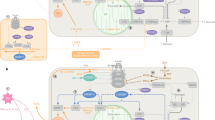
Abstract
Neuroimmunology and immunometabolism are burgeoning topics of study, but the intersection of these two fields is scarcely considered. This interplay is particularly prevalent within adipose tissue, where immune cells and the sympathetic nervous system (SNS) have an important role in metabolic homeostasis and pathology, namely in obesity. In the present Review, we first outline the established reciprocal adipose–SNS relationship comprising the neuroendocrine loop facilitated primarily by adipose tissue-derived leptin and SNS-derived noradrenaline. Next, we review the extensive crosstalk between adipocytes and resident innate immune cells as well as the changes that occur in these secretory and signalling pathways in obesity. Finally, we discuss the effect of SNS adrenergic signalling in immune cells and conclude with exciting new research demonstrating an immutable role for SNS-resident macrophages in modulating SNS–adipose crosstalk. We posit that the latter point constitutes the existence of a new field — neuroimmunometabolism.
Key points
-
Adipose tissue is innervated by sympathetic nerves that release noradrenaline, which drives thermogenesis in brown adipose tissue and lipolysis and/or beiging in white adipose tissue.
-
Adipocytes release leptin and other adipokines that signal through the hypothalamus to control whole-body metabolism, including modulation of sympathetic output to adipose tissue.
-
The polarization of adipose-resident macrophages is a prominent effector in obesity and insulin resistance. This polarization is sensitive to signalling from other immune cells, adipocytes and sympathetic nerves.
-
Adipokines, such as leptin and adiponectin, directly modulate the activation status of macrophages and other immune cells. Adipokine release changes drastically in obesity.
-
Noradrenaline released by sympathetic nerves directly modulates macrophages and other immune cells by signalling through various subtypes of adrenoreceptor.
-
Small populations of macrophages have been discovered by several groups in various adipose depots that closely associate with sympathetic nerves and play a novel role in modulating sympathetic innervation of adipose tissue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chu, D. T. et al. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab. Syndr. 12, 1095–1100 (2018).
Bachman, E. S. et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Saltiel, A. R. & Olefsky, J. M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127, 1–4 (2017).
Galton, D. J. & Bray, G. A. Studies on lipolysis in human adipose cells. J. Clin. Invest. 46, 621–629 (1967).
Goodner, C. J., Tustison, W. A., Davidson, M. B., Chu, P. C. & Conway, M. J. Studies of substrate regulation in fasting. I. Evidence for central regulation of lipolysis by plasma glucose mediated by the sympathetic nervous system. Diabetes 16, 576–589 (1967).
Bogdonoff, M. D., Weissler, A. M. & Merritt, F. L. The effect of autonomic ganglionic blockade upon serum free fatty acid levels in man. J. Clin. Invest. 39, 959–965 (1960).
Fredholm, B. & Rosell, S. Effects of adrenergic blocking agents on lipid mobilization from canine subcutaneous adipose tissue after sympathetic nerve stimulation. J. Pharmacol. Exp. Ther. 159, 1–7 (1968).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Bartness, T. J. & Song, C. K. Brain-adipose tissue neural crosstalk. Physiol. Behav. 91, 343–351 (2007).
Youngstrom, T. G. & Bartness, T. J. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am. J. Physiol. 268, R744–R751 (1995).
Bartness, T. J., Shrestha, Y. B., Vaughan, C. H., Schwartz, G. J. & Song, C. K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell Endocrinol. 318, 34–43 (2010).
Bowers, R. R. et al. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R1167–R1175 (2004).
Bamshad, M., Aoki, V. T., Adkison, M. G., Warren, W. S. & Bartness, T. J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 275, R291–R299 (1998).
Bamshad, M., Song, C. K. & Bartness, T. J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 276, R1569–R1578 (1999).
Pereira, M. M. et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat. Commun. 8, 14967 (2017).
Blaszkiewicz, M. W. et al. Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLOS ONE 14, e0221766 (2019).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27, 226–236.e3 (2018).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692.e3 (2017).
Francois, M. et al. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann. NY Acad. Sci. 1454, 3–13 (2019).
Morrison, S. F., Ramamurthy, S. & Young, J. B. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J. Neurosci. 20, 9264–9271 (2000).
Giordano, A. et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1243–R1255 (2006).
Uyama, N., Geerts, A. & Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. A. Discov. Mol. Cell Evol. Biol. 280, 808–820 (2004).
