Abstract
Insulin action in the human brain modulates eating behaviour, whole-body metabolism and body fat distribution1,2. In particular, brain insulin action increases whole-body insulin sensitivity, but these studies were mainly performed in lean men3,4. Here we investigate metabolic and hypothalamic effects of brain insulin action in women with a focus on the impact of menstrual cycle (ClinicalTrials.gov registration: NCT03929419).
Eleven women underwent four hyperinsulinemic–euglycemic clamps, two in the follicular phase and two in the luteal phase. Brain insulin action was introduced using nasal insulin spray5,6,7 and compared to placebo spray in a fourfold crossover design with change in glucose infusion rate as the primary endpoint. Here we show that during the follicular phase, more glucose has to be infused after administration of nasal insulin than after administration of placebo. This remains significant after adjustment for blood glucose and insulin. During the luteal phase, no significant influence of brain insulin action on glucose infusion rate is detected after adjustment for blood glucose and insulin (secondary endpoint). In 15 other women, hypothalamic insulin sensitivity was assessed in a within-subject design by functional magnetic resonance imaging with intranasal insulin administration8. Hypothalamus responsivity is influenced by insulin in the follicular phase but not the luteal phase.
Our study therefore highlights that brain insulin action improves peripheral insulin sensitivity also in women but only during the follicular phase. Thus, brain insulin resistance could contribute to whole-body insulin resistance in the luteal phase of the menstrual cycle.
Similar content being viewed by others
Main
Over the last decade, the brain was characterized as an insulin-sensitive organ1. Insulin crosses the blood–brain barrier9 and acts in specialized neurons and glia cells1. In humans, insulin regulates brain activity in specific regions, including the hypothalamus10, which in turn influences outflows toward the periphery2 and modulates food intake and whole-body metabolism2. Studies performed in healthy men found that insulin action in the brain suppresses endogenous glucose production3,11 and stimulates glucose uptake into peripheral tissues3. However, this may require postprandial metabolic conditions12,13, as nasal insulin did not modulate endogenous glucose production under systemic fasting insulin concentrations in most12,14, but not all, clinical trials11.
Nasal insulin administration has been repeatedly used to study insulin action in the human brain15. Using this delivery route, substantial amounts of insulin are delivered into the brain5, while only a tiny fraction reaches the bloodstream6. Thus, nasal insulin allows selective stimulation of brain insulin action without major peripheral side effects. Using this approach, studies have addressed brain-derived modulation of peripheral metabolism in men with normal weight and obesity (for review, see Kullmann et al.2).
Initial studies on this topic have already revealed that a substantial number of individuals have a reduced or even absent response to insulin in the brain16, a phenomenon termed brain insulin resistance17. In affected individuals, brain insulin cannot sufficiently modulate peripheral metabolism2. These individuals are predisposed for subsequent weight gain and visceral fat accumulation18. The most prominent phenotype linked to human brain insulin resistance is obesity1, although other factors, such as genetic background19, elevated circulating free fatty acids20 and impaired insulin transport across the blood–brain barrier21,22, have also been implicated. The detailed regulatory mechanisms for brain insulin resistance, however, are still largely unknown.
Of note, experimental studies suggest considerable sex differences in brain insulin sensitivity. Intracerebroventricular injection of insulin markedly reduced food intake in male rodents, whereas feeding was unaffected in female rodents23. In line with these findings, intranasal insulin delivery to the human brain reduced food intake in lean men but not in women24. However, some studies reported effects of brain insulin on postprandial snacking in women25. Nevertheless, daily nasal insulin administration over 8 weeks reduced body weight and body fat content only in men, whereas women showed no reduction in body adiposity26.
To date, no study has investigated sex differences in the regulation of whole-body metabolism by brain insulin action. Because most previous experiments were conducted in healthy young men, here, we aimed to investigate the influence of brain insulin administration on regional brain activity and whole-body glucose metabolism in young women. Because sex hormones are suspected to have a regulatory impact on brain insulin action, we studied naturally cycling women during both the follicular and luteal phases of the menstrual cycle.
Hyperinsulinemic–euglycemic clamp study
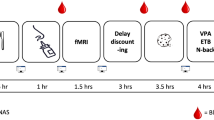
For the hyperinsulinemic–euglycemic clamp study, we examined 11 healthy naturally cycling women twice during the follicular phase and twice during the luteal phase (Fig. 1a). Although their body weight and body fat content were not different between phases (both P ≥ 0.3), there were differences in sex hormones that ensured our cycle phase determination (Supplementary Table 1). Progesterone and 17-OH-progesterone were higher in the luteal phase, while follicle-stimulating hormone (FSH) was lower. Luteinizing hormone (LH) and estradiol concentrations were not significantly different between phases. The androgens dehydroepiandrosterone (DHEA)-sulfate and androstenedione were higher in the luteal phase, but there were no differences in total and calculated free testosterone. The concentrations of prolactin, anti-Müllerian hormone and morning cortisol were comparable between cycle phases.
