Abstract
Adaptive thermogenesis by brown adipose tissue (BAT) dissipates calories as heat, making it an attractive anti-obesity target. Yet how BAT contributes to circulating metabolite exchange remains unclear. Here, we quantified metabolite exchange in BAT and skeletal muscle by arteriovenous metabolomics during cold exposure in fed male mice. This identified unexpected metabolites consumed, released and shared between organs. Quantitative analysis of tissue fluxes showed that glucose and lactate provide ~85% of carbon for adaptive thermogenesis and that cold and CL316,243 trigger markedly divergent fuel utilization profiles. In cold adaptation, BAT also dramatically increases nitrogen uptake by net consuming amino acids, except glutamine. Isotope tracing and functional studies suggest glutamine catabolism concurrent with synthesis via glutamine synthetase, which avoids ammonia buildup and boosts fuel oxidation. These data underscore the ability of BAT to function as a glucose and amino acid sink and provide a quantitative and comprehensive landscape of BAT fuel utilization to guide translational studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the extended tables. R scripts to conduct bootstrapping to calculate the confidence interval of the difference between tissue and serum area under the curve are available on GitHub at https://github.com/johnnl15/Bootstrapping_AUC_NitrogenFL_BATLiverSerum.Source data are provided with this paper.
References
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Rothwell, N. J. & Stock, M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281, 31–35 (1979).
Feldmann, H. M., Golozoubova, V., Cannon, B. & Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 (2009).
von Essen, G., Lindsund, E., Cannon, B. & Nedergaard, J. Adaptive facultative diet-induced thermogenesis in wild-type but not in UCP1-ablated mice. Am. J. Physiol. Endocrinol. Metab. 313, E515–E527 (2017).
Hung, C. M. et al. Rictor/mTORC2 loss in the Myf5 lineage reprograms brown fat metabolism and protects mice against obesity and metabolic disease. Cell Rep. 8, 256–271 (2014).
Jung, S. M. et al. Non-canonical mTORC2 signaling regulates brown adipocyte lipid catabolism through SIRT6-FoxO1. Mol. Cell 75, 807–822 e808 (2019).
Nedergaard, J. & Cannon, B. Diet-induced thermogenesis: principles and pitfalls. Methods Mol. Biol. 2448, 177–202 (2022).
Betz, M. J. & Enerback, S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat. Rev. Endocrinol. 14, 77–87 (2018).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Nedergaard, J., Bengtsson, T. & Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 (2007).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Chen, K. Y. et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J. Biol. Chem. 295, 1926–1942 (2020).
Wolfrum, C. & Gerhart-Hines, Z. Fueling the fire of adipose thermogenesis. Science 375, 1229–1231 (2022).
Seki, T. et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 608, 421–428 (2022).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688 (2020).
Villarroya, F., Cereijo, R., Villarroya, J. & Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 13, 26–35 (2017).
Cereijo, R. et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab. 28, 750–763 (2018).
Wang, Z. et al. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 21, e50085 (2020).
Trayhurn, P. Fatty acid synthesis in vivo in brown adipose tissue, liver and white adipose tissue of the cold-acclimated rat. FEBS Lett. 104, 13–16 (1979).
Foster, D. O., Frydman, M. L. & Usher, J. R. Nonshivering thermogenesis in the rat. I. The relation between drug-induced changes in thermogenesis and changes in the concentration of plasma cyclic AMP. Can. J. Physiol. Pharmacol. 55, 52–64 (1977).
Foster, D. O. & Frydman, M. L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can. J. Physiol. Pharmacol. 56, 110–122 (1978).
Foster, D. O. & Frydman, M. L. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 57, 257–270 (1979).
Lopez-Soriano, F. J. & Alemany, M. Effect of cold-temperature exposure and acclimation on amino acid pool changes and enzyme activities of rat brown adipose tissue. Biochim. Biophys. Acta 925, 265–271 (1987).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606 (2019).
Berg, F., Gustafson, U. & Andersson, L. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet. 2, e129 (2006).
Hou, L. et al. Pig has no uncoupling protein 1. Biochem. Biophys. Res. Commun. 487, 795–800 (2017).
Dou, H. et al. Aryl hydrocarbon receptor (AhR) regulates adipocyte differentiation by assembling CRL4B ubiquitin ligase to target PPARγ for proteasomal degradation. J. Biol. Chem. 294, 18504–18515 (2019).
Gnad, T. et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014).