Brito, N. A., Brito, M. N. & Bartness, T. J. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1445–R1452 (2008).
Young, J. B., Saville, E., Rothwell, N. J., Stock, M. J. & Landsberg, L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J. Clin. Invest. 69, 1061–1071 (1982).
Murano, I., Barbatelli, G., Giordano, A. & Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 214, 171–178 (2009).
Guilherme, A., Henriques, F., Bedard, A. H. & Czech, M. P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 15, 207–225 (2019).
Bartness, T. J. & Song, C. K. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J. Lipid Res. 48, 1655–1672 (2007).
Bartness, T. J., Vaughan, C. H. & Song, C. K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34, S36–S42 (2010).
Foster, M. T. & Bartness, T. J. Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1630–R1637 (2006).
Garretson, J. T. et al. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol. Metab. 5, 626–634 (2016).
Murphy, K. T. et al. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 304, E1338–E1347 (2013).
Shi, H. & Bartness, T. J. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R514–R520 (2005).
Kreier, F. et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat–functional implications. J. Clin. Invest. 110, 1243–1250 (2002).
Garofalo, M. A., Kettelhut, I. C., Roselino, J. E. & Migliorini, R. H. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J. Auton. Nerv. Syst. 60, 206–208 (1996).
Cohen, P. et al. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108, 1113–1121 (2001).
Scott, M. M. et al. Leptin targets in the mouse brain. J. Comp. Neurol. 514, 518–532 (2009).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Ramseyer, V. D. & Granneman, J. G. Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 5, 119–129 (2016).
Coppari, R. & Bjorbaek, C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat. Rev. Drug Discov. 11, 692–708 (2012).
Caron, A., Lee, S., Elmquist, J. K. & Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 19, 153–165 (2018).
Elias, C. F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Scarpace, P. J. & Matheny, M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am. J. Physiol. 275, E259–E264 (1998).
Bell, B. B., Harlan, S. M., Morgan, D. A., Guo, D. F. & Rahmouni, K. Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol. Metab. 8, 1–12 (2018).
Zhang, Y. et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 31, 1873–1884 (2011).
Ruan, H. B. et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159, 306–317 (2014).
Youngstrom, T. G. & Bartness, T. J. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am. J. Physiol. 275, R1488–R1493 (1998).
Niijima, A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J. Auton. Nerv. Syst. 73, 19–25 (1998).
Niijima, A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci. Lett. 262, 125–128 (1999).
Penn, D. M., Jordan, L. C., Kelso, E. W., Davenport, J. E. & Harris, R. B. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1613–R1621 (2006).
Roelfsema, F., Boelen, A., Kalsbeek, A. & Fliers, E. Regulatory aspects of the human hypothalamus-pituitary-thyroid axis. Best Pract. Res. Clin. Endocrinol. Metab. 31, 487–503 (2017).
Lopez, M., Alvarez, C. V., Nogueiras, R. & Dieguez, C. Energy balance regulation by thyroid hormones at central level. Trends Mol. Med. 19, 418–427 (2013).
Alvarez-Crespo, M. et al. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol. Metab. 5, 271–282 (2016).
Martinez-Sanchez, N. et al. Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 26, 212–229.e12 (2017).
Martinez-Sanchez, N. et al. Thyroid hormones induce browning of white fat. J. Endocrinol. 232, 351–362 (2017).
Seoane-Collazo, P. et al. SF1-specific AMPKalpha1 deletion protects against diet-induced obesity. Diabetes 67, 2213–2226 (2018).
Lopez, M. Hypothalamic AMPK and energy balance. Eur. J. Clin. Invest. 48, e12996 (2018).
Silva, J. E. & Larsen, P. R. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305, 712–713 (1983).
Rubio, A., Raasmaja, A., Maia, A. L., Kim, K. R. & Silva, J. E. Effects of thyroid hormone on norepinephrine signaling in brown adipose tissue. I. Beta 1- and beta 2-adrenergic receptors and cyclic adenosine 3′,5′-monophosphate generation. Endocrinology 136, 3267–3276 (1995).
Weiner, J., Hankir, M., Heiker, J. T., Fenske, W. & Krause, K. Thyroid hormones and browning of adipose tissue. Mol. Cell Endocrinol. 458, 156–159 (2017).
Solmonson, A. & Mills, E. M. Uncoupling proteins and the molecular mechanisms of thyroid thermogenesis. Endocrinology 157, 455–462 (2016).
