Abstract
The lateral hypothalamic area (LHA) regulates food intake and energy balance. Although LHA neurons innervate adipose tissues, the identity of neurons that regulate fat is undefined. Here we show that GABRA5-positive neurons in LHA (GABRA5LHA) polysynaptically project to brown and white adipose tissues in the periphery. GABRA5LHA are a distinct subpopulation of GABAergic neurons and show decreased pacemaker firing in diet-induced obesity mouse models in males. Chemogenetic inhibition of GABRA5LHA suppresses fat thermogenesis and increases weight gain, whereas gene silencing of GABRA5 in LHA decreases weight gain. In the diet-induced obesity mouse model, GABRA5LHA are tonically inhibited by nearby reactive astrocytes releasing GABA, which is synthesized by monoamine oxidase B (Maob). Gene silencing of astrocytic Maob in LHA facilitates fat thermogenesis and reduces weight gain significantly without affecting food intake, which is recapitulated by administration of a Maob inhibitor, KDS2010. We propose that firing of GABRA5LHA suppresses fat accumulation and selective inhibition of astrocytic GABA is a molecular target for treating obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The accession numbers are listed in Supplementary Table 1. The sequences of the shRNAs used in this study have been provided in the supplementary figures. The viruses used in this study were provided by and are available from the IBS Virus Facility (https://www.ibs.re.kr/virusfacility) and Korea Institute of Science and Technology Virus Facility upon request (http://virus.kist.re.kr). Microscopy data reported in this paper will be shared by the lead contact upon request. This paper does not report any original code. Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request. Source data are provided with this paper.
Change history
16 October 2023
A Correction to this paper has been published: https://doi.org/10.1038/s42255-023-00923-7
References
Kong, D. et al. GABAergic RIP–Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151, 645–657 (2012).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Flament-Durand, J. The hypothalamus: anatomy and functions. Acta Psychiatr. Belg. 80, 364–375 (1980).
Bernardis, L. L. & Bellinger, L. L. The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci. Biobehav. Rev. 17, 141–193 (1993).
Palkovits, M., Makara, G. B., Leranth, C. & Van Cuc, H. Intrahypothalamic terminals of stress conducting fibers. Brain Res. 190, 399–407 (1980).
Stuber, G. D. & Wise, R. A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19, 198–205 (2016).
Cerri, M. & Morrison, S. F. Activation of lateral hypothalamic neurons stimulates brown adipose tissue thermogenesis. Neuroscience 135, 627–638 (2005).
Contreras, C., Nogueiras, R., Dieguez, C., Rahmouni, K. & Lopez, M. Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox Biol. 12, 854–863 (2017).
Lee, J., Raycraft, L. & Johnson, A. W. The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells. Physiol. Behav. 229, 113234 (2021).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
Bittencourt, J. C. Anatomical organization of the melanin-concentrating hormone peptide family in the mammalian brain. Gen. Comp. Endocrinol. 172, 185–197 (2011).
Oldfield, B. J. et al. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience 110, 515–526 (2002).
Berthoud, H. R., Patterson, L. M., Sutton, G. M., Morrison, C. & Zheng, H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem. Cell Biol. 123, 147–156 (2005).
Tupone, D., Madden, C. J., Cano, G. & Morrison, S. F. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 31, 15944–15955 (2011).
Contreras, C. et al. The brain and brown fat. Ann. Med. 47, 150–168 (2015).
Kakizaki, M. et al. Differential roles of each orexin receptor signaling in obesity. iScience 20, 1–13 (2019).
Zink, A. N., Bunney, P. E., Holm, A. A., Billington, C. J. & Kotz, C. M. Neuromodulation of orexin neurons reduces diet-induced adiposity. Int. J. Obes. 42, 737–745 (2018).
Kosse, C., Schone, C., Bracey, E. & Burdakov, D. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc. Natl Acad. Sci. USA 114, 4525–4530 (2017).
Karnani, M. M., Szabo, G., Erdelyi, F. & Burdakov, D. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J. Physiol. 591, 933–953 (2013).