Neither the follicular nor the luteal phase showed differences in sex hormone concentrations between the insulin and placebo spray days (all P ≥ 0.07).
Fasting insulin concentrations were comparable between study days (F3,39 = 1.78, PANOVA = 0.3). After initiation of the hyperinsulinemic–euglycemic clamp, insulin concentrations were also comparable before spray administration (135 ± 20 pmol liter–1; F3,39 = 1.93, PANOVA = 0.1; Extended Data Fig. 1). After administration of insulin nasal spray, there was an increase in circulating insulin concentrations by 65 ± 58 pmol liter–1 that peaked after 10 min and was mimicked on placebo days by an intravenous (i.v.) insulin bolus (achieved increase in serum insulin by 30 ± 45 pmol liter–1 with peak after 10 min). This increase in serum insulin in the first 10 min as well as in the 20 min after spray administration was comparable between study days (F3,39 = 1.54 and PANOVA = 0.2 and F3,39 = 0.86 and PANOVA = 0.5, respectively; Extended Data Fig. 1). Thirty minutes after spray administration, serum insulin concentrations were again in the range of what was measured before spray application on all study days. Also, at the end of the hyperinsulinemic–euglycemic clamp, insulin concentrations were comparable between study days (F3,39 = 2.43, PANOVA = 0.1; Extended Data Fig. 1).
Plasma glucose concentrations were within the target range and comparable on all study days before spray administration (F3,39 = 1.03, PANOVA = 0.4; Extended Data Fig. 2) in the 30 min following spray administration (F3,39 = 0.31, PANOVA = 0.8; Extended Data Fig. 2) and at the end (F3,38 = 0.26, PANOVA = 0.9; Extended Data Fig. 2) of the hyperinsulinemic–euglycemic clamp experiments.
There were no interactions between the administered type of nasal spray and cycle phase on insulin courses (t514 = −0.55, Ptime × spray × phase = 0.6) and on achieved glucose courses (t1,025 = −1.34, Ptime × spray × phase = 0.2) over the whole hyperinsulinemic–euglycemic clamps. The courses of C-peptide (t514 = −1.51, Ptime × spray × phase = 0.1) and free fatty acid suppression (t172 = 0.02, Ptime × spray × phase = 1.0) were also comparable between days.
Regarding our primary research question, spray-induced changes in glucose infusion rates were significantly different between cycle phases (t1,075 = 2.85, Ptime × spray × phase = 0.004). This remained significant after adjustment for plasma glucose and insulin concentrations (t464 = 2.53, Ptime × spray × phase = 0.01).
In the follicular phase, the glucose infusion rate had to be increased more after intranasal insulin administration than after administration of placebo spray (t525 = −6.83, Ptime × spray < 0.0001; Fig. 2a). This was independent of insulin and glucose concentrations (t230 = −5.66, adjusted Ptime × spray < 0.0001).
a,b, Absolute changes in glucose infusion rates after administration of intranasal insulin (filled circles) or intranasal placebo and i.v. insulin bolus (open circles). Results in the follicular (a) and luteal (b) phases are shown. P values are for time × spray interactions tested by two-sided linear mixed models adjusted for serum insulin and glucose; presented are means, and error bars represent s.e.m.; N = 11 participants except for the follicular phase where N = 10 for the insulin spray.
In the luteal phase, the different courses in glucose infusion rate between insulin and placebo spray (t550 = −2.83, Ptime × spray = 0.005; Fig. 2b) may result from differences in the achieved plasma glucose and insulin concentrations, as they were no longer detectable after respective adjustments (t234 = −1.69, adjusted Ptime × spray = 0.09).
To test if the different responses to nasal insulin between cycle phases are secondary to specific hormone levels, we tested for interactions between measured hormone levels and the nasal spray effect on glucose infusion rates. Neither estradiol nor progesterone nor 17-OH progesterone showed such interactions (all Ptime × spray × hormone ≥ 0.2). However, the estradiol:progesterone ratio interacted with spray (placebo versus insulin) and time (t210 = −2.89, Ptime × spray × hormone = 0.004). This model suggests that a high estradiol:progesterone ratio, as present during the follicular phase, is linked to a stronger effect of intranasal insulin on glucose infusion rates than placebo spray.
Furthermore, comparable interactions were detected for testosterone (t1,075 = 3.7, Ptime × spray × hormone = 0.0002). However, this was not present for calculated free testosterone (t1,075 = 0.37, Ptime × spray × hormone = 0.7) and was also not present for other tested androgens (androstenedione (t1,075 = 1.75, Ptime × spray × hormone = 0.08) and DHEA-sulfate (t1,075 = −0.64, Ptime × spray × hormone = 0.5)).