Lahesmaa, M. et al. Regulation of human brown adipose tissue by adenosine and A2A receptors - studies with [(15)O]H2O and [(11)C]TMSX PET/CT. Eur. J. Nucl. Med. Mol. Imaging 46, 743–750 (2019).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Yoneshiro, T. et al. Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. eLife https://doi.org/10.7554/eLife.66865 (2021).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Keinan, O. et al. Glycogen metabolism links glucose homeostasis to thermogenesis in adipocytes. Nature 599, 296–301 (2021).
Jung, S. M. et al. In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep. 36, 109459 (2021).
Jabbour, H. N. & Sales, K. J. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol. Metab. 15, 398–404 (2004).
Cekic, C. & Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 16, 177–192 (2016).
Di Virgilio, F., Sarti, A. C., Falzoni, S., De Marchi, E. & Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 18, 601–618 (2018).
Boldyrev, A. A., Aldini, G. & Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 93, 1803–1845 (2013).
Schaalan, M. F., Ramadan, B. K. & Abd Elwahab, A. H. Synergistic effect of carnosine on browning of adipose tissue in exercised obese rats; a focus on circulating irisin levels. J. Cell. Physiol. 233, 5044–5057 (2018).
Anderson, E. J. et al. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Invest. 128, 5280–5293 (2018).
Wolfe, R. R. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality? J. Int Soc. Sports Nutr. 14, 30 (2017).
Laha, A., Singh, M., George, A. K., Homme, R. P. & Tyagi, S. C. Dysregulation of 1-carbon metabolism and muscle atrophy: potential roles of forkhead box O proteins and PPARγ co-activator-1α. Can. J. Physiol. Pharmacol. 97, 1013–1017 (2019).
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 (2014).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Heine, M. et al. Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated brown adipose tissue in mice. Cell Metab. 28, 644–655 (2018).
Fischer, A. W. et al. Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation. Cell Metab. 33, 547–564 (2021).
Fischer, A. W. et al. Brown adipose tissue lipoprotein and glucose disposal is not determined by thermogenesis in uncoupling protein 1-deficient mice. J. Lipid Res. 61, 1377–1389 (2020).
Berbee, J. F. et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 6, 6356 (2015).
Wade, G., McGahee, A., Ntambi, J. M. & Simcox, J. Lipid transport in brown adipocyte thermogenesis. Front. Physiol. 12, 787535 (2021).
Simcox, J. et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 26, 509–522 (2017).
M, U. D. et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab. 28, 207–216 (2018).
Adlanmerini, M. et al. Circadian lipid synthesis in brown fat maintains murine body temperature during chronic cold. Proc. Natl Acad. Sci. USA 116, 18691–18699 (2019).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Mottillo, E. P. et al. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res. 55, 2276–2286 (2014).
Sanchez-Gurmaches, J. et al. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab. 27, 195–209 (2018).
Veliova, M. et al. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep. 21, e49634 (2020).
Mills, E. L. et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018).
Bisbach, C. M. et al. Succinate can shuttle reducing power from the hypoxic retina to the O(2)-rich pigment epithelium. Cell Rep. 31, 107606 (2020).
Heim, T. & Hull, D. The blood flow and oxygen consumption of brown adipose tissue in the new-born rabbit. J. Physiol. 186, 42–55 (1966).
Foster, D. O. & Frydman, M. L. Brown adipose tissue: the dominant site of nonshivering thermogenesis in the rat. Exp. Suppl. 32, 147–151 (1978).
Foster, D. O., Depocas, F. & Frydman, M. L. Noradrenaline-induced calorigenesis in warm- and cold-acclimated rats: relations between concentration of noradrenaline in arterial plasma, blood flow to differently located masses of brown adipose tissue, and calorigenic response. Can. J. Physiol. Pharmacol. 58, 915–924 (1980).
Bean, C. et al. The mitochondrial protein Opa1 promotes adipocyte browning that is dependent on urea cycle metabolites. Nat. Metab. 3, 1633–1647 (2021).
Ramirez, A. K. et al. Integrating extracellular flux measurements and genome-scale modeling reveals differences between brown and white adipocytes. Cell Rep. 21, 3040–3048 (2017).
Wang, C. H. et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaz8664 (2020).
Labbe, S. M. et al. mTORC1 is required for brown adipose tissue recruitment and metabolic adaptation to cold. Sci. Rep. 6, 37223 (2016).