Backberg, M., Ultenius, C., Fritschy, J. M. & Meister, B. Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J. Neuroendocrinol. 16, 589–604 (2004).
Bonnavion, P., Mickelsen, L. E., Fujita, A., de Lecea, L. & Jackson, A. C. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J. Physiol. 594, 6443–6462 (2016).
Shin, S. Y. et al. Identification of the adrenoceptor subtypes expressed on GABAergic neurons in the anterior hypothalamic area and rostral zona incerta of GAD65–eGFP transgenic mice. Neurosci. Lett. 422, 153–157 (2007).
Hassani, O. K., Henny, P., Lee, M. G. & Jones, B. E. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur. J. Neurosci. 32, 448–457 (2010).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Jung, S. et al. A forebrain neural substrate for behavioral thermoregulation. Neuron 110, 266–279.e9 (2022).
de Vrind, V. A. J., Rozeboom, A., Wolterink-Donselaar, I. G., Luijendijk-Berg, M. C. M. & Adan, R. A. H. Effects of GABA and leptin receptor-expressing neurons in the lateral hypothalamus on feeding, locomotion, and thermogenesis. Obesity 27, 1123–1132 (2019).
Zhang, Y., Reichel, J. M., Han, C., Zuniga-Hertz, J. P. & Cai, D. Astrocytic process plasticity and IKKβ/NF-κB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 25, 1091–1102 e1094 (2017).
Chun, H. et al. Astrocytic proBDNF and tonic GABA distinguish active versus reactive astrocytes in hippocampus. Exp. Neurobiol. 27, 155–170 (2018).
Chun, H. & Lee, C. J. Reactive astrocytes in Alzheimer’s disease: a double-edged sword. Neurosci. Res. 126, 44–52 (2018).
Yoon, B. E. et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J. Physiol. 592, 4951–4968 (2014).
Rossi, M. A. et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364, 1271–1274 (2019).
Yang, L., Qi, Y. & Yang, Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep. 11, 798–807 (2015).
Chen, N. et al. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. eLife 5, e18716 (2016).
Varela, L. et al. Hunger-promoting AgRP neurons trigger an astrocyte-mediated feed-forward autoactivation loop in mice. J. Clin. Invest. 131, e144239 (2021).
Bouyakdan, K. et al. The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J. Clin. Invest. 129, 2417–2430 (2019).
McDougal, D. H., Hermann, G. E. & Rogers, R. C. Astrocytes in the nucleus of the solitary tract are activated by low glucose or glucoprivation: evidence for glial involvement in glucose homeostasis. Front. Neurosci. 7, 249 (2013).
Chari, M. et al. Glucose transporter-1 in the hypothalamic glial cells mediates glucose sensing to regulate glucose production in vivo. Diabetes 60, 1901–1906 (2011).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Garcia-Caceres, C. et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166, 867–880 (2016).
Gonzalez-Garcia, I. & Garcia-Caceres, C. Hypothalamic astrocytes as a specialized and responsive cell population in obesity. Int. J. Mol. Sci. 22, 6176 (2021).
Buckman, L. B., Thompson, M. M., Moreno, H. N. & Ellacott, K. L. Regional astrogliosis in the mouse hypothalamus in response to obesity. J. Comp. Neurol. 521, 1322–1333 (2013).
Moraes, J. C. et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE 4, e5045 (2009).
Lizarbe, B., Cherix, A., Duarte, J. M. N., Cardinaux, J. R. & Gruetter, R. High-fat diet consumption alters energy metabolism in the mouse hypothalamus. Int. J. Obes. 43, 1295–1304 (2019).
Yoon, B. E., Woo, J. & Lee, C. J. Astrocytes as GABA-ergic and GABA-ceptive cells. Neurochem. Res. 37, 2474–2479 (2012).
Jo, S. et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 20, 886–896 (2014).
Brickley, S. G. & Mody, I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73, 23–34 (2012).
Caraiscos, V. B. et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl Acad. Sci. USA 101, 3662–3667 (2004).
Hortnagl, H. et al. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 236, 345–372 (2013).
