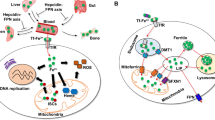
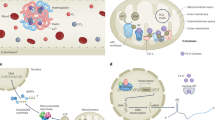
Abstract
Vitamin K is essential for several physiological processes, such as blood coagulation, in which it serves as a cofactor for the conversion of peptide-bound glutamate to γ-carboxyglutamate in vitamin K-dependent proteins. This process is driven by the vitamin K cycle facilitated by γ-carboxyglutamyl carboxylase, vitamin K epoxide reductase and ferroptosis suppressor protein-1, the latter of which was recently identified as the long-sought-after warfarin-resistant vitamin K reductase. In addition, vitamin K has carboxylation-independent functions. Akin to ubiquinone, vitamin K acts as an electron carrier for ATP production in some organisms and prevents ferroptosis, a type of cell death hallmarked by lipid peroxidation. In this Perspective, we provide an overview of the diverse functions of vitamin K in physiology and metabolism and, at the same time, offer a perspective on its role in ferroptosis together with ferroptosis suppressor protein-1. A comparison between vitamin K and ubiquinone, from an evolutionary perspective, may offer further insights into the manifold roles of vitamin K in biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mladenka, P. et al. Vitamin K—sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 80, 677–698 (2022).
Tabb, M. M. et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 278, 43919–43927 (2003).
Nowicka, B. & Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta 1797, 1587–1605 (2010).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Mishima, E. & Conrad, M. Nutritional and metabolic control of ferroptosis. Annu. Rev. Nutr. 42, 275–309 (2022).
Dam, H. The antihaemorrhagic vitamin of the chick. Biochem. J. 29, 1273–1285 (1935).
Dam, H. et al. Isolierung des vitamins K in hochgereinigter form. Helv. Chim. Acta 22, 310–313 (1939).
McKee, R. W., Binkley, S. B., MacCorquodale, D. W., Thayer, S. A. & Doisy, E. A. The isolation of vitamins K1 and K2. J. Am. Chem. Soc. 61, 1295–1295 (1939).
Lehmann, J. Vitamin K as a prophylactic in 13,000 infants. Lancet 243, 493–494 (1944).
Esmon, C. T., Sadowski, J. A. & Suttie, J. W. A new carboxylation reaction. The vitamin K-dependent incorporation of H-14-CO3− into prothrombin. J. Biol. Chem. 250, 4744–4748 (1975).
Stenflo, J., Fernlund, P., Egan, W. & Roepstorff, P. Vitamin K-dependent modifications of glutamic acid residues in prothrombin. Proc. Natl Acad. Sci. USA 71, 2730–2733 (1974).
Stenflo, J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J. Biol. Chem. 251, 355–363 (1976).
Stenflo, J. & Jonsson, M. Protein S, a new vitamin K-dependent protein from bovine plasma. FEBS Lett. 101, 377–381 (1979).
Villa, J. K. D., Diaz, M. A. N., Pizziolo, V. R. & Martino, H. S. D. Effect of vitamin K in bone metabolism and vascular calcification: a review of mechanisms of action and evidences. Crit. Rev. Food Sci. Nutr. 57, 3959–3970 (2017).
Bouckaert, J. H. & Said, A. H. Fracture healing by vitamin K. Nature 185, 849 (1960).
Walker, C. S. et al. On a potential global role for vitamin K-dependent gamma-carboxylation in animal systems. Evidence for a gamma-glutamyl carboxylase in Drosophila. J. Biol. Chem. 276, 7769–7774 (2001).
Brown, M. A. et al. Precursors of novel Gla-containing conotoxins contain a carboxy-terminal recognition site that directs gamma-carboxylation. Biochemistry 44, 9150–9159 (2005).
Li, T., Yang, C. T., Jin, D. & Stafford, D. W. Identification of a Drosophila vitamin K-dependent gamma-glutamyl carboxylase. J. Biol. Chem. 275, 18291–18296 (2000).
Schofield, F. W. A brief account of a disease in cattle simulating hemorrhagic septicaemia due to feeding sweet clover. Can. Vet. J. 3, 3274–3278 (1922).
Campbell, H. A. & Link, K. P. Studies on the hemorrhagic sweet clover disease: iv. The isolation and crystallization of the hemorrhagic agent. J. Biol. Chem. 138, 21–33 (1941).
Overman, R. S. et al. Studies on the hemorrhagic sweet clover disease: xiii. Anticoagulant activity and structure in the 4-hydroxycoumarin group. J. Biol. Chem. 153, 5–24 (1944).