Functional magnetic resonance imaging study
We investigated 15 women for the functional magnetic resonance imaging (fMRI) study (Fig. 1b). Just as in study group 1, body weight and body fat contents were comparable between cycle phases (both P ≥ 0.2), and there were differences in sex hormones (Supplementary Table 2). Progesterone, 17-OH-progesterone and estradiol concentrations were higher in the luteal phase. FSH and LH levels did not differ between phases. The studied androgens were also comparable between phases, while calculated free testosterone was higher in the follicular phase. Neither fasting glucose nor insulin nor C-peptide levels differed between phases (all P ≥ 0.1).
On the basis of our previous findings3,8, we assessed the insulin-induced response of hypothalamic cerebral blood flow (CBF) as a readout for hypothalamic insulin action. For this purpose, a hypothalamus mask was created based on the WFU pickatlas, which resulted in a mean CBF of 23 voxels. Insulin administration significantly decreased hypothalamic CBF only in the follicular phase from before administration to 30 min after nasal spray application (t14 = −2.57, P = 0.02). In the luteal phase, insulin induced no such effect (t14 = −0.467, P = 0.648; Fig. 3a,b). However, no significant interaction was observed between cycle phase and spray application (P = 0.5). Furthermore, no significant correlations with the tested sex hormones were observed for hypothalamic insulin response (P ≥ 0.1).
a, Hypothalamus region of interest in coronal (top), sagittal (middle) and axial section planes (bottom). b, Absolute changes in CBF in the hypothalamus from before treatment to 30 min after intranasal insulin administration in the follicular (left) and luteal (right) phases of the menstrual cycle. In the follicular phase, insulin induced a significant reduction in hypothalamic CBF, whereas this effect was absent in the luteal phase. Bar graphs are shown with means and individual data points, and error bars represent s.e.m. Data were analysed by two-sided paired t-tests; *P = 0.02; N = 15 participants in both cycle phases.
In the follicular phase of the menstrual cycle, brain insulin action improved peripheral insulin sensitivity in lean women, similar to what has previously been described in lean men. Such a response was not detectable in the luteal phase. In our functional neuroimaging study, consistent findings were obtained with modulation of hypothalamic activity by insulin in the follicular phase but not in the luteal phase. However, insulin response in the hypothalamus was not statistically different between cycle phases.
Our findings demonstrate that brain insulin sensitivity is not a fixed trait but can be rapidly regulated, with metabolic consequences for the entire body. The current study corroborates that insulin action in the human brain modulates peripheral insulin sensitivity3,4,11 and demonstrates that this mechanism is not limited to lean men but is also present in lean women. This underscores the brain’s function in ‘fine-tuning’ whole-body metabolism to rapidly adapt peripheral energy fluxes to current energy availability2.
However, in lean women, this function of insulin in the brain appears to be evident only in the follicular phase. Here, the effects on peripheral insulin sensitivity were comparable in timing and magnitude to previous findings obtained in lean men3,4.
How this would relate to women who are overweight/obese is unclear. Most likely, they would behave as men with obesity3,4,27 and would not respond to brain insulin delivery, regardless of their menstrual cycle phase. This hypothesis is in line with neuroimaging studies that demonstrated hypothalamic insulin resistance in individuals of both sexes who were overweight or obese8,28. However, appropriate interventional studies are needed to experimentally investigate this assumption.
Peripheral insulin sensitivity changes during the menstrual cycle, with relative peripheral insulin resistance occurring in the luteal phase compared to in the follicular phase29. Our results suggest that changes in brain insulin responsiveness could be one mechanism that contributes to this phenomenon. The failure of brain insulin to exert its effects on peripheral insulin sensitivity in the luteal phase is likely due to relative hypothalamic insulin resistance in this cycle phase. In line with experimental evidence from animals30, previous correlational findings from humans indicate a crucial role of insulin action in the hypothalamus for the modulation of whole-body glucose metabolism3,4. Other brain regions may also contribute to this complex regulation2. Based on our current results, we hypothesize that hypothalamic insulin resistance in the luteal phase may be responsible for cycle-dependent differences in brain-derived modulation of peripheral insulin sensitivity.
The hypothalamus is crucial not only for peripheral insulin sensitivity and whole-body glucose homeostasis but also for upstream regulation of sex hormones and thereby the menstrual cycle. However, both functions depend on distinct loci of the hypothalamus. The precise anatomical and functional interplay between gonadotropin-releasing hormone-producing neurons in the preoptic area and neurons crucial for whole-body glucose homeostasis in the medial hypothalamus is still largely unexplored. Of note, brain-specific deletion of insulin receptors altered physiologic gonadotropin secretion in rodents of both sexes31, suggesting a reciprocal interplay of brain insulin and the menstrual cycle.