Muller, T. D., Klingenspor, M. & Tschop, M. H. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nat. Metab. 3, 1134–1136 (2021).
Kurylowicz, A. et al. Obesity is associated with a decrease in expression but not with the hypermethylation of thermogenesis-related genes in adipose tissues. J. Transl. Med. 13, 31 (2015).
Cero, C. et al. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 6, e139160 (2021).
Whittle, A. J. et al. Soluble LR11/SorLA represses thermogenesis in adipose tissue and correlates with BMI in humans. Nat. Commun. 6, 8951 (2015).
Orozco-Solis, R. et al. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab. 23, 467–478 (2016).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Cao, L. et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 14, 324–338 (2011).
Grujic, D. et al. β3-Adrenergic receptors on white and brown adipocytes mediate β3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J. Biol. Chem. 272, 17686–17693 (1997).
Schneeberger, M. et al. Regulation of energy expenditure by brainstem GABA neurons. Cell 178, 672–685 e612 (2019).
Bartness, T. J., Kay Song, C., Shi, H., Bowers, R. R. & Foster, M. T. Brain–adipose tissue cross talk. Proc. Nutr. Soc. 64, 53–64 (2005).
Ryu, V., Watts, A. G., Xue, B. & Bartness, T. J. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R324–R337 (2017).
You, H., Chu, P., Guo, W. & Lu, B. A subpopulation of Bdnf-e1-expressing glutamatergic neurons in the lateral hypothalamus critical for thermogenesis control. Mol. Metab. 31, 109–123 (2020).
Ryu, V., Garretson, J. T., Liu, Y., Vaughan, C. H. & Bartness, T. J. Brown adipose tissue has sympathetic-sensory feedback circuits. J. Neurosci. 35, 2181–2190 (2015).
Farrant, M. & Nusser, Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 6, 215–229 (2005).
Bhattarai, J. P. et al. Tonic extrasynaptic GABA(A) receptor currents control gonadotropin-releasing hormone neuron excitability in the mouse. Endocrinology 152, 1551–1561 (2011).
Lee, S. et al. Channel-mediated tonic GABA release from glia. Science 330, 790–796 (2010).
Park, J. H. et al. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer’s disease. Sci. Adv. 5, eaav0316 (2019).
Recena Aydos, L. et al. Nonalcoholic fatty liver disease induced by high-fat diet in C57bl/6 models. Nutrients 11, 3067 (2019).
Burdakov, D. & Karnani, M. M. Ultra-sparse connectivity within the lateral hypothalamus. Curr. Biol. 30, 4063–4070 e4062 (2020).
Jennings, J. H. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Elias, C. F. et al. Melanin-concentrating hormone projections to areas involved in somatomotor responses. J. Chem. Neuroanat. 35, 188–201 (2008).
Harthoorn, L. F., Sane, A., Nethe, M. & Van Heerikhuize, J. J. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell. Mol. Neurobiol. 25, 1209–1223 (2005).
Bamshad, M., Song, C. K. & Bartness, T. J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 276, R1569–R1578 (1999).
Bartness, T. J., Vaughan, C. H. & Song, C. K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34, S36–S42 (2010).
Richard, D. & Picard, F. Brown fat biology and thermogenesis. Front. Biosci. 16, 1233–1260 (2011).
Izawa, S. et al. Melanin-concentrating hormone-producing neurons in the hypothalamus regulate brown adipose tissue and thus contribute to energy expenditure. J. Physiol. https://doi.org/10.1113/jp281241 (2021).
Morrison, S. F., Madden, C. J. & Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756 (2014).
Nguyen, N. L., Randall, J., Banfield, B. W. & Bartness, T. J. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R375–R386 (2014).
Morrison, S. F., Madden, C. J. & Tupone, D. Central control of brown adipose tissue thermogenesis. Front. Endocrinol. 3, 5 (2012).
Pandit, S. et al. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia 68, 1065–1080 (2020).
Chun, H., Lim, J., Park, K. D. & Lee, C. J. Inhibition of monoamine oxidase B prevents reactive astrogliosis and scar formation in stab wound injury model. Glia https://doi.org/10.1002/glia.24110 (2021).
