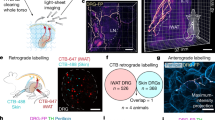
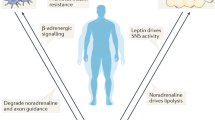
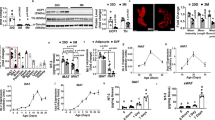
Abstract
Homeostatic regulation of adipose tissue is critical for the maintenance of energy balance and whole-body metabolism. The peripheral nervous system provides bidirectional neural communication between the brain and adipose tissue, thereby providing homeostatic control. Most research on adipose innervation and nerve functions has been limited to the sympathetic nerves and their neurotransmitter norepinephrine. In recent years, more work has focused on adipose sensory nerves, but the contributions of subsets of sensory nerves to metabolism and the specific roles contributed by sensory neuropeptides are still understudied. Advances in imaging of adipose innervation and newer tissue denervation techniques have confirmed that sensory nerves contribute to the regulation of adipose functions, including lipolysis and browning. Here, we summarize the historical and latest findings on the regulation, function and plasticity of adipose tissue sensory nerves that contribute to metabolically important processes such as lipolysis, vascular control and sympathetic axis cross-talk.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bartness, T. J., Vaughan, C. H. & Song, C. K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34, S36–S42 (2010).
Bartness, T. J., Shrestha, Y. B., Vaughan, C. H., Schwartz, G. J. & Song, C. K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 318, 34–43 (2010).
Wang, Y. et al. The role of somatosensory innervation of adipose tissues. Nature 609, 569–574 (2022).
Bartness, T. & Kay Song, C. Innervation of brown adipose tissue and its role in thermogenesis. Can. J. Diabetes 29, 420–428 (2005).
Youngstrom, T. G. & Bartness, T. J. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am. J. Physiol. 275, R1488–R1493 (1998).
Harris, R. B. S. Denervation as a tool for testing sympathetic control of white adipose tissue. Physiol. Behav. 190, 3–10 (2018).
Harris, R. B. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity 20, 1355–1364 (2012).
Makwana, K. et al. Sensory neurons expressing calcitonin gene-related peptide alpha regulate adaptive thermogenesis and diet-induced obesity. Mol. Metab. 45, 101161 (2021).
Nguyen, N. L. T., Xue, B. & Bartness, T. J. Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters. Physiol. Behav. 190, 28–33 (2018).
Blaszkiewicz, M. et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLoS ONE 14, e0221766 (2019).
Almuklass, A. M., Capobianco, R. A., Feeney, D. F., Alvarez, E. & Enoka, R. M. Sensory nerve stimulation causes an immediate improvement in motor function of persons with multiple sclerosis: a pilot study. Mult. Scler. Relat. Disord. 38, 101508 (2020).
Dhaka, A., Earley, T. J., Watson, J. & Patapoutian, A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 28, 566–575 (2008).
Fishman, R. B. & Dark, J. Sensory innervation of white adipose tissue. Am. J. Physiol. 253, R942–R944 (1987).
Song, C. K., Schwartz, G. J. & Bartness, T. J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R501–R511 (2009).
Stefanidis, A. et al. Insights into the neurochemical signature of the Innervation of Beige Fat. Mol. Metab. 11, 47–58 (2018).
Giordano, A. et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1243–R1255 (2006).
Ryu, V., Garretson, J. T., Liu, Y., Vaughan, C. H. & Bartness, T. J. Brown adipose tissue has sympathetic-sensory feedback circuits. J. Neurosci. 35, 2181–2190 (2015).
Vaughan, C. H. & Bartness, T. J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1049–R1058 (2012).
Garretson, J. T. et al. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol. Metab. 5, 626–634 (2016).
Liu, B. X. et al. Distribution, morphological characterization, and resiniferatoxin-susceptibility of sensory neurons that innervate rat perirenal adipose tissue. Front Neuroanat. 13, 29 (2019).
Marzvanyan, A. & Alhawaj, A. F. in StatPearls (StatPearls Publishing, 2023).
Quick, K. et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2, 120068 (2012).
Sita, G., Hrelia, P., Graziosi, A., Ravegnini, G. & Morroni, F. TRPM2 in the brain: role in health and disease. Cells https://doi.org/10.3390/cells7070082 (2018).
Gavva, N. R. et al. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol. Pain. 8, 36 (2012).
Duitama, M. et al. TRP channels role in pain associated with neurodegenerative diseases. Front Neurosci. 14, 782 (2020).
Christie, S., Wittert, G. A., Li, H. & Page, A. J. Involvement of TRPV1 channels in energy homeostasis. Front Endocrinol. 9, 420 (2018).