It is tempting to speculate on the potential physiologic functions of regulating brain insulin sensitivity across the menstrual cycle. One major function of the menstrual cycle is the preparation of the uterus and the rest of the body for a potential upcoming pregnancy. During the follicular phase, glucose fluxes over the human body must be precisely relayed to areas with high energy need, including the uterus with the growing endometrium. Besides modulating whole-body energy fluxes2, insulin sensitivity is crucial for both the uterus and ovaries32. Insulin signalling plays a critical role in the proliferation of the endometrium33 and development of the preovulatory follicle34. Of note, rodents selectively lacking brain insulin receptors display impaired follicular maturation31, underpinning the crucial role of brain insulin action in oocyte maturation. Proper brain-derived enhancement of peripheral insulin sensitivity may support insulin-sensitive processes in the uterus and ovaries. These brain insulin-mediated mechanisms are thought to be especially important in the postprandial state12 when vast amounts of energy must be guided through the body.
In the luteal phase, lack of the brain-derived modulation of peripheral insulin sensitivity could promote energy storage in adipose tissue, as suggested by findings of progressive fat accumulation in humans with brain insulin resistance18. Besides the major effects of sex hormones, these natural changes in brain-derived modulation of adipose energy storage throughout the menstrual cycle could be one contributor to differences in body fat distribution between sexes35.
Although we detected an association between testosterone levels and the brain’s ability to enhance peripheral insulin sensitivity, the lack of association with free testosterone argues against a major role in the regulation of brain insulin sensitivity. However, estradiol:progesterone ratio was linked to brain-derived improvement of peripheral insulin sensitivity. This is contrary to findings in healthy men, where estrogen administration did not alter the response in eating behaviour after administration of nasal insulin36. However, influences of sex hormones on insulin sensitivity have long been suspected37, and sex-specific effects of gonadal hormones are plausible. Precise molecular mechanisms of potential hormonal influences on brain insulin responsiveness need to be further tested in upcoming studies.
We studied only a limited number of participants and did not include women taking oral contraceptives. The detected differences are rather small and come with large confidence intervals, and replication is necessary before final conclusions can be drawn. Furthermore, nasal insulin delivery is not the physiological route. Despite the lack of data on the exact amount of insulin reaching the brain, previous trials found that the used dose of 160 U resulted in substantial effects on brain activity6,7, comparable to oral glucose ingestion, and this dose has been widely used in previous trials on acute brain effects of insulin38. While we mimicked spillover of nasal insulin into the systemic circulation with statistically comparable circulating concentrations between study days, there was still variation in individual study participants that might potentially have impacted our results. The limited sample size might also underlie the fact that differences in hypothalamic insulin response did not reach statistical significance, while significant interactions between spray and cycle phase were detected in the hyperinsulinemic–euglycemic clamp experiments. In our hyperinsulinemic–euglycemic clamp experiments, we unfortunately did not achieve stable tracer enrichment of d-[6,6-2H2]-glucose and were therefore not able to disentangle endogenous glucose production and glucose disappearance. Previous findings from healthy men indicate that brain insulin most likely modulates both3. Furthermore, our experiments were performed in a single-blind fashion, and the investigators were aware of the condition (nasal insulin versus placebo). In our fMRI experiment, no placebo administration was used as a control condition. However, in our previous placebo-controlled assessments6,8,39 (see Supplementary Material 2 for more details), the placebo nasal spray did not impact hypothalamic blood flow.
Taken together, insulin delivery to the brain improves peripheral insulin sensitivity in lean women, comparable to what has previously been described in lean men. However, this response was limited to the follicular phase of the menstrual cycle and appears to be absent in the luteal phase. The underlying mechanism most likely involves hypothalamic insulin resistance during the luteal phase. Brain insulin resistance could therefore contribute to the long-known peripheral insulin resistance in the luteal phase of the menstrual cycle. This could also be involved in worse glycemic control in the luteal phase in women with type I diabetes40. Our findings need to be taken into consideration both in mechanistic studies as well as in upcoming therapeutic approaches that address insulin action in the brain.
Methods
The study (NCT03929419) received approval by the local ethics committee (Ethics Committee of the Medical Faculty of the Eberhard Karls University and the University Hospital of Tübingen) and was conducted according to the relevant guidelines and regulations. Data acquisition was performed at the University Hospital of Tübingen between April 2019 and March 2021.
Study design and participants
We analysed two different study populations. In the first group (N = 11), we performed two clamp experiments per cycle phase using a single-blind crossover design (Extended Data Fig. 3). The second group (N = 15) was subjected to one fMRI experiment per cycle phase (Extended Data Fig. 4). The initial cycle phase for the experiments was chosen in a random order. For both groups, young, healthy, naturally cycling women who neither took hormonal contraceptives nor any other medication were recruited (for participant characteristics, see Table 1; for inclusion and exclusion criteria, see Supplementary Table 3). After providing informed written consent, participants were screened for eligibility, where medical history and a blood count were obtained. To ensure a regular menstrual cycle, participants provided information about the length of their last three cycles, which most of them tracked via an app.