Nam, M. H. et al. KDS2010, a newly developed reversible MAO-B inhibitor, as an effective therapeutic candidate for Parkinson’s disease. Neurotherapeutics 18, 1729–1747 (2021).
Shim, H. S., Park, H. J., Woo, J., Lee, C. J. & Shim, I. Role of astrocytic GABAergic system on inflammatory cytokine-induced anxiety-like behavior. Neuropharmacology 160, 107776 (2019).
Sa, M. et al. Unaltered tonic inhibition in the arcuate nucleus of diet-induced obese mice. Exp. Neurobiol. 31, 147–157 (2022).
Lee, N., Sa, M., Hong, Y. R., Lee, C. J. & Koo, J. Fatty acid increases cAMP-dependent lactate and MAO-B-dependent GABA production in mouse astrocytes by activating a galphas protein-coupled receptor. Exp. Neurobiol. 27, 365–376 (2018).
Chao, D. H. M. et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 34, 1532–1547.e6 (2022).
Chun, H. et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci. 23, 1555–1566 (2020).
Cheung, B. M., Cheung, T. T. & Samaranayake, N. R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 4, 171–181 (2013).
Kim, G. W., Lin, J. E., Blomain, E. S. & Waldman, S. A. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin. Pharmacol. Ther. 95, 53–66 (2014).
Heo, J. Y. et al. Aberrant tonic inhibition of dopaminergic neuronal activity causes motor symptoms in animal models of Parkinson’s disease. Curr. Biol. 30, 276–291 e279 (2020).
Chun, H. et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci. 23, 1555–U1542 (2020).
Park, Y. G. et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. https://doi.org/10.1038/nbt.4281 (2018).
Tschop, M. H. et al. A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 (2011).
Virtue, S. & Vidal-Puig, A. GTTs and ITTs in mice: simple tests, complex answers. Nat. Metab. 3, 883–886 (2021).
Kwak, H. et al. Astrocytes control sensory acuity via tonic inhibition in the thalamus. Neuron 108, 691–706. e610 (2020).
Nam, M. H. et al. Excessive astrocytic GABA causes cortical hypometabolism and impedes functional recovery after subcortical stroke. Cell Rep. 32, 107861 (2020).
Woo, D. H. et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40 (2012).
Acknowledgements
We are grateful to all members of the C.J.L. laboratory at the IBS for helpful discussions and comments. We acknowledge T.S.K. at the Research Solution Center of IBS for help with imaging data using the 3D cell culture imaging microscope. S.J. and S.-Y.K. from Seoul National University for help with the measurement of BAT temperature. S.P. from the Korea Advanced Institute of Science and Technology for help with the ANCOVA analysis. This study was supported by Center for Cognition and Sociality (IBS-R001-D2) to C.J.L. from the IBS, Republic of Korea. W.K. was supported by a Young Scientist Fellowship (IBS-R001-Y1) from the IBS. J.-W.S. was supported by the NRF (2022R1A2C3005613).
Author information
Authors and Affiliations
Contributions
M.S., E.-S.Y., W.K., M.G.P., H.-J.J., Y.R.Y., M.B., J.-H.L., J.L., W.W., J.K., J.-H.K., Y.S., B.K., H.A. and S.E.L. performed the experiments. K.D.P., P.-G.S., J.-W.S. and C.J.L. supervised the analysis. M.S. and C.J.L. designed all the experiments and wrote the manuscript with input from the co-authors.
Corresponding author
Ethics declarations
Competing interests
K.D.P. and C.J.L. are inventors on a patent for KDS2010 (US11053190) and related to MAO-B inhibition as an anti-obesity therapy (PCT/KR2015/010104). M.S., K.D.P., and C.J.L. are inventors on a patent for reversible MAO-B inhibitors for treating obesity (KR-10-1746060). These patents have been licensed to Neurobiogen, which develops KDS2010 for the treatment of Alzheimer’s disease and obesity. Additionally, C.J.L. and K.D.P. also received honoraria for speaking engagements from Neurobiogen. The other authors declare no conflict of interest.