Holmes, R. W. & Love, J. Suicide attempt with warfarin, a bishydroxycoumarin-like rodenticide. JAMA 148, 935–937 (1952).
Whitlon, D. S., Sadowski, J. A. & Suttie, J. W. Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition. Biochemistry 17, 1371–1377 (1978).
Berkner, K. L. Vitamin K-dependent carboxylation. Vitam. Horm. 78, 131–156 (2008).
Bell, R. G. & Matschiner, J. T. Warfarin and the inhibition of vitamin K activity by an oxide metabolite. Nature 237, 32–33 (1972).
Sherman, P. A. & Sander, E. G. Vitamin K epoxide reductase: evidence that vitamin K dihydroquinone is a product of vitamin K epoxide reduction. Biochem. Biophys. Res. Commun. 103, 997–1005 (1981).
Tie, J. K. & Stafford, D. W. Structural and functional insights into enzymes of the vitamin K cycle. J. Thromb. Haemost. 14, 236–247 (2016).
Wu, S. M., Cheung, W. F., Frazier, D. & Stafford, D. W. Cloning and expression of the cDNA for human gamma-glutamyl carboxylase. Science 254, 1634–1636 (1991).
Li, T. et al. Identification of the gene for vitamin K epoxide reductase. Nature 427, 541–544 (2004).
Rost, S. et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427, 537–541 (2004).
Wallin, R. & Hutson, S. Vitamin K-dependent carboxylation. Evidence that at least two microsomal dehydrogenases reduce vitamin K1 to support carboxylation. J. Biol. Chem. 257, 1583–1586 (1982).
Shearer, M. J. & Okano, T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu. Rev. Nutr. 38, 127–151 (2018).
Chu, P. H., Huang, T. Y., Williams, J. & Stafford, D. W. Purified vitamin K epoxide reductase alone is sufficient for conversion of vitamin K epoxide to vitamin K and vitamin K to vitamin KH2. Proc. Natl Acad. Sci. USA 103, 19308–19313 (2006).
Wallin, R., Patrick, S. D. & Ballard, J. O. Vitamin K antagonism of coumarin intoxication in the rat. Thromb. Haemost. 55, 235–239 (1986).
Lowenthal, J. & Macfarlane, J. A. The nature of the antagonism between vitamin K and indirect anticoagulants. J. Pharmacol. Exp. Ther. 143, 273–277 (1964).
Ingram, B. O., Turbyfill, J. L., Bledsoe, P. J., Jaiswal, A. K. & Stafford, D. W. Assessment of the contribution of NAD(P)H-dependent quinone oxidoreductase 1 (NQO1) to the reduction of vitamin K in wild-type and NQO1-deficient mice. Biochem. J. 456, 47–54 (2013).
Ross, D. et al. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Interact. 129, 77–97 (2000).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Kaye, J. B. et al. Warfarin pharmacogenomics in diverse populations. Pharmacotherapy 37, 1150–1163 (2017).
Nutescu, E., Chuatrisorn, I. & Hellenbart, E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J. Thromb. Thrombolysis 31, 326–343 (2011).
Jin, D. Y. et al. A genome-wide CRISPR-Cas9 knockout screen identifies FSP1 as the warfarin-resistant vitamin K reductase. Nat. Commun. 14, 828 (2023).
Napolitano, M., Mariani, G. & Lapecorella, M. Hereditary combined deficiency of the vitamin K-dependent clotting factors. Orphanet J. Rare Dis. 5, 21 (2010).
Brenner, B. et al. A missense mutation in gamma-glutamyl carboxylase gene causes combined deficiency of all vitamin K-dependent blood coagulation factors. Blood 92, 4554–4559 (1998).
De Vilder, E. Y., Debacker, J. & Vanakker, O. M. GGCX-associated phenotypes: an overview in search of genotype–phenotype correlations. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18020240 (2017).
Oldenburg, J. et al. Congenital deficiency of vitamin K-dependent coagulation factors in two families presents as a genetic defect of the vitamin K-epoxide-reductase-complex. Thromb. Haemost. 84, 937–941 (2000).
Pauli, R. M., Lian, J. B., Mosher, D. F. & Suttie, J. W. Association of congenital deficiency of multiple vitamin K-dependent coagulation factors and the phenotype of the warfarin embryopathy: clues to the mechanism of teratogenicity of coumarin derivatives. Am. J. Hum. Genet. 41, 566–583 (1987).
Azuma, K. et al. Liver-specific gamma-glutamyl carboxylase-deficient mice display bleeding diathesis and short life span. PLoS ONE 9, e88643 (2014).