Villarroya, J. et al. New insights into the secretory functions of brown adipose tissue. J. Endocrinol. 243, R19–R27 (2019).
Scheele, C. & Wolfrum, C. Brown adipose crosstalk in tissue plasticity and human metabolism. Endocr. Rev. https://doi.org/10.1210/endrev/bnz007 (2020).
Niemann, B. et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature https://doi.org/10.1038/s41586-022-05041-0 (2022).
Gnad, T. et al. Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metab. 34, 649 (2022).
Yoo, H., Antoniewicz, M. R., Stephanopoulos, G. & Kelleher, J. K. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J. Biol. Chem. 283, 20621–20627 (2008).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2011).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2011).
McCormack, J. G. & Denton, R. M. Evidence that fatty acid synthesis in the interscapular brown adipose tissue of cold-adapted rats is increased in vivo by insulin by mechanisms involving parallel activation of pyruvate dehydrogenase and acetyl-coenzyme A carboxylase. Biochem. J. 166, 627–630 (1977).
Shimazu, T. & Takahashi, A. Stimulation of hypothalamic nuclei has differential effects on lipid synthesis in brown and white adipose tissue. Nature 284, 62–63 (1980).
Yu, X. X., Lewin, D. A., Forrest, W. & Adams, S. H. Cold elicits the simultaneous induction of fatty acid synthesis and β-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J. 16, 155–168 (2002).
Weir, G. et al. Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab. 27, 1348–1355 (2018).
Imai, K., Tsujisaki, M. & Yachi, A. [Application of monoclonal antibodies to cancer therapy: idiotype mapping of monoclonal antibodies to tumor-associated antigens]. Gan To Kagaku Ryoho 315, 1051–1059 (1988).
Spinelli, J. B. et al. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358, 941–946 (2017).
Isler, D., Hill, H. P. & Meier, M. K. Glucose metabolism in isolated brown adipocytes under β-adrenergic stimulation. Quantitative contribution of glucose to total thermogenesis. Biochem. J. 245, 789–793 (1987).
Ma, S. W. & Foster, D. O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can. J. Physiol. Pharmacol. 64, 609–614 (1986).
Saggerson, E. D., McAllister, T. W. & Baht, H. S. Lipogenesis in rat brown adipocytes. Effects of insulin and noradrenaline, contributions from glucose and lactate as precursors and comparisons with white adipocytes. Biochem. J. 251, 701–709 (1988).
Lopez-Soriano, F. J. & Alemany, M. Activities of enzymes of amino acid metabolism in rat brown adipose tissue. Biochem. Int. 12, 471–478 (1986).
Lopez-Soriano, F. J. et al. Amino acid and glucose uptake by rat brown adipose tissue. Effect of cold-exposure and acclimation. Biochem. J. 252, 843–849 (1988).
Kaikaew, K., Grefhorst, A. & Visser, J. A. Sex differences in brown adipose tissue function: sex hormones, glucocorticoids, and their crosstalk. Front. Endocrinol. 12, 652444 (2021).
Keuper, M. & Jastroch, M. The good and the BAT of metabolic sex differences in thermogenic human adipose tissue. Mol. Cell. Endocrinol. 533, 111337 (2021).
Ouellet, V. et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 (2012).
Carneheim, C., Nedergaard, J. & Cannon, B. β-adrenergic stimulation of lipoprotein lipase in rat brown adipose tissue during acclimation to cold. Am. J. Physiol. 246, E327–E333 (1984).
Schreiber, R. et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab. 26, 753–763 (2017).
Wollenberger, A., Ristau, O. & Schoffa, G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflug. Arch. Gesamt. Physiol. Menschen Tiere 270, 399–412 (1960).
Heinrich, P. et al. Correcting for natural isotope abundance and tracer impurity in MS-, MS/MS- and high-resolution-multiple-tracer-data from stable isotope labeling experiments with IsoCorrectoR. Sci. Rep. 8, 17910 (2018).
Respress, J. L. & Wehrens, X. H. Transthoracic echocardiography in mice. J. Vis. Exp. https://doi.org/10.3791/1738 (2010).
Scherrer-Crosbie, M. & Thibault, H. B. Echocardiography in translational research: of mice and men. J. Am. Soc. Echocardiogr. 21, 1083–1092 (2008).
Fasshauer, M. et al. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol. Cell. Biol. 21, 319–329 (2001).
Isidor, M. S. et al. An siRNA-based method for efficient silencing of gene expression in mature brown adipocytes. Adipocyte 5, 175–185 (2016).