Peer review
Peer review information
Nature Metabolism thanks Paulo Kofuji for his contribution to the peer review of this work. Primary Handling editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Inhibition of GABRA5LHA by DREADD.
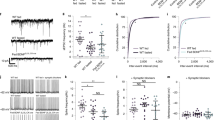
a, Experimental timeline for GABRA5LHA under chemogenetic inhibition by DREADD hM4Di with CNO. b, GABRA5:mCherry mice were injected with AAV-mGABRA5-EGFP-Cre and Cre-activatable AAV carrying mCh (AAV-hSyn-DIO-mCh). c, Ex vivo cell-attached patch-clamp recording of GABRA5LHA under chemogenetic inhibition. Representative traces of cell attached recording in GABRA5::mCherry (top) and GABRA5::hM4Di (bottom) in LHA. Both groups were treated of CNO (5 μM) with bath application. d, Quantification of firing rate in GABRA5::mCherry and GABRA5::hM4Di (n= 9, 8 cells, respectively). e, Representative images of AAV-mGABRA5-EGFP-Cre expression in LHA. Scale bar, 100 μm. f, Representative images of GABRA5::hM4Di and GABRA5:mCherry mice in LHA. Scale bar, 20 μm. g, Percentage of c-FOS in GABRA5-positive neurons of GABRA5::hM4Di and GABRA5:mCherry mice (n = 10, 10 slices from n = 3, 4 mice, respectively). h, Quantification of c-FOS expression in GABRA5-positive neurons in each group (n = 641, 466 cells from n = 3, 4 mice, respectively). Data were presented as mean ± SEM. p values were determined by Mann Whitney test (d,g,h). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 2 Development and validation of shRNAs for Gabra5 in vitro. and in vivo.
a, Candidate sequences for GABRA5 shRNA are shown with vector information. Each shRNA was cloned in pSicoR vector to express under U6 promoter together with a cytomegalovirus (CMV) promoter driving expression of mCherry reporter gene. b, Fluorescence images of HEK293-T cells co-transfected with shRNA vectors (shRNA-mCh, red, bottom) with GABRA5 full clone (GABRA5-GFP, green, top). Scale bar, 100 μm. c, Gene expression level of Gabra5 quantified by qRT-PCR in the co-transfected HEK293T cells, normalized to Scrambled-shRNA. d, Schematic and representative images of GABRA5-shRNA-carrying lenti virus express mCherry in LHA. Scale bar, 100 μm. e, Representative images of GABRA5-IR (GFP) and mCherry in LHA of Scrambled and shGABRA5 group. Scale bar, 20 μm. Scale bar, 10 μm. f, Quantified expression of GABRA5 in mCherry-positive cells in LHA of Scrambled and shGABRA5 group (n= 96, 64 cells from n = 3, 3 mice, respectively). Data were presented as mean ± SEM. p values were determined by Unpaired t-test (f). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 3 Knockdown of Gabra5 in LHA with normal chow diet and HFD.
a,b, Experimental scheme and timeline for LHA injection. c, Schematic diagram of Scrambled (top) and shGABRA5 (bottom) virus constructs. d, Curves of bodyweight in Scrambled and shGABRA5 mice with chow diet (n = 9, 9 mice per group). e, Representative images of each organ between Scrambled and shGABRA5 group with chow diet. Scale bar, 1 cm. f, Average food intake per day in Scrambled and shGABRA5 group (n = 7, 8 mice per group). g, Quantification of organ weight in Scrambled and shGABRA5 group with chow diet (n = 6 mice per group). h, Average weight of iBAT (n = 6 mice per group). i, Time course of blood glucose levels during the glucose tolerance test (GTT) in Scrambled and shGABRA5 group with chow diet (n = 7, 8 mice per group). j, Quantification of area of curve (AOC) of GTT. k, Time course of blood glucose levels during the insulin tolerance test (ITT) in Scrambled and shGABRA5 group with chow diet (n = 7, 8 mice per group). l, Quantification of area of curve (AOC) of ITT. m, Timeline of the experiment. n, Time course of blood glucose levels during the glucose tolerance test (GTT) in Scrambled and shGABRA5 group with HFD (n = 8, 8 mice per group). o, Time course of blood glucose levels during the insulin tolerance test (ITT) in Scrambled and shGABRA5 group with HFD (n = 8, 8 mice per group). p, Quantification of area of curve (AOC) of GTT. q, Quantification of area of curve (AOC) of ITT. r, Representative infra-red thermograph images showing the surface temperature of the intrascapular brown adipose tissue (TBAT) and the tail temperature (TTail) in Scrambled and shGABRA5 group (n = 9, 8 mice, respectively). s, Quantification of TBAT. t, Quantification of TBAT. Data were presented as mean ± SEM. p values were determined by Two-way ANOVA with Šídák’s multiple comparisons test (d,g,i,k,n,o), Unpaired t-test (f,p,q,s,t), or Mann Whitney test (h,j,l). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 4 Expression of iBAT or iWAT innervating neurons in LHA and expression of GABRA5LHA. Related to Supplementary Movie S1.