Zhu, A. et al. Fatal hemorrhage in mice lacking gamma-glutamyl carboxylase. Blood 109, 5270–5275 (2007).
Shiba, S. et al. Vitamin K-dependent gamma-glutamyl carboxylase in sertoli cells is essential for male fertility in mice. Mol. Cell Biol. https://doi.org/10.1128/MCB.00404-20 (2021).
Azuma, K. et al. Osteoblast-specific gamma-glutamyl carboxylase-deficient mice display enhanced bone formation with aberrant mineralization. J. Bone Miner. Res. 30, 1245–1254 (2015).
Spohn, G. et al. VKORC1 deficiency in mice causes early postnatal lethality due to severe bleeding. Thromb. Haemost. 101, 1044–1050 (2009).
LeBlanc, J. G. et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168 (2013).
Shearer, M. J., McBurney, A. & Barkhan, P. Studies on the absorption and metabolism of phylloquinone (vitamin K1) in man. Vitam. Horm. 32, 513–542 (1974).
Narushima, K., Takada, T., Yamanashi, Y. & Suzuki, H. Niemann-pick C1-like 1 mediates alpha-tocopherol transport. Mol. Pharmacol. 74, 42–49 (2008).
Takada, T. et al. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci. Transl. Med. 7, 275ra223 (2015).
Goncalves, A. et al. Intestinal scavenger receptors are involved in vitamin K1 absorption. J. Biol. Chem. 289, 30743–30752 (2014).
Hirota, Y. et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J. Biol. Chem. 288, 33071–33080 (2013).
Nakagawa, K. et al. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 468, 117–121 (2010).
Okano, T. et al. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 283, 11270–11279 (2008).
Thijssen, H. H. & Drittij-Reijnders, M. J. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br. J. Nutr. 75, 121–127 (1996).
Shearer, M. J. Vitamin K in parenteral nutrition. Gastroenterology 137, S105–S118 (2009).
Araki, S. & Shirahata, A. Vitamin K deficiency bleeding in infancy. Nutrients https://doi.org/10.3390/nu12030780 (2020).
Zipursky, A. Prevention of vitamin K deficiency bleeding in newborns. Br. J. Haematol. 104, 430–437 (1999).
Shea, M. K. et al. Vitamin K status and cognitive function in adults with chronic kidney disease: the chronic renal insufficiency cohort. Curr. Dev. Nutr. 6, nzac111 (2022).
Berkner, K. L. & Runge, K. W. Vitamin K-dependent protein activation: normal gamma-glutamyl carboxylation and disruption in disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23105759 (2022).
Hayes, D. M. Neonatal anemia due to water-soluble vitamin K analogue: case report. N. C. Med. J. 22, 270–271 (1961).
Ansbacher, S., Corwin, W. C. & Thomas, B. G. H. Toxicity of menadione, menadiol and esters. J. Pharmacol. Exp. Ther. 75, 111 (1942).
Loor, G. et al. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 49, 1925–1936 (2010).
Goto, S. et al. Prodrugs for skin delivery of menahydroquinone-4 an active form of vitamin K2(20) could overcome the photoinstability and phototoxicity of vitamin K2(20). Int. J. Mol. Sci. 20, 2548 (2019).
Ichikawa, T., Horie-Inoue, K., Ikeda, K., Blumberg, B. & Inoue, S. Vitamin K2 induces phosphorylation of protein kinase A and expression of novel target genes in osteoblastic cells. J. Mol. Endocrinol. 39, 239–247 (2007).
Ohsaki, Y. et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation. J. Nutr. Biochem. 21, 1120–1126 (2010).
Hirota, Y. & Suhara, Y. New aspects of vitamin K research with synthetic ligands: transcriptional activity via SXR and neural differentiation activity. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20123006 (2019).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Vervoort, L. M., Ronden, J. E. & Thijssen, H. H. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem. Pharmacol. 54, 871–876 (1997).
Li, J. et al. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J. Neurosci. 23, 5816–5826 (2003).
Kolbrink, B. et al. Vitamin K1 inhibits ferroptosis and counteracts a detrimental effect of phenprocoumon in experimental acute kidney injury. Cell. Mol. Life Sci. 79, 387 (2022).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Misima, E. et al. DHODH inhibitors sensitize cancer cells to ferroptosis via FSP1 inhibition. Res. Sq. https://doi.org/10.21203/rs.3.rs-2190326/v1 (2022).
Hiratsuka, T. et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science 321, 1670–1673 (2008).