The respective phase of the menstrual cycle was identified using calendar-based counting and serum sex hormone analysis41. Taking the individual cycle length into account, the start of the current and subsequent cycles were recorded to determine the cycle day of the experiment retrospectively. For this, the onset of their last menstruation was considered the first day of the current cycle and denoted the start of the follicular phase. The follicular phase was assumed starting 2 d after the start of the current menstrual cycle but no more than 17 d apart from the start of the subsequent cycle. The luteal phase was assigned in the time frame of a maximum of 10 d but no less than 3 d before the start of the subsequent cycle (Fig. 1). For final cycle phase verification, sex hormones (LH, FSH, estradiol and progesterone) were analysed, as proposed previously41.
Participants were instructed to restrain from heavy physical activity, smoking and alcohol within the 24 h before the experiments.
Hyperinsulinemic–euglycemic clamp (performed in study group 1)
The applied protocol was similar to our previous hyperinsulinemic–euglycemic clamps in men3,4. Experiments were conducted in the morning after an overnight fast. Participants underwent two hyperinsulinemic–euglycemic clamp experiments per cycle phase in a single-blind randomized order. Hence, only study participants were blinded for the type of spray, whereas study personnel were not blinded due to safety reasons. A venous cannula was placed in one arm that was warmed to facilitate arterialized blood sampling. Another cannula was placed into the contralateral antecubital vein for infusions. The experiment started with an intravenous insulin bolus of 6.25 mU × kg−1, followed by constant i.v. insulin infusion at 0.25 mU × kg−1 × min−1 for 3.5 h (Insuman Rapid, Sanofi). Nasal spray was administered 1.5 h after initiation of insulin infusion (time point: 0 min). On one day, participants received 160 U of human insulin (eight puffs in each nostril over 4 min; insulin Actrapid, Novo Nordisk), and vehicle was administered as placebo on the other day. Together with placebo spray, insulin infusion was increased by 0.17 mU × kg−1 × min−1 for 15 min after the first placebo spray puff. This resulted in an i.v. insulin bolus of 2.5 mU × kg−1 over 15 min and was performed to mimic spillover of nasal insulin into the systemic circulation, as done previously3,42.
During the experiment, blood samples were taken every 5 min to measure blood glucose, and the infusion rate of 20% dextrose was adjusted to maintain euglycemia with a targeted glucose concentration of 5 mmol litr–1. Additional blood samples were taken to determine the concentrations of other hormones. The infused glucose was enriched with d-[6,6-2H2]-glucose, although no stable levels were achieved, and tracer enrichment was therefore not further statistically analysed.
In this paper, the time of spray administration was used as time point 0 min. This makes it easier to understand the timeline of the effects of nasal insulin versus placebo and to facilitate comparison with previous studies using the same clamp protocol in men3. As a result, the nomenclature of the time frame in ClinicalTrials.gov and the paper differs, with the time frame 60–90 min after clamp initiation in ClinicalTrials.gov referring to the time before spray administration (that is 0 min in the paper) and the time frame 150–210 min in ClinicalTrials.gov referring to the end of the clamps, which corresponds to 90–120 min in this paper.
Analytic procedures
Blood glucose was measured immediately on site by the glucose oxidase method (Biosen C-Line, EKF Diagnostics). Technical errors prevented valid measurement of seven glucose values, which were therefore excluded from analyses. Except for androstenedione, which was measured from samples stored at −80 °C, all other analytes were handled on ice and measured immediately in the central laboratory of the University Hospital of Tübingen. Concentrations of circulating testosterone, insulin, C-peptide, DHEA-sulfate, LH, FSH, cortisol, estradiol and prolactin were measured on an ADVIA Centaur XPT, and sexual hormone-binding globulin and androstenedione were measured on IMMULITE immunoassay systems (Siemens Healthineers). Anti-Müllerian hormone concentrations were determined using the cobas e411 analyser (Roche Diagnostics). Plasma concentrations of albumin as well as total non-esterified fatty acids (enzymatic method, WAKO Chemicals) were measured using the ADVIA XPT clinical chemical analyser (Siemens Healthineers). HbA1c measurements were performed using a Tosoh glycohemoglobin analyser (HLC-723G8, Tosoh Bioscience). 17-OH-Progesterone was measured on the IDS-iSYS Immunoanalyzer (Immunodiagnostic Systems). Based on serum testosterone, sexual hormone-binding globulin and albumin levels, free testosterone in serum was calculated as previously reported43.
Hormone measurements were performed in a routine diagnostic laboratory under accreditation with the German accredited body (DAkkS). Internal and external quality control was performed at all times during the study, including proficiency testing four times per year, and passed at all times.
Brain fMRI measurement (performed in study group 2)
After completing the hyperinsulinemic–euglycemic clamp experiments, we decided to follow up by studying insulin effects in the hypothalamus. However, once the amendment was approved by the Ethics Committee, we were unable to retain all initial participants of the clamp study and therefore recruited 15 new participants who underwent pulsed arterial spin labelling measurement to determine CBF. After the basal fMRI measurement, 160 U of nasal insulin were administered. A second fMRI measurement was performed 30 min later.