a, Fluorescence images of serial sections along the AP axis of LHA from mice injected with PRV–EGFP virus in iBAT and iWAT 5 days after injection. Sections from AP -1.34 mm to AP -1.94 mm were stained with DAPI (blue). Scale bar, 100 μm. b, Experimental scheme of AAV-mGABRA5-EGFP-Cre virus injection and sagittal view of the mouse brain. c, Ventral view of the mouse brain from 3D Allen brain atlas. LHA is colored green and PAG is colored magenta. White rectangle indicates the region of interest. d, Ventral image of LHA-injected mice bilaterally with AAV-mGABRA5-EGFP-Cre virus. Scale bar, 300 μm.
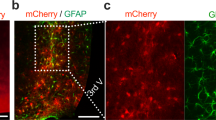
Extended Data Fig. 5 Astrocytes in LHA show hypertrophy in moderate obesity.
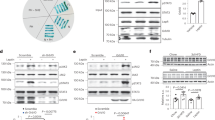
a, Timeline of the experiment. b, Left, Immunostaining for GFAP-Cre-mCh (red) in LHA of Chow and HFD group. Middle, GFAP (magenta), GABA (green), DAPI (blue) in LHA of Chow and HFD group. Scale bar, 30 μm. Right, magnified images taken from the boxed areas in the middle images with 3D rendering, showing GFAP colocalized with GABA immunostaining. Scale bar, 15 μm. c, The number of process intersections at each radial distance from soma in GFAP+ astrocytes in each group (n = 7, 7 slices from 3 chow mice, 3 HFD mice). d,e, Quantification of GFAP intensity (d) and GABA intensity (n = 445, 438 cells from 3 chow mice, 3 HFD mice) (e) in GFAP+ astrocytes (n = 372, 438 cells from 3 chow mice, 3 HFD mice). f, Quantification for 3D volumes of GFAP+ astrocytes in each group (n=776, 1103 cells from 3 chow mice, 3 HFD mice). g, Number of summarized intersections of GFAP+ astrocytes in each group (n = 734, 1299 cells from 3 chow mice, 3 HFD mice). h, Quantification of ramification index (n =793, 1285 cells from 3 chow mice, 3 HFD mice). i, The ending radius of GFAP+ astrocytes in LHA (n = 7, 7 slices from 3 chow mice, 3 HFD mice). j, The number of GFAP+ astrocytes in LHA (n = 7, 7 slices from 3 chow mice, 3 HFD mice). k, Timeline of the experiment. l, GABRA5 expression in LHA of Chow and HFD mice. m, Expression level of GABRA5 in medial, central and lateral part of the LHA was not significantly changed in HFD mice compared to chow mice. Scale bar, 20 μm. Scale bar, 5 μm. n, Top, schematic diagram. Bottom, GABRA5-shRNA-carrying lenti virus express mCherry in LHA. Scale bar, 100 μm. o, Maob-IR was shown in GFP. Scale bar, 20 μm. Scale bar, 15 μm. p, Expression of Maob in GFAP-positive astrocytes in LHA was significantly lower in shMAOB group compared to Scrambled group (n=697, 345 cells from 3, 3 mice, respectively). q, Time course of blood glucose levels during the glucose tolerance test (GTT) in Scrambled and shMAOB group with HFD (n = 8, 8 mice, respectively). r, Quantification of area of curve (AOC) of GTT. s, Time course of blood glucose levels during the insulin tolerance test (ITT) in Scrambled and shMAOB group with HFD (n = 8, 8 mice per group). t, Quantification of area of curve (AOC) of ITT. Data were presented as mean ± SEM. p values were determined by Two-way ANOVA with Šídák’s multiple comparisons test (c,q,s), Mann-Whitney test (d-j,p), or Unpaired t-test (r,t). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 6 Knockdown of astrocytic Maob in LHA with normal chow diet and HFD.