Mauvais-Jarvis, F., Clegg, D. J. & Hevener, A. L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 34, 309–338 (2013).
Lopez, M. & Tena-Sempere, M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol. Metab. 26, 411–421 (2015).
de Souza, F. S. et al. The estrogen receptor alpha colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur. J. Pharmacol. 660, 181–187 (2011).
Diano, S., Kalra, S. P., Sakamoto, H. & Horvath, T. L. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Res. 812, 256–259 (1998).
Hirosawa, M. et al. Ablation of estrogen receptor alpha (ERalpha) prevents upregulation of POMC by leptin and insulin. Biochem. Biophys. Res. Commun. 371, 320–323 (2008).
Xu, Y. et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 14, 453–465 (2011).
Martinez de Morentin, P. B. et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 20, 41–53 (2014).
Miao, Y. F. et al. An ERbeta agonist induces browning of subcutaneous abdominal fat pad in obese female mice. Sci. Rep. 6, 38579 (2016).
Rodriguez-Cuenca, S. et al. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol. Biochem. 20, 877–886 (2007).
Palin, S. L. et al. 17Beta-estradiol and anti-estrogen ICI:compound 182,780 regulate expression of lipoprotein lipase and hormone-sensitive lipase in isolated subcutaneous abdominal adipocytes. Metabolism 52, 383–388 (2003).
Pedersen, S. B., Kristensen, K., Hermann, P. A., Katzenellenbogen, J. A. & Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 89, 1869–1878 (2004).
Monjo, M., Rodriguez, A. M., Palou, A. & Roca, P. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology 144, 4923–4930 (2003).
Rodriguez, A. M., Monjo, M., Roca, P. & Palou, A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol. Life Sci. 59, 1714–1723 (2002).
Lapid, K., Lim, A., Clegg, D. J., Zeve, D. & Graff, J. M. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat. Commun. 5, 5196 (2014).
Goke, R., Fehmann, H. C. & Goke, B. Glucagon-like peptide-1(7-36) amide is a new incretin/enterogastrone candidate. Eur. J. Clin. Invest. 21, 135–144 (1991).
Beiroa, D. et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358 (2014).
Nogueiras, R. et al. Direct control of peripheral lipid deposition by CNS GLP-1 receptor sigaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J. Neurosci. 29, 5916–5925 (2009).
Chen, J. et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell Physiol. Biochem. 42, 1165–1176 (2017).
Challa, T. D. et al. Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J. Biol. Chem. 287, 6421–6430 (2012).
Krieger, J. P. et al. Glucagon-like peptide-1 regulates brown adipose tissue thermogenesis via the gut-brain axis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R708–R720 (2018).
Lopez, M., Tena-Sempere, M. & Dieguez, C. Cross-talk between orexins (hypocretins) and the neuroendocrine axes (hypothalamic-pituitary axes). Front. Neuroendocrinol. 31, 113–127 (2010).
Backberg, M., Hervieu, G., Wilson, S. & Meister, B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur. J. Neurosci. 15, 315–328 (2002).
Berthoud, H. R., Patterson, L. M., Sutton, G. M., Morrison, C. & Zheng, H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem. Cell Biol. 123, 147–156 (2005).
Sellayah, D., Bharaj, P. & Sikder, D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 14, 478–490 (2011).
Pino, M. F., Divoux, A., Simmonds, A. V., Smith, S. R. & Sparks, L. M. Investigating the effects of Orexin-A on thermogenesis in human deep neck brown adipose tissue. Int. J. Obes. 41, 1646–1653 (2017).
Digby, J. E. et al. Orexin receptor expression in human adipose tissue: effects of orexin-A and orexin-B. J. Endocrinol. 191, 129–136 (2006).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
McLaughlin, T., Ackerman, S. E., Shen, L. & Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Invest. 127, 5–13 (2017).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Lumeng, C. N., DelProposto, J. B., Westcott, D. J. & Saltiel, A. R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246 (2008).
Stein, M., Keshav, S., Harris, N. & Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 (1992).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Sartipy, P. & Loskutoff, D. J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 100, 7265–7270 (2003).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Sartipy, P. & Loskutoff, D. J. Expression profiling identifies genes that continue to respond to insulin in adipocytes made insulin-resistant by treatment with tumor necrosis factor-alpha. J. Biol. Chem. 278, 52298–52306 (2003).
Weisberg, S. P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Amano, S. U. et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19, 162–171 (2014).
Inouye, K. E. et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56, 2242–2250 (2007).