Cavanaugh, D. J. et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 31, 10119–10127 (2011).
Baboota, R. K. et al. Capsaicin induces ‘brite’ phenotype in differentiating 3T3-L1 preadipocytes. PLoS ONE 9, e103093 (2014).
Kanno, M., Akishima, S., Ohta, J., Hara, S. & Honda, M. A case of acute postinfarction mitral insufficiency and cardiogenic shock caused by total rupture of a papillary muscle. Kyobu Geka 44, 515–518 (1991).
Cline, D. L., Short, L. I., Forster, M. A. M. & Gray, S. L. Adipose tissue expression of PACAP, VIP, and their receptors in response to cold stress. J. Mol. Neurosci. 68, 427–438 (2019).
Jia, M. Q. et al. Orexin receptor type 2 agonism inhibits thermogenesis in brown adipose tissue by attenuating afferent innervation. J. Biomed. Res. 36, 195–207 (2022).
Conner, W. E., Lin, D. S. & Colvis, C. Differential mobilization of fatty acids from adipose tissue. J. Lipid Res. 37, 290–298 (1996).
Raclot, T. & Groscolas, R. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J. Lipid Res. 34, 1515–1526 (1993).
Snoke, D. B. et al. Linoleate-rich safflower oil diet increases linoleate-derived bioactive lipid mediators in plasma, and brown and white adipose depots of healthy mice. Metabolites https://doi.org/10.3390/metabo12080743 (2022).
Miller, J. L. et al. A peroxidized omega-3-enriched polyunsaturated diet leads to adipose and metabolic dysfunction. J. Nutr. Biochem. 64, 50–60 (2019).
Alsalem, M. et al. The contribution of the endogenous TRPV1 ligands 9-HODE and 13-HODE to nociceptive processing and their role in peripheral inflammatory pain mechanisms. Br. J. Pharmacol. 168, 1961–1974 (2013).
Inoue, N., Matsunaga, Y., Satoh, H. & Takahashi, M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci. Biotechnol. Biochem. 71, 380–389 (2007).
Snitker, S. et al. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am. J. Clin. Nutr. 89, 45–50 (2009).
Yoshioka, M., Doucet, E., Drapeau, V., Dionne, I. & Tremblay, A. Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. Br. J. Nutr. 85, 203–211 (2001).
Baskaran, P., Krishnan, V., Ren, J. & Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 173, 2369–2389 (2016).
Lee, E. et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 29, 3182–3192 (2015).
Li, L. et al. Lack of TRPV1 aggravates obesity-associated hypertension through the disturbance of mitochondrial Ca2+ homeostasis in brown adipose tissue. Hypertens. Res 45, 789–801 (2022).
Ohyama, K. et al. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 65, 1410–1423 (2016).
Saito, M., Matsushita, M., Yoneshiro, T. & Okamatsu-Ogura, Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men. Front. Endocrinol. 11, 222 (2020).
Motter, A. L. & Ahern, G. P. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 582, 2257–2262 (2008).
Takaishi, M. et al. Reciprocal effects of capsaicin and menthol on thermosensation through regulated activities of TRPV1 and TRPM8. J. Physiol. Sci. 66, 143–155 (2016).
Niijima, A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J. Auton. Nerv. Syst. 73, 19–25 (1998).
Murphy, K. T. et al. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 304, E1338–E1347 (2013).
Levi-Montalcini, R. & Angeletti, P. U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev. Biol. 6, 653–659 (1963).
Yoo, S., Lim, J. Y. & Hwang, S. W. Sensory TRP channel interactions with endogenous lipids and their biological outcomes. Molecules 19, 4708–4744 (2014).
Guilherme, A., Henriques, F., Bedard, A. H. & Czech, M. P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 15, 207–225 (2019).
Shaw, J. E. & Ramwell, P. W. Release of prostaglandin from rat epididymal fat pad on nervous and hormonal stimulation. J. Biol. Chem. 243, 1498–1503 (1968).
Smith, J. A., Amagasu, S. M., Eglen, R. M., Hunter, J. C. & Bley, K. R. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br. J. Pharmacol. 124, 513–523 (1998).
Shi, Z. et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J. Appl. Physiol. 112, 1008–1014 (2012).
Ryu, V., Watts, A. G., Xue, B. & Bartness, T. J. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R324–R337 (2017).
de Kloet, A. D. & Herman, J. P. Fat-brain connections: adipocyte glucocorticoid control of stress and metabolism. Front. Neuroendocrinol. 48, 50–57 (2018).
do Carmo, J. M. et al. Obesity-induced hypertension: brain signaling pathways. Curr. Hypertens. Rep. 18, 58 (2016).
Pyner, S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J. Chem. Neuroanat. 38, 197–208 (2009).