a,b, Experimental scheme and timeline for LHA injection. c, Schematic diagram of Scrambled (top) and shMAOB (bottom) virus constructs. d, Curves of bodyweight in Scrambled and shMAOB group with chow diet (n = 9, 10 mice per group). e, Representative images of each organ between Scrambled and shMAOB group with chow diet. Scale bar, 1 cm. f, Average food intake per day in Scrambled and shMAOB group (n = 9, 10 mice per group). g, Quantification of organ weight in Scrambled and shMAOB group with chow diet (n = 6, 6 mice per group). h, Average weight of iBAT (n = 6, 6 mice per group). i, Time course of blood glucose levels during the glucose tolerance test (GTT) in Scrambled and shMAOB group with chow diet (n = 9, 9 mice per group). j, Quantification of area of curve (AOC) of GTT. k, Time course of blood glucose levels during the insulin tolerance test (ITT) in Scrambled and shMAOB group with chow diet (n = 9, 9 mice per group). l, Quantification of area of curve (AOC) of ITT. m, Timeline and schematic images of the experiment. n, Representative images of LHA from PRV-EGFP injected (green) mice stained against GABAAR-α5 (magenta) with Cre signals (top). Scale bar, 50 μm. Magnification of arrowed cells (bottom). Scale bar, 10 μm. o,p, Quantification between GABRA5 and PRV retrogradely labeled from iBAT at 5 days post-injection (n = 6, 7 slices from 3, 3 mice per group). Data were presented as mean ± SEM. p values were determined by Two-way ANOVA with Šídák’s multiple comparisons test (d,g,i,k), Unpaired t-test (f,j,l), or Mann-Whitney test (h,l,o,p). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 7 KDS2010 reduces astrocytic Maob and fats without affecting locomotion.
a, Curves representing the kinetics of change in body weight among HFD, HFD with KDS2010, and HFD with selegiline mice over the 22 weeks following HFD treatment. Light blue shaded box indicates period of KDS2010 or selegiline treatment in drinking water (n= 8, 8, 8, and 8 mice, respectively). b, Cumulative food intake in HFD, HFD with KDS2010, HFD with selegiline mice over the 22 weeks (n= 8, 8, 8, and 8 mice, respectively). c,d, Quantification of percentage change of fat mass (c) and lean mass (d) before (at 15 weeks) and after (at 22 weeks) KDS2010 or selegiline treatment (n= 8, 8, 8, and 8 mice, respectively). e, Representative images of each organ of chow, chow with KDS2010, HFD, HFD with KDS2010 mice. Scale bar, 1 cm. f. Timeline of the experiment. g, Real-time monitoring curve of locomotion in chow, KDS2010-treated chow, HFD, and KDS2010-treated HFD mice (n = 11, 11, 12, and 10 mice for respective group). h, Quantification of locomotor activity in light and dark cycle. i, Quantification of Maob intensity in GFAP-positive cells in chow, chow with KDS2010, HFD, and HFD with KDS2010 group (n = 464, 463, 337, and 296 cells from 4, 4, 3, and 3 mice per group, respectively). j, Representative images for Maob (green) and GFAP (magenta) in LHA of chow, chow with KDS2010, HFD, and HFD with KDS2010 group. Scale bar, 10 μm. Data were presented as mean ± SEM. p values were determined by One-way ANOVA with Tukey’s multiple comparisons test (c,d,h) or Kruskal-Wallis test (i). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 8 A less-BBB-permeable Maob inhibitor, KDS1524 does not reduce astrogliosis in LHA.