Meganathan, R. & Kwon, O. Biosynthesis of menaquinone (vitamin K2) and ubiquinone (coenzyme Q). EcoSal Plus https://doi.org/10.1128/ecosalplus.3.6.3.3 (2009).
Brettel, K. & Leibl, W. Electron transfer in photosystem I. Biochim. Biophys. Acta 1507, 100–114 (2001).
Wang, L. et al. The phytol phosphorylation pathway is essential for the biosynthesis of phylloquinone, which is required for photosystem I stability in Arabidopsis. Mol. Plant 10, 183–196 (2017).
Vos, M. et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 336, 1306–1310 (2012).
Cerqua, C. et al. Vitamin K2 cannot substitute coenzyme Q10 as electron carrier in the mitochondrial respiratory chain of mammalian cells. Sci. Rep. 9, 6553 (2019).
Hirota, Y. et al. Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. PLoS ONE 10, e0125737 (2015).
Nashimoto, S., Takekawa, Y., Takekuma, Y., Sugawara, M. & Sato, Y. Transport via Niemann–Pick C1 Like 1 contributes to the intestinal absorption of ubiquinone. Drug Metab. Pharmacokinet. 35, 527–533 (2020).
Ilbert, M. & Bonnefoy, V. Insight into the evolution of the iron oxidation pathways. Biochim. Biophys. Acta 1827, 161–175 (2013).
Bergdoll, L., Ten Brink, F., Nitschke, W., Picot, D. & Baymann, F. From low- to high-potential bioenergetic chains: thermodynamic constraints of Q-cycle function. Biochim. Biophys. Acta 1857, 1569–1579 (2016).
Distefano, A. M. et al. Heat stress induces ferroptosis-like cell death in plants. J. Cell Biol. 216, 463–476 (2017).
Aguilera, A. et al. C-ferroptosis is an iron-dependent form of regulated cell death in cyanobacteria. J. Cell Biol. https://doi.org/10.1083/jcb.201911005 (2022).
Bogacz, M. & Krauth-Siegel, R. L. Tryparedoxin peroxidase deficiency commits trypanosomes to ferroptosis-type cell death. Elife https://doi.org/10.7554/eLife.37503 (2018).
Shen, Q., Liang, M., Yang, F., Deng, Y. Z. & Naqvi, N. I. Ferroptosis contributes to developmental cell death in rice blast. New Phytol. 227, 1831–1846 (2020).
Perez, M. A., Magtanong, L., Dixon, S. J. & Watts, J. L. Dietary lipids induce ferroptosis in Caenorhabditis elegans and human cancer cells. Dev. Cell 54, 447–454 (2020).
Conrad, M. et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 32, 602–619 (2018).
Vats, K. et al. Keratinocyte death by ferroptosis initiates skin inflammation after UVB exposure. Redox Biol. 47, 102143 (2021).
Singhal, R. & Shah, Y. M. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505 (2020).
Acknowledgements
M.C. acknowledges funding from the Deutsche Forschungsgemeinschaft (CO 291/9-1, 461385412; and the Priority Program SPP 2306 (CO 291/9-1, 461385412; CO 291/10-1, 461507177)), the German Federal Ministry of Education and Research (BMBF) FERROPath (01EJ2205B), the Else Kröner-Fresenius-Stiftung (projects 2019_T12 and 2020_EKTP19) and the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. GA 884754). E.M. is funded by JSPS KAKENHI (20KK0363 and 18K08198).
Author information
Authors and Affiliations
Contributions
E.M., A.W., T.S. and M.C. jointly wrote this article.
Corresponding authors
Ethics declarations
Competing interests
E.M. has filed a patent related to the treatment of ferroptosis-associated diseases with vitamin K (WO2022075444A1). M.C. is a cofounder and shareholder of ROSCUE Therapeutics, which is developing ferroptosis inhibitors. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Martin Shearer and Yoshihisa Hirota for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishima, E., Wahida, A., Seibt, T. et al. Diverse biological functions of vitamin K: from coagulation to ferroptosis. Nat Metab 5, 924–932 (2023). https://doi.org/10.1038/s42255-023-00821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00821-y
This article is cited by
-
Identification of a murder caused by brodifacoum poisoning based on clinical examinations and LC-MS/MS results
International Journal of Legal Medicine (2024)
-
Targeting ferroptosis for treating kidney disease
Clinical and Experimental Nephrology (2024)
-
Cyclodextrins and their potential applications for delivering vitamins, iron, and iodine for improving micronutrient status
Drug Delivery and Translational Research (2024)