Data acquisition
Scanning was conducted with a 3T whole-body Siemens scanner (MAGNETOM Prisma) with a 20-channel coil.
As recently reported8,44, pulsed arterial spin labelling images were obtained with a PICORE-Q2TIPS (proximal inversion with control for off-resonance effects (quantitative imaging of perfusion by using a single subtraction sequence)) by applying a frequency offset corrected inversion pulse and echo planar imaging readout for acquisition. In addition, high-resolution T1-weighted anatomical images were obtained.
Arterial spin labelling image processing
For image preprocessing, ASLtbx with SPM12 (Wellcome Trust Centre for Neuroimaging) was implemented. In detail, functional images were motion corrected, co-registered to the individual anatomical image and smoothed with a full-width at half-maximum of 6 mm. Perfusion images were generated by calculating the control tag differences by using surround subtraction. We used a unique M0 value extracted from a region of interest in the cerebrospinal fluid to quantify CBF (ml × 100 g−1 × min−1) and applied the general kinetic model for absolute perfusion quantification. CBF was extracted from the hypothalamus as our region of interest, based on recent findings3,8.
Safety
We observed a short-lasting burning sensation in the nose immediately after administration of both types of nasal sprays in most participants. No other adverse effects were observed. Importantly, there were no cases of allergic reactions or severe hypoglycemia.
Statistical analysis
Statistical analyses for clinical characteristics and the hyperinsulinemic–euglycemic clamp as well as block randomization for the clamp study were performed using R 4.2.1 (ref. 45). Normal distribution was tested by visual inspection of QQ-plots.
Differences in metabolite/hormone levels across study days were tested by one-way analysis of variance (ANOVA). The courses of dynamic variables, such as glucose infusion rates, across clamps were modelled for all time points. We tested interactions between elapsed time during the clamp (standardized) and the appropriate grouping variables (phase: follicular versus luteal; spray: placebo versus insulin) using linear mixed models with the interaction terms as fixed effects. In some models, additional adjustment for plasma glucose and insulin was performed, as indicated. The participants and clamp order of the four investigations were included as random intercept and random slope, respectively. Glucose infusion rates were tested relative to the glucose infusion rate before spray application at 90 min of the clamp. We used the lme4 library46 (version 1.1-31) to fit mixed linear models, and P values were computed using Satterthwaite’s method as implemented in the lmerTest library47 (version 3.1-3). Details on the mixed models are provided in Supplementary Table 4.
Mean hypothalamic CBF before and after nasal insulin application was compared by paired t-tests (two tailed) for the follicular and luteal phases separately. The interaction between cycle phase and spray application was tested using a linear mixed model with the interaction term as a fixed effect.
As the fMRI study (study group 2) did not include a placebo spray control, we evaluated hypothalamic CBF response before and 30 min after placebo administration based on previous publications8,39. No significant change in hypothalamic blood flow was observed in 110 healthy participants (P > 0.05). On a whole-brain level, a significant CBF increase was identified only in the occipital region 30 min after placebo spray application (family-wise error-corrected P value of <0.05, corrected for multiple comparisons; see Supplementary Material 1). Furthermore, reproducibility and reliability were assessed for the global and hypothalamic CBF measures in participants with two measurement time points separated by 2 to 6 weeks without a lifestyle or pharmaceutical intervention in between (see Supplementary Material 2).
For the hyperinsulinemic–euglycemic clamp study, a sample size of N = 10 was calculated (α = 0.05, power (1 – β error probability) = 0.9, G*Power) based on an effect size of Cohen’s dz = 1.178, which was achieved in our previous work investigating glucose infusion rate in response to intranasal insulin in lean men20. To compensate for potential dropouts, N = 2 were additionally recruited.
For the fMRI study, the sample size was chosen based on previous studies using fMRI in combination with intranasal insulin versus placebo showing moderate-to-high within-group (dz = 0.7) and high between-group differences (dz = 0.9) in hypothalamus insulin action8,48.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated during the current study are shared with researchers upon reasonable request. Requests will be promptly reviewed by the Data Access Steering Committee of the Institute of Diabetes Research and Metabolic Diseases, Tübingen, Germany. Any data and materials that can be shared will be released via a material transfer agreement.
References
Scherer, T., Sakamoto, K. & Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. 17, 468–483 (2021).
Kullmann, S. et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 8, 524–534 (2020).
Heni, M. et al. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66, 1797–1806 (2017).
Heni, M. et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 63, 4083–4088 (2014).
Born, J. et al. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 5, 514–516 (2002).
Kullmann, S. et al. Dose-dependent effects of intranasal insulin on resting-state brain activity. J. Clin. Endocrinol. Metab. 103, 253–262 (2018).