a, Experimental timeline for oral administration of KDS2010 or KDS1524 with HFD. b, Chemical structure of KDS1524. c, Cerebrospinal fluid (CSF) concentration after intravenous and oral administration at a dose of 5, 10 mg/kg of KDS1524 (n= 4 rat). d, Curves representing the kinetics of change in body weight in gram of chow, HFD, HFD with KDS1524 and HFD with KDS2010 mice over 16 days following HFD treatment. KDS2010 or KDS1524 was administered by oral injection (n= 8, 8, 8, and 8 mice, respectively). e, Curves representing the kinetics of change in body increase in the percentage of chow, HFD, HFD with KDS1524 and HFD with KDS2010 mice (n= 8, 8, 8, and 8 mice, respectively). f, Quantification of percentage change of body increase of HFD with KDS1524 and HFD with KDS2010 compared to HFD mice. g, Top, Immunostaining for GABA (green) and GFAP (magenta) in LHA of HFD, HFD with KDS1524 and HFD with KDS2010 group. Scale bar, 30 μm. Bottom, magnified images taken from the boxed areas in the top images with 3D rendering, showing GFAP colocalized with GABA immunostaining. Scale bar, 15 μm. h, The number of process intersections at each radial distance from soma in GFAP+ astrocytes in each group (n = 4, 5, and 4 slices from 4, 4, and 4 mice). i,j, Quantification of GFAP intensity (n = 88, 116, and 68 cells) (i) and GABA intensity (n = 118, 93, and 65 cells) (j) in GFAP+ astrocytes from 4, 4, and 4 mice, respectively. k,l, Quantification for 3D volumes (n = 323, 318, and 104 cells) (k), number of summarized intersections (n = 234, 160, and 165 cells) (l) of GFAP+ astrocytes from 4, 4, and 4 mice, respectively. m, Quantification of ramification index (n = 235, 180, and 190 cells from 4, 4, and 4 mice, respectively). n, The ending radius of GFAP+ astrocytes in LHA (n = 4, 4, and 4 slices per group from 4, 4, and 4 mice). o, The number of GFAP+ astrocytes in LHA (n = 4, 5, and 4 slices per group from 4, 4, and 4 mice). Data were presented as mean ± SEM. p values were determined by Two-way ANOVA with Tukey’s multiple comparisons test (h) or One-way ANOVA with Kruskal-Wallis test (i,j,k,l,m,n,o). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Extended Data Fig. 9 Model of obesity and anti-obesity mechanisms.
Left, in the chronic HFD-fed DIO mouse model, the expression of the GABA-synthesizing enzyme, Maob, increases in reactive astrocytes within the LHA. This elevated astrocytic GABA production leads to the tonic suppression of GABAergic GABRA5LHA activity. The subsequent inhibition of pacemaker firing in GABRA5LHA exacerbates obesity by reducing thermogenesis and lipolysis in both BAT and WAT. Right, the elevated tonic inhibition can be reversed by either gene-silencing of Maob or pharmacological treatment targeting Maob, or by gene-silencing of Gabra5 in the LHA of the DIO mouse model. These interventions release the inhibition on the pacemaker firing of GABRA5LHA, thereby enhancing fat-burning and potentially preventing obesity.
Supplementary information
Supplementary Table 1
Resources used in the experiments.
Supplementary Video 1
GABAergic GABRA5LHA project to outside LHA.
Source data
Source Data Figs. 1–8
Statistical source data for each figure.
Source Data Extended Data Figs. 1–8
Statistical source data for each extended data figure.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sa, M., Yoo, ES., Koh, W. et al. Hypothalamic GABRA5-positive neurons control obesity via astrocytic GABA. Nat Metab 5, 1506–1525 (2023). https://doi.org/10.1038/s42255-023-00877-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00877-w