Kirk, E. A., Sagawa, Z. K., McDonald, T. O., O’Brien, K. D. & Heinecke, J. W. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes 57, 1254–1261 (2008).
Bai, Y. & Sun, Q. Macrophage recruitment in obese adipose tissue. Obes. Rev. 16, 127–136 (2015).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Erridge, C. & Samani, N. J. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 29, 1944–1949 (2009).
Huang, S. et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 53, 2002–2013 (2012).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Rogero, M. M. & Calder, P. C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients 10, E432 (2018).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Kosteli, A. et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 (2010).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Morigny, P., Houssier, M., Mouisel, E. & Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 125, 259–266 (2016).
Sun, K., Tordjman, J., Clement, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013).
Fujisaka, S. et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia 56, 1403–1412 (2013).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Yin, J. et al. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 296, E333–E342 (2009).
Engin, A. Adipose tissue hypoxia in obesity and its impact on preadipocytes and macrophages: hypoxia hypothesis. Adv. Exp. Med. Biol. 960, 305–326 (2017).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Miles, P. D. et al. TNF-alpha-induced insulin resistance in vivo and its prevention by troglitazone. Diabetes 46, 1678–1683 (1997).
Tang, X. et al. An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARgamma, adipogenesis, and insulin-responsive hexose transport. Proc. Natl Acad. Sci. USA 103, 2087–2092 (2006).
Ruan, H., Hacohen, N., Golub, T. R., Van Parijs, L. & Lodish, H. F. Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 51, 1319–1336 (2002).
Peraldi, P., Hotamisligil, G. S., Buurman, W. A., White, M. F. & Spiegelman, B. M. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 271, 13018–13022 (1996).
Ruan, H. et al. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 51, 3176–3188 (2002).
Dahlman, I. et al. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes 55, 1792–1799 (2006).
Cawthorn, W. P. & Sethi, J. K. TNF-alpha and adipocyte biology. FEBS Lett. 582, 117–131 (2008).
Uysal, K. T., Wiesbrock, S. M., Marino, M. W. & Hotamisligil, G. S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389, 610–614 (1997).
Nguyen, K. D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014).
Fischer, K. et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 23, 623–630 (2017).
Reitman, M. L. How does fat transition from white to beige? Cell Metab. 26, 14–16 (2017).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Bolus, W. R. et al. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol. Metab. 8, 86–95 (2018).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Hams, E., Locksley, R. M., McKenzie, A. N. & Fallon, P. G. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J. Immunol. 191, 5349–5353 (2013).
Nguyen, M. T. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 282, 35279–35292 (2007).
Lee, B. C. et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab. 23, 685–698 (2016).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012).
Zhu, Q. & Scherer, P. E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat. Rev. Nephrol. 14, 105–120 (2018).
Tsiotra, P. C., Pappa, V., Raptis, S. A. & Tsigos, C. Expression of the long and short leptin receptor isoforms in peripheral blood mononuclear cells: implications for leptin’s actions. Metabolism 49, 1537–1541 (2000).
Mattioli, B., Straface, E., Quaranta, M. G., Giordani, L. & Viora, M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 174, 6820–6828 (2005).
Zhao, Y., Sun, R., You, L., Gao, C. & Tian, Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem. Biophys. Res. Commun. 300, 247–252 (2003).
Caldefie-Chezet, F., Poulin, A., Tridon, A., Sion, B. & Vasson, M. P. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 69, 414–418 (2001).
Kato, H. et al. Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int. Arch. Allergy Immunol. 155, 335–344 (2011).
Suzukawa, M. et al. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 186, 5254–5260 (2011).
Dib, L. H., Ortega, M. T., Fleming, S. D., Chapes, S. K. & Melgarejo, T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology 155, 40–46 (2014).
Gutierrez, D. A. & Hasty, A. H. Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity. J. Endocrinol. 212, 343–351 (2012).
Gove, M. E., Sherry, C. L., Pini, M. & Fantuzzi, G. Generation of leptin receptor bone marrow chimeras: recovery from irradiation, immune cellularity, cytokine expression, and metabolic parameters. Obesity 18, 2274–2281 (2010).
Acedo, S. C., Gambero, S., Cunha, F. G., Lorand-Metze, I. & Gambero, A. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In Vitro Cell Dev. Biol. Anim. 49, 473–478 (2013).
Wong, C. K., Cheung, P. F. & Lam, C. W. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur. J. Immunol. 37, 2337–2348 (2007).
