flow cytometry (e) in Primary brown adipocytes of JunBCreERT2 mice. f. JunB+ adipocytes were increased by the treatment of 20 ng/mL TNFα or 20 ng/mL IL-6 for 24 hrs post primary adipogenesis. g. Quantification of JunB-expressing adipocytes in Figure f. h. JunB+ adipocytes were increased by the treatment of 20 ng/mL TNFα or 20 ng/mL IL-6 for 24 hrs post primary adipogenesis. Preadiocytes were isolated from AdipoChaser-YFP mice and differentiated into adipocytes. i. Quantification of JunB-expressing adipocytes in Figure h. All data in this Figure were analyzed by unpaired two-sided T-Test.
Extended Data Fig. 3 Both JunB FKO and BKO mice display enhanced energy expenditure and cold adaptation, related to Fig. 3.
10-week-old male were individually housed in Phenotyping Systems (Sable Systems International) coupled with a temperature controllable chamber. a. Respiratory exchange ratio (RER) was decreased in adipocyte-specific JunB KO (JunB FKO) mice compared to control littermates under cold stress conditions. Food intake (b) and motor activities (c) were little affected by JunB deficiency in fat. d. mRNA levels of JunB were significantly downregulated in BAT but not in gWAT of UCP1+ cell-specific JunB KO (JunB BKO) mice compared to the controls. e. JunB BKO mice exhibited enhanced basal and cold-induced O2 consumption throughout light and dark cycles compared with controls. f. The quantified data of Extended Data Fig. 3e using CaIR. There was little difference in food intake (g) despite slightly reduced motor activities under cold stress conditions (h) between JunB BKO and control mice. i. JunB deficiency significantly increased oxygen consumption in primary brown adipocytes (4 mice/group). j. The quantified data of Extended Data Fig. 3i using CaIR. k. Ablation of JunB-expressing adipocytes upregulated the expression levels of Ucp1, Ppargc1α, and Errα (7 mice/group). Extended Data Fig. 3i was analyzed by ANOVA analysis and the unpaired two-sided T-Test was used for the rest figures. Data are presented as the mean ± SEM. *P < 0.05 or **P < 0.01.
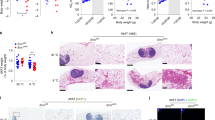
Extended Data Fig. 4 Both JunB FKO and BKO mice exhibit improved diet-induced insulin resistance, related to Fig. 4.
6-week-old male JunB FKO and control mice were fed with HFD for 16 weeks and used for the studies (4a-h). The percentage of larger adipocytes was decreased accompanied with an increase in smaller adipocytes in iWAT (a) and BAT (b) of HFD-fed JunB BKO mice compared to controls. Depletion of JunB in adipocytes improved glucose (c) and insulin (d) tolerance after 16-week HFD feeding. JunB FKO mice displayed improved hepatic steatosis (e) and triglyceride content in the liver (8 mice for NC and 6 mice for HFD). (f) of JunB FKO mice compared with control mice under HFD feeding conditions. g-h. O2 consumption was significantly increased in JunB FKO mice compared to controls after feeding with HFD for 12 weeks. Extended Data Fig. 4a–d were analyzed by ANOVA analysis. Extended Data Fig. 4f was analyzed with unpaired two-sided T-Test. Data are presented as the mean ± SEM. *P < 0.05 or **P < 0.01.
Extended Data Fig. 5 Depletion of JunB in adipocytes increased O2 consumption with insignificant anti-obesity effect under HFD condition, related to Fig. 4.
Under HFD feeding conditions, JunB FKO displayed similar RER (a) and food intake (b), while exhibited a significant decrease in motor activities (c) compared to control mice. There was no significant difference in body mass (d), fat mass, fat percentage (e), the image (f) and weight (g) of individual organs such as gWAT, iWAT, BAT and liver between JunB FKO and control mice when challenged with HFD.
Extended Data Fig. 6 SnRNA-seq analysis of JunB KO BAT, related to Fig. 5.
a. The sorting strategy of isolated nuclei. The nuclei were first sorted based on forward scatter height (FSC) and side scatter height (SSC), and singlets were then sorted based on the combination of area and heights of FSC followed with sorting of DAPI-positive events used for the snRNA-Seq. b. Heat map of gene signature for each population of brown adipocyte nuclei (AD1-AD10). c. Feature plots for Adipoq, JunB, Ucp1, Ppargc1a, Cyp2e1, Fuca1, Slc12a2, Bmpr1a, Gm19951, Acly, Aco1, Flvcr1, Fam13a and Kcnd2.
Extended Data Fig. 7 JunB deficiency induces a shift of low to high-thermogenic adipocytes in BAT, related to Fig. 5.
a. JunB is mainly expressed in UCP1low adipocytes (expression levels <2 described in Fig. 5f). b-c. Majority of YFP+ (JunB+) differentiated adipocytes are Mitochondriallow indicated by the staining with MitoTracker.(4 mice/group) d. YFP+ (JunB+) differentiated adipocytes showed significantly upregulated expression of thermogenic genes compared to YFP− (JunB−) adipocytes(3 mice/group). The partition (e) and pseudotime (f) trajectory of adipocytes analyzed by Monocle. g. Violin plots for Adipoq, Plin1, JunB, Ucp1, Erra, and Ppargc1α for each sub-population of adipocytes in JunB KO and control BAT. h. Volcano plot showing the up regulation of Ucp1, Erra and Ppargc1a in JunB KO BAT compared to control. The unpaired two-sided T-Test was used to analyze Extended Data Fig. 4c, d.
Extended Data Fig. 8 JunB deficiency enhances adipocyte thermogenic capacity in vivo, related to Fig. 5.
a. Feature plots for Adipoq, Junb, Ucp1 and Ppargc1a in JunB KO adipocytes and control cells. b. Mapping the present data sets of BAT snRNAseq with the published work by Sun et al (PMID: 33116305). c. UMAP of KO BAT adipocytes and controls after mapping with published work by Sun et al (PMID: 33116305).
Extended Data Fig. 9 JunB deficiency increases thermogenic capacity in primary brown adipocytes, related to Fig. 5.
a. JunB FKO mice displayed increased mitochondriahigh and decreased mitochondrialow adipocytes in BAT. LD, lipid droplet; N, nucleus. b. The quantified data of mitochondriahigh and mitochondrialow adipocytes from Figure a (n = 4, mice/group). c. JunB deficiency had little effect on the primary adipogenesis. d. Quantification of lipid droplet area of JunB FKO and control primary adipocytes in Figure c. e. Gene Set Enrichment Analysis (GSEA) of SnRNA-Seq in JunB FKO and Control mice. Extended Data Fig. 9b was analyzed by ANOVA analysis, Data are presented as the mean ± SEM. *P < 0.05; **P < 0.01.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Ding, X., Wang, C. et al. Depletion of JunB increases adipocyte thermogenic capacity and ameliorates diet-induced insulin resistance. Nat Metab 6, 78–93 (2024). https://doi.org/10.1038/s42255-023-00945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00945-1