Edwin Thanarajah, S. et al. Modulation of midbrain neurocircuitry by intranasal insulin. Neuroimage 194, 120–127 (2019).
Kullmann, S. et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 38, 1044–1050 (2015).
Rhea, E. M. & Banks, W. A. A historical perspective on the interactions of insulin at the blood–brain barrier. J. Neuroendocrinol. 33, e12929 (2021).
Kullmann, S., Heni, M., Fritsche, A. & Preissl, H. Insulin action in the human brain: evidence from neuroimaging studies. J. Neuroendocrinol. 27, 419–423 (2015).
Dash, S., Xiao, C., Morgantini, C., Koulajian, K. & Lewis, G. F. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes 64, 766–774 (2015).
Plomgaard, P. et al. Nasal insulin administration does not affect hepatic glucose production at systemic fasting insulin levels. Diabetes Obes. Metab. 21, 993–1000 (2018).
Gancheva, S. & Roden, M. Central regulation of glucose metabolism in humans: fact or fiction? Diabetes 65, 2467–2469 (2016).
Gancheva, S. et al. Effects of intranasal insulin on hepatic fat accumulation and energy metabolism in humans. Diabetes 64, 1966–1975 (2015).
Hallschmid, M. Intranasal insulin. J. Neuroendocrinol. 33, e12934 (2021).
Tschritter, O. et al. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc. Natl Acad. Sci. USA 103, 12103–12108 (2006).
Arnold, S. E. et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 14, 168–181 (2018).
Kullmann, S. et al. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat. Commun. 11, 1841 (2020).
Heni, M., Kullmann, S., Preissl, H., Fritsche, A. & Häring, H.-U. Impaired insulin action in the human brain: causes and metabolic consequences. Nat. Rev. Endocrinol. 11, 701–711 (2015).
Tschritter, O. et al. The insulin effect on cerebrocortical theta activity is associated with serum concentrations of saturated nonesterified fatty acids. J. Clin. Endocrinol. Metab. 94, 4600–4607 (2009).
Rhea, E. M., Banks, W. A. & Raber, J. Insulin resistance in peripheral tissues and the brain: a tale of two sites. Biomedicines 10, 1582 (2022).
Heni, M. et al. Evidence for altered transport of insulin across the blood–brain barrier in insulin-resistant humans. Acta Diabetol. 51, 679–681 (2014).
Clegg, D. J., Riedy, C. A., Smith, K. A. B., Benoit, S. C. & Woods, S. C. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 52, 682–687 (2003).
Benedict, C., Kern, W., Schultes, B., Born, J. & Hallschmid, M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab. 93, 1339–1344 (2008).
Hallschmid, M., Higgs, S., Thienel, M., Ott, V. & Lehnert, H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 61, 782–789 (2012).
Hallschmid, M. et al. Intranasal insulin reduces body fat in men but not in women. Diabetes 53, 3024–3029 (2004).
Xiao, C., Dash, S., Stahel, P. & Lewis, G. F. Effects of intranasal insulin on endogenous glucose production in insulin resistant men. Diabetes Obes. Metab. 20, 1751–1754 (2018).
Kullmann, S. et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care 45, 398–406 (2021).
Yeung, E. H. et al. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle study. J. Clin. Endocrinol. Metab. 95, 5435–5442 (2010).
Ruud, J., Steculorum, S. M. & Brüning, J. C. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat. Commun. 8, 15259 (2017).
Brüning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Pauli, J. M., Raja-Khan, N., Wu, X. & Legro, R. S. Current perspectives of insulin resistance and polycystic ovary syndrome. Diabet. Med. 28, 1445–1454 (2011).
Sekulovski, N., Whorton, A. E., Shi, M., Hayashi, K. & MacLean, J. A. Insulin signaling is an essential regulator of endometrial proliferation and implantation in mice. FASEB J. 35, e21440 (2021).
Das, D. & Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 84, 444–459 (2017).
Li, C., Ford, E. S., Zhao, G., Balluz, L. S. & Giles, W. H. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am. J. Clin. Nutr. 90, 1457–1465 (2009).
Krug, R., Mohwinkel, L., Drotleff, B., Born, J. & Hallschmid, M. Insulin and estrogen independently and differentially reduce macronutrient intake in healthy men. J. Clin. Endocrinol. Metab. 103, 1393–1401 (2018).
Bruns, C. M. & Kemnitz, J. W. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J. 45, 160–169 (2004).
Schmid, V. et al. Safety of intranasal human insulin: a review. Diabetes Obes. Metab. 20, 1563–1577 (2018).
Wagner, L. et al. Brain insulin responsiveness is linked to age and peripheral insulin sensitivity. Diabetes Obes. Metab. 25, 2171–2180 (2023).
Tatulashvili, S. et al. Ambulatory glucose profile according to different phases of the menstrual cycle in women living with type 1 diabetes. J. Clin. Endocrinol. Metab. 107, 2793–2800 (2022).