Santos-Alvarez, J., Goberna, R. & Sanchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 194, 6–11 (1999).
Yokota, T. et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96, 1723–1732 (2000).
Wulster-Radcliffe, M. C., Ajuwon, K. M., Wang, J., Christian, J. A. & Spurlock, M. E. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun. 316, 924–929 (2004).
Wolf, A. M., Wolf, D., Rumpold, H., Enrich, B. & Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 323, 630–635 (2004).
Ohashi, K. et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285, 6153–6160 (2010).
Hui, X. et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 22, 279–290 (2015).
Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 7, 947–953 (2001).
Tsatsanis, C. et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem. Biophys. Res. Commun. 335, 1254–1263 (2005).
Cheng, X., Folco, E. J., Shimizu, K. & Libby, P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J. Biol. Chem. 287, 36896–36904 (2012).
Folco, E. J., Rocha, V. Z., Lopez-Ilasaca, M. & Libby, P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J. Biol. Chem. 284, 25569–25575 (2009).
Park, P. H., McMullen, M. R., Huang, H., Thakur, V. & Nagy, L. E. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J. Biol. Chem. 282, 21695–21703 (2007).
Fruebis, J. et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl Acad. Sci. USA 98, 2005–2010 (2001).
Pajvani, U. B. et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin: implications for metabolic regulation and bioactivity. J. Biol. Chem. 278, 9073–9085 (2003).
Kang, K. et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7, 485–495 (2008).
Ouchi, N. et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454–457 (2010).
Han, C. Y. et al. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes 56, 2260–2273 (2007).
Li, P. et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 21, 239–247 (2015).
Spite, M. et al. Deficiency of the leukotriene B4 receptor, BLT-1, protects against systemic insulin resistance in diet-induced obesity. J. Immunol. 187, 1942–1949 (2011).
Cereijo, R. et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab. 28, 750–763.e6 (2018).
Scanzano, A. & Cosentino, M. Adrenergic regulation of innate immunity: a review. Front. Pharmacol. 6, 171 (2015).
Grailer, J. J., Haggadone, M. D., Sarma, J. V., Zetoune, F. S. & Ward, P. A. Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J. Innate. Immun. 6, 607–618 (2014).
Noh, H. et al. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 92, 101–113 (2017).
Tan, K. S. et al. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 19, 251–260 (2007).
Grisanti, L. A. et al. beta2-Adrenergic receptor-dependent chemokine receptor 2 expression regulates leukocyte recruitment to the heart following acute injury. Proc. Natl. Acad Sci. USA 113, 15126–15131 (2016).
Shen, H. M., Sha, L. X., Kennedy, J. L. & Ou, D. W. Adrenergic receptors regulate macrophage secretion. Int. J. Immunopharmacol. 16, 905–910 (1994).
Piazza, O. et al. Effect of alpha2-adrenergic agonists and antagonists on cytokine release from human lung macrophages cultured in vitro. Transl Med. UniSa. 15, 67–73 (2016).
Silvestri, M., Oddera, S., Lantero, S. & Rossi, G. A. β2-agonist-induced inhibition of neutrophil chemotaxis is not associated with modification of LFA-1 and Mac-1 expression or with impairment of polymorphonuclear leukocyte antibacterial activity. Respir. Med. 93, 416–423 (1999).
Butchers, P. R., Vardey, C. J. & Johnson, M. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br. J. Pharmacol. 104, 672–676 (1991).
Noguchi, T. et al. Effect of beta2-adrenergic agonists on eosinophil adhesion, superoxide anion generation, and degranulation. Allergol. Int. 64, S46–S53 (2015).
Moriyama, S. et al. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359, 1056–1061 (2018).
Takamoto, T. et al. Norepinephrine inhibits human natural killer cell activity in vitro. Int. J. Neurosci. 58, 127–131 (1991).
Wu, H. et al. beta2-adrenoceptor signaling reduction in dendritic cells is involved in the inflammatory response in adjuvant-induced arthritic rats. Sci. Rep. 6, 24548 (2016).
Prinz, M. & Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 20, 136–144 (2017).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Wolf, Y. et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18, 665–674 (2017).
Young, P., Arch, J. R. & Ashwell, M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14 (1984).
Cousin, B. et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci. 103, 931–942 (1992).
Fukui, Y., Masui, S., Osada, S., Umesono, K. & Motojima, K. A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes 49, 759–767 (2000).