Seravalle, G. & Grassi, G. Sympathetic nervous system, hypertension, obesity and metabolic syndrome. High. Blood Press. Cardiovasc Prev. 23, 175–179 (2016).
Ding, L. et al. Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS ONE 8, e83771 (2013).
Xiong, X. Q. et al. Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension 60, 1280–1286 (2012).
Dalmasso, C., Leachman, J. R., Osborn, J. L. & Loria, A. S. Sensory signals mediating high blood pressure via sympathetic activation: role of adipose afferent reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R379–R389 (2020).
Cui, B. P. et al. Ionotropic glutamate receptors in paraventricular nucleus mediate adipose afferent reflex and regulate sympathetic outflow in rats. Acta Physiol. 209, 45–54 (2013).
Kalil, G. Z. & Haynes, W. G. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens. Res. 35, 4–16 (2012).
Garcia-Mesa, Y. et al. Involvement of cutaneous sensory corpuscles in non-painful and painful diabetic neuropathy. J. Clin. Med. https://doi.org/10.3390/jcm10194609 (2021).
Agashe, S. & Petak, S. Cardiac autonomic neuropathy in diabetes mellitus. Methodist Debakey Cardiovasc. J. 14, 251–256 (2018).
Azpiroz, F. & Malagelada, C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia 59, 404–408 (2016).
He, Z., Yin, G., Li, Q. Q., Zeng, Q. & Duan, J. Diabetes mellitus causes male reproductive dysfunction: a review of the evidence and mechanisms. In Vivo 35, 2503–2511 (2021).
Willows, J. W. et al. Age-related changes to adipose tissue and peripheral neuropathy in genetically diverse HET3 mice differ by sex and are not mitigated by rapamycin longevity treatment. Aging Cell https://doi.org/10.1111/acel.13784 (2023).
Blaszkiewicz, M. et al. The involvement of neuroimmune cells in adipose innervation. Mol. Med. 26, 126 (2020).
Blaszkiewicz, M. et al. Adipose tissue myeloid-lineage neuroimmune cells express genes important for neural plasticity and regulate adipose innervation. Front. Endocrinol. 13, 864925 (2022).
Willows, J. W. et al. Schwann cells contribute to demyelinating diabetic neuropathy and nerve terminal structures in white adipose tissue. iScience 26, 106189 (2023).
Feldman, E. L., Nave, K. A., Jensen, T. S. & Bennett, D. L. H. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93, 1296–1313 (2017).
Al-Ani, F. S., Al-Nimer, M. S. & Ali, F. S. Dyslipidemia as a contributory factor in etiopathogenesis of diabetic neuropathy. Indian J. Endocrinol. Metab. 15, 110–114 (2011).
Vincent, A. M., Hinder, L. M., Pop-Busui, R. & Feldman, E. L. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J. Peripher. Nerv. Syst. 14, 257–267 (2009).
Stino, A. M., Rumora, A. E., Kim, B. & Feldman, E. L. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J. Peripher. Nerv. Syst. 25, 76–84 (2020).
O’Brien, P. D. et al. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis. Model. Mech. https://doi.org/10.1242/dmm.042101 (2020).
Gustavsson, C. et al. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PLoS ONE 5, e12699 (2010).
Barreto, J., Karathanasis, S. K., Remaley, A. & Sposito, A. C. Role of LOX-1 (lectin-like oxidized low-density lipoprotein receptor 1) as a cardiovascular risk predictor: mechanistic insight and potential clinical use. Arterioscler. Thromb. Vasc. Biol. 41, 153–166 (2021).
Vincent, A. M. et al. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 58, 2376–2385 (2009).
Patwardhan, A. M. et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J. Clin. Invest. 120, 1617–1626 (2010).
Ding, L. et al. Reduced lipolysis response to adipose afferent reflex involved in impaired activation of adrenoceptor-cAMP-PKA-hormone sensitive lipase pathway in obesity. Sci. Rep. 6, 34374 (2016).
Osaka, T. et al. Temperature- and capsaicin-sensitive nerve fibers in brown adipose tissue attenuate thermogenesis in the rat. Pflugers Arch. 437, 36–42 (1998).
Blondin, D. P. et al. Human brown adipocyte thermogenesis is driven by beta2-AR stimulation. Cell Metab. 32, 287–300 (2020).
Benedek, G., Szikszay, M. & Obal, F. Impaired thermoregulation against cold in capsaicin pretreated rats. Pflugers Arch. 399, 243–245 (1983).
Cui, J. & Himms-Hagen, J. Rapid but transient atrophy of brown adipose tissue in capsaicin-desensitized rats. Am. J. Physiol. 262, R562–R567 (1992).