Spinelli, J. B., Kelley, L. P. & Haigis, M. C. An LC–MS approach to quantitative measurement of ammonia isotopologues. Sci. Rep. 7, 10304 (2017).
Acknowledgements
We thank all members of the Jang and Guertin laboratories for the discussion. We thank J. Park for help with bootstrapping and statistical methods and F. Roberts and T. Cashman for their technical assistance with blood flow measurements. This work was funded by R01DK116005, R01DK127175 and R01DK094004 to D.A.G.; the AASLD Foundation Pinnacle Research Award in Liver Disease, The Edward Mallinckrodt, Jr. Foundation Award, R01DK127175 and R01 AA029124 to C.J.; F31DK129018 to J.A.H.; T32GM008620 and F31DK134173 to J.L.; Basic Science Research Program of the Ministry of Education (South Korea) NRF-2019R1A6A1A10073079 to S.M.J.; and R01HL118100 and R01HL141377 to C.M.T.
Author information
Authors and Affiliations
Contributions
D.A.G. and C.J. conceived the project and supervised the study. G.P. performed sample processing and LC–MS analysis for the AV experiments. J.A.H. performed most of the animal experiments, sample preparation and the experiments and analysis related to GS activity, including ammonia-tracing studies. J.L. performed sample processing and LC–MS analysis for glucose and glutamine-tracing experiments. S.M.J. helped develop the AV collection strategy and assisted in early animal experiments and performed animal experiments for glutamine tracing. T.P.F. performed Doppler imaging and analysis. E.D.K. assisted with animal dissections for AV experiments and ammonia tracing and provided protein samples for the tissue panel and adipogenesis panel. H.L. assisted with the glutamine- and ammonia-tracing experiments and animal colony management. S.M.F. and Q.C. assisted with AV experiments and ammonia tracing and animal dissections for blood flow, respectively. J.B.S. assisted with ammonia-tracing experiments. C.M.T. assisted with blood flow experiments. G.P., J.A.H., J.L., S.M.J., C.J. and D.A.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Brandon Faubert and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Establishment of AV metabolomics for BAT and hind limb in mice.
a, Schematic of blood vessels used for AV sampling. The Sulzer’s vein (SV) and femoral vein (FV) were used to characterize BAT and hind limb activity. Systemic arterial blood was collected from the left ventricle (LV). Made with BioRender.com. b, Different biological scenarios reflected by AV gradients across BAT. Positive and negative values indicate net release and absorption, while net zero values indicate metabolite bypass (neither uptake nor release). intracellular futile cycling (release equal to uptake) or intercellular cross-exchange between adipocytes and non-adipocytes. c, Heat map shows different metabolite abundances between LV, SV and FV blood collected from mice adapted to mild (22 °C) or severe cold (6 °C). Box 1 highlights metabolites more abundant in BAT-draining blood (SV) than blood from other sites, regardless of temperature, boxes 2-3 highlight metabolites more abundant in BAT-draining blood (SV) or systemic arterial blood (LV) and temperature-sensitive and box 4 highlights metabolites sensitive to temperature across organs. Each column shows an individual mouse.
Extended Data Fig. 2 Characterization of BAT in TN, CC, AC and CL.
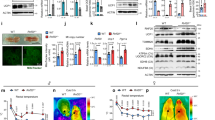
a, Daily food intake of TN and CC-adapted mice. Data are mean ± s.e. ****p = 3×10−12 by unpaired two-tailed Student’s t-test. b, Final body weight of TN, CC, AC, CL-treated mice. Data are mean ± s.e. **p = 0.001 and p = 0.009 by one-way ANOVA with Tukey’s multiple comparisons test. c, Western blot of key markers in BAT from mice in TN, CC, AC and CL. S.E., short exposure; L.E., long exposure. d, H&E images of BAT from mice in TN, CC, AC and CL. Scale bar = 50 μm.
Extended Data Fig. 4 Quantitative analysis of BAT total carbon and nitrogen influx and efflux in TN and CL.
Colors indicate different metabolite categories. Metabolites are ordered based on their relative contributions from greatest to least. Fatty acids from lipoprotein particles are indicated as ‘LIPID’ after each fatty acid species (for example, C16:0 LIPID).
Extended Data Fig. 5 Temperature-dependent glutamine carbon usage by BAT and liver.