Sanchez-Gurmaches, J., Hung, C. M. & Guertin, D. A. Emerging complexities in adipocyte origins and identity. Trends Cell Biol. 26, 313–326 (2016).
Shabalina, I. G. et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 5, 1196–1203 (2013).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Barquissau, V. et al. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol. Metab. 5, 352–365 (2016).
Hanssen, M. J. et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 65, 1179–1189 (2016).
Cypess, A. M. et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
Patsouris, D. et al. Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep. 13, 1538–1544 (2015).
Sidossis, L. S. et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 22, 219–227 (2015).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Hakansson, M. L., Brown, H., Ghilardi, N., Skoda, R. C. & Meister, B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 18, 559–572 (1998).
Schwartz, M. W. et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46, 2119–2123 (1997).
Stephens, T. W. et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377, 530–532 (1995).
Korner, J., Savontaus, E., Chua, S. C. Jr., Leibel, R. L. & Wardlaw, S. L. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J. Neuroendocrinol. 13, 959–966 (2001).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
van den Top, M., Lee, K., Whyment, A. D., Blanks, A. M. & Spanswick, D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat. Neurosci. 7, 493–494 (2004).
Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Lu, D. et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371, 799–802 (1994).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Seeley, R. J. et al. Melanocortin receptors in leptin effects. Nature 390, 349 (1997).
Siegel-Axel, D. I. & Haring, H. U. Perivascular adipose tissue: an unique fat compartment relevant for the cardiometabolic syndrome. Rev. Endocr. Metab. Disord. 17, 51–60 (2016).
Henrichot, E. et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 25, 2594–2599 (2005).
Withers, S. B. et al. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler. Thromb. Vasc. Biol. 31, 908–913 (2011).
Yamashita, A. et al. Medial and adventitial macrophages are associated with expansive atherosclerotic remodeling in rabbit femoral artery. Histol. Histopathol. 23, 127–136 (2008).
Almabrouk, T. A. M. et al. High fat diet attenuates the anticontractile activity of aortic PVAT via a mechanism involving AMPK and reduced adiponectin secretion. Front. Physiol. 9, 51 (2018).
Greenstein, A. S. et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119, 1661–1670 (2009).
Chatterjee, T. K. et al. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ. Res. 104, 541–549 (2009).
Candela, J., Wang, R. & White, C. Microvascular endothelial dysfunction in obesity is driven by macrophage-dependent hydrogen sulfide depletion. Arterioscler. Thromb. Vasc. Biol. 37, 889–899 (2017).
Aghamohammadzadeh, R. et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J. Am. Coll. Cardiol. 62, 128–135 (2013).
Noblet, J. N., Owen, M. K., Goodwill, A. G., Sassoon, D. J. & Tune, J. D. Lean and obese coronary perivascular adipose tissue impairs vasodilation via differential inhibition of vascular smooth muscle K+ channels. Arterioscler. Thromb. Vasc. Biol. 35, 1393–1400 (2015).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Madden, K. S. Sympathetic neural-immune interactions regulate hematopoiesis, thermoregulation and inflammation in mammals. Dev. Comp. Immunol. 66, 92–97 (2017).
Lopez, M., Nogueiras, R., Tena-Sempere, M. & Dieguez, C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 12, 421–432 (2016).
Contreras, C., Nogueiras, R., Dieguez, C., Rahmouni, K. & Lopez, M. Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox. Biol. 12, 854–863 (2017).
Jiang, Y., Berry, D. C. & Graff, J. M. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 (2017).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed to discussion of the content, C.M.L. wrote the article and C.M.L. and A.I.D. reviewed and/or edited the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Arborizations
-
Tree-like, branching arrangement of neuronal processes.
Rights and permissions
About this article
Cite this article
Larabee, C.M., Neely, O.C. & Domingos, A.I. Obesity: a neuroimmunometabolic perspective. Nat Rev Endocrinol 16, 30–43 (2020). https://doi.org/10.1038/s41574-019-0283-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-019-0283-6
This article is cited by
-
Secretin-dependent signals in the ventromedial hypothalamus regulate energy metabolism and bone homeostasis in mice
Nature Communications (2024)
-
The sympathetic nervous system in heart failure revisited
Heart Failure Reviews (2023)
-
Dietary regulation in health and disease
Signal Transduction and Targeted Therapy (2022)
-
Roles and mechanisms of exosomal non-coding RNAs in human health and diseases
Signal Transduction and Targeted Therapy (2021)
-
Liver disease in obesity and underweight: the two sides of the coin. A narrative review
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2021)