Schaumberg, M. A., Jenkins, D. G., Janse de Jonge, X. A. K., Emmerton, L. M. & Skinner, T. L. Three-step method for menstrual and oral contraceptive cycle verification. J. Sci. Med. Sport 20, 965–969 (2017).
Heni, M. et al. Insulin action in the hypothalamus increases second-phase insulin secretion in humans. Neuroendocrinology 110, 929–937 (2020).
Vermeulen, A., Verdonck, L. & Kaufman, J. M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 84, 3666–3672 (1999).
Kullmann, S. et al. Exercise restores brain insulin sensitivity in sedentary adults who are overweight and obese. JCI Insight 7, e161498 (2022).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Kuznetsova, A., Brockhoff, P. & Christensen, R. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017).
Kullmann, S. et al. Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology 97, 176–182 (2013).
Acknowledgements
We thank all the participants for their participation in this trial. We especially thank I. Wagener, A. Eberle, M. Borutta, L. Wagner, D. Neuscheler and E. Metzinger for their excellent technical assistance. This study was partly supported by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD: 01GI0925).
Funding
Open access funding provided by Helmholtz Zentrum München - Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH).
Author information
Authors and Affiliations
Contributions
J.H., C.B. and R.W. researched and analysed data. S.K. researched, analysed and supervised the fMRI study. L.F., K.P., A.V., S.G., S.T. and A.P. researched data. A.L.B., H.P. and M.R. contributed to discussion. H.-U.H. and A.F. contributed to the design of the trial and discussed data. M.H. designed the trial, researched data, supervised the project and drafted the manuscript together with S.K. and J.H. All authors contributed to discussion and approved the final version of the paper before submission.
Corresponding author
Ethics declarations
Competing interests
M.R. served on scientific advisory boards of Bristol-Myers Squibb, Eli Lilly, Fishawack Group, Gilead Sciences, Novo Nordisk and Target RWE and received investigator-initiated support from Boehringer Ingelheim, Nutricia/Danone and Sanofi-Aventis. A.F. reports lecture fees from Sanofi, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, AstraZeneca and Synlab and serves as a consultant for Novo Nordisk and Sanofi. R.W. reports lecture fees from Novo Nordisk and Sanofi. R.W. served on an advisory board for Akcea Therapeutics, Daiichi Sankyo, Sanofi and Novo Nordisk. S.K. reports lecture fees from Novo Nordisk and serves as a consultant for Boehringer Ingelheim. Outside of the current work, M.H. reports research grants from Boehringer Ingelheim and Sanofi to the University Hospital of Tübingen, participation on an advisory board for Boehringer Ingelheim, Sanofi and Amryt and lecture fees from Amryt, Sanofi, Eli Lilly, Novo Nordisk and Boehringer Ingelheim. All other authors have no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Alexandra DiFeliceantonio and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Results from the hyperinsulinemic-euglycemic clamp study – individual courses of serum insulin around nasal insulin administration / mimicking by iv insulin bolus.
Presented are the individual courses of serum insulin during the clamp after intranasal insulin spray (green and orange circles) vs placebo spray combined with simultaneous iv insulin bolus (yellow and blue circles) in both phases of the menstrual cycle. Presented are individual data points; N = 11 participants except for the follicular phase where N = 10 for the insulin spray.
Extended Data Fig. 2 Results from the hyperinsulinemic-euglycemic clamp study – individual courses of plasma glucose around nasal insulin administration / mimicking by iv insulin bolus.
Presented are the individual courses of plasma glucose during the clamp after intranasal insulin spray (green and orange circles) vs placebo spray combined with simultaneous iv insulin bolus (yellow and blue circles) in both phases of the menstrual cycle. Presented is a dot plot with means; error bars represent SEM; N = 11 participants except for the follicular phase where N = 10 for the insulin spray.
Extended Data Fig. 3 CONSORT flow diagram - hyperinsulinemic-euglycemic clamp study.
CONSORT flow chart of the study course of the hyperinsulinemic-euglycemic clamp study.
Extended Data Fig. 4 CONSORT flow diagram – fMRI study.
CONSORT flow chart of the study course of the fMRI study.
Supplementary information
Supplementary Information
Supplementary Tables 1–4 and Materials 1 and 2.
Supplementary Data 1
Translated final study protocol of the trial.
Supplementary Data 2
CONSORT checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hummel, J., Benkendorff, C., Fritsche, L. et al. Brain insulin action on peripheral insulin sensitivity in women depends on menstrual cycle phase. Nat Metab 5, 1475–1482 (2023). https://doi.org/10.1038/s42255-023-00869-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00869-w
This article is cited by
-
The insulin resistant brain: impact on whole-body metabolism and body fat distribution
Diabetologia (2024)
-
Brain insulin action in women
Nature Reviews Endocrinology (2023)