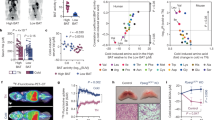
a, Glutamine fractional labeling (both carbon and nitrogen) at 5 min after tracer administration in BAT for TN, MC and CC. Data are mean ± s.e. N = 6 mice per temperature condition. b, Heat map shows median of the total 13C-labeled carbons in metabolites in BAT, liver and serum for TN, MC and CC, scaled for each metabolite and organ. N = 6 mice for TN, N = 6 mice for MC, N = 7 mice for CC at 2.5 min, N = 6 mice for all temperature conditions at 5 min, N = 6 mice for TN, N = 6 mice for MC, N = 5 mice for CC at 15 min for BAT and N = 6 mice for all temperature conditions at 15 min for liver and serum. c, Total normalized labeling fraction of carbon atoms in representative TCA intermediates in BAT. Data are mean ± s.e. ****p < 0.0001 by two-way ANOVA with post-hoc Tukey HSD Test. Malate MC vs TN **p = 0.0021, Succinate CC vs TN ***p = 0.0002 and MC vs TN **p = 0.0027. N = 6 mice for TN, N = 6 mice for MC, N = 7 mice for CC at 2.5 min, N = 6 mice for all temperature conditions at 5 min, N = 6 mice for TN, N = 6 mice for MC, N = 5 mice for CC at 15 min. d, Normalized carbon labeling fraction of representative TCA intermediates at 5 min after tracer administration. Data are mean ± s.e. N = 6 mice per temperature condition. e, Schematic of TCA cycle labeling from glutamine. Conventional TCA cycle predicts M + 4 labeling of succinate, malate and citrate from glutamine tracer, whereas reversed TCA cycle (that is, reductive carboxylation) predicts M + 5 labeling of citrate. PC flux can also generate M + 1 citrate with labeled CO2 incorporation. Citrate can be used for de novo lipogenesis. Made with BioRender.com.
Extended Data Fig. 6 Temperature-dependent glutamine nitrogen usage by BAT.
a, Heat map shows median of total 15N-labeled nitrogen in BAT metabolites for TN, MC and CC. N = 6 mice for TN, N = 6 mice for MC, N = 7 mice for CC at 2.5 min, N = 6 mice for all temperature conditions at 5 min, N = 6 mice for TN, N = 6 mice for MC, N = 5 mice for CC at 15 min. b, Schematic of nitrogen exchange reactions between glutamine, glutamate and keto acids. Made with BioRender.com.
Extended Data Fig. 7 Cold-induced glutamine synthetase in BAT.
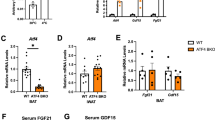
a, qRT–PCR comparing Glul gene expression in BAT for TN and CC. N = 8 mice per condition. Data are mean ± s.e. ***p = 0.0003 by unpaired two-tailed Student’s t-test. b, Western blot of GS in brown adipocytes during adipogenesis. c, Western blot of GS in different tissues from mice at 22 °C. iB, interscapular BAT; sB, subcutaneous BAT; iW, inguinal white fat; pW, perigonadal white fat; LV, liver; Q, quadricep; S, spleen; H, heart; Lu, lung; B, brain; K, kidney. d-g,15N1-labeled glutamine and glutamate abundances in liver (d,e) or serum (f,g) after 15N-ammonia tracer administration. N = 5 mice were used for each time point except TN 15-minute and CC 5-minute N = 4 mice were used. Data are mean ± s.e. h, Full graph of oxygen consumption rate in mature brown adipocytes transfected with control or Glul targeting siRNAs with or without norepinephrine (NE) stimulation. N = 15 biological replicates. Data are mean ± s.e.
Supplementary information
Source data
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, G., Haley, J.A., Le, J. et al. Quantitative analysis of metabolic fluxes in brown fat and skeletal muscle during thermogenesis. Nat Metab 5, 1204–1220 (2023). https://doi.org/10.1038/s42255-023-00825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00825-8
This article is cited by
-
MCT1 helps brown fat suck up succinate
Nature Metabolism (2024)
-
Monocarboxylate transporters facilitate succinate uptake into brown adipocytes
Nature Metabolism (2024)
-
A waste product’s unexpected role in wasting
Nature Metabolism (2024)
-
Plasmalogens and Octanoylcarnitine Serve as Early Warnings for Central Retinal Artery Occlusion
Molecular Neurobiology (2024)
-
Brown fat has a sweet tooth
Nature Metabolism (2023)