Podsednik, A., Cabrejo, R. & Rosen, J. Adipose tissue uses in peripheral nerve surgery. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23020644 (2022).
Uretsky, B. F. Sensory reinnervation of the heart after cardiac transplantation. N. Engl. J. Med. 326, 66–67 (1992).
Kakizaki, M. et al. Differential roles of each orexin receptor signaling in obesity. iScience 20, 1–13 (2019).
Makela, K. A. et al. Plasma orexin-A levels do not undergo circadian rhythm in young healthy male subjects. Front. Endocrinol. 9, 710 (2018).
Fischer, A. W., Schlein, C., Cannon, B., Heeren, J. & Nedergaard, J. Intact innervation is essential for diet-induced recruitment of brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 316, E487–E503 (2019).
Himms-Hagen, J., Cui, J. & Lynn Sigurdson, S. Sympathetic and sensory nerves in control of growth of brown adipose tissue: effects of denervation and of capsaicin. Neurochem. Int. 17, 271–279 (1990).
Cui, J. & Himms-Hagen, J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. Am. J. Physiol. 262, R568–R573 (1992).
Mancini, C. et al. Identification of biomarkers of brown adipose tissue aging highlights the role of dysfunctional energy and nucleotide metabolism pathways. Sci. Rep. 11, 19928 (2021).
Shi, H. & Bartness, T. J. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R514–R520 (2005).
Watts, A. G. & Grill, H. J. Tim Bartness (1953-2015). Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R385–R387 (2016).
Nguyen, N. L. et al. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R132–R145 (2017).
Ryu, V. & Bartness, T. J. Short and long sympathetic-sensory feedback loops in white fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R886–R900 (2014).
Aveseh, M., Koushkie-Jahromi, M., Nemati, J. & Esmaeili-Mahani, S. Serum calcitonin gene-related peptide facilitates adipose tissue lipolysis during exercise via PIPLC/IP3 pathways. Endocrine 61, 462–472 (2018).
Huesing, C. et al. Sympathetic innervation of inguinal white adipose tissue in the mouse. J. Comp. Neurol. 529, 1465–1485 (2021).
Russell, F. A., King, R., Smillie, S. J., Kodji, X. & Brain, S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142 (2014).
Willows, J. W. et al. Visualization and analysis of whole depot adipose tissue neural innervation. iScience 24, 103127 (2021).
Ibrahim, M. M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 11, 11–18 (2010).
Frei, I. C. et al. Adipose mTORC2 is essential for sensory innervation in white adipose tissue and whole-body energy homeostasis. Mol. Metab. 65, 101580 (2022).
Chang, H. H., Yang, S. S. & Chang, S. J. Perivascular adipose tissue modulation of neurogenic vasorelaxation of rat mesenteric arteries. J. Cardiovasc. Pharmacol. 75, 21–30 (2020).
Abu Bakar, H., Robert Dunn, W., Daly, C. & Ralevic, V. Sensory innervation of perivascular adipose tissue: a crucial role in artery vasodilatation and leptin release. Cardiovasc. Res. 113, 962–972 (2017).
Kawasaki, H., Takasaki, K., Saito, A. & Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 335, 164–167 (1988).
Saito, A. & Yamamoto, M. Acute oral toxicity of capsaicin in mice and rats. J. Toxicol. Sci. 21, 195–200 (1996).
Vaughan, C. H., Zarebidaki, E., Ehlen, J. C. & Bartness, T. J. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol. 537, 199–225 (2014).
Akagi, A. et al. Non-carcinogenicity of capsaicinoids in B6C3F1 mice. Food Chem. Toxicol. 36, 1065–1071 (1998).
Surh, Y. J. & Lee, S. S. Capsaicin, a double-edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci. 56, 1845–1855 (1995).
Fischer, M. J. M., Ciotu, C. I. & Szallasi, A. The mysteries of capsaicin-sensitive afferents. Front. Physiol. 11, 554195 (2020).
Iadarola, M. J. & Gonnella, G. L. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain. J. 6, 95–107 (2013).
Karai, L. et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J. Clin. Invest. 113, 1344–1352 (2004).
Acknowledgements
K.L.T. was supported by start-up funding from The Ohio State University College of Medicine, as well as NIH grant R01DH114320, a NIDDK Diabetic Complications Consortium (DIACOMP) award EEIR:SCR_001415 and a W.M. Keck Foundation award. We thank J. W. Willows for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
G.M. and K.L.T. contributed equally to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, G., Townsend, K.L. The metabolic and functional roles of sensory nerves in adipose tissues. Nat Metab 5, 1461–1474 (2023). https://doi.org/10.1038/s42255-023-00868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00868-x