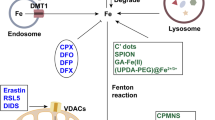
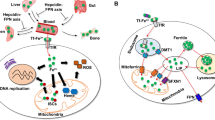
Abstract
Ferroptosis, spurred by excess labile iron and lipid peroxidation, is implicated in various diseases. Advances have been made in comprehending the lipid-peroxidation side of ferroptosis, but the exact role of iron in driving ferroptosis remains unknown. Although iron overload is characterized in multiple disease states, the potential role of ferroptosis within them remains undefined. This overview focuses on the ‘ferro’ side of ferroptosis, highlighting iron dysregulation in human diseases and potential therapeutic strategies targeting iron regulation and metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aisen, P., Enns, C. & Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 33, 940–959 (2001).
Ponka, P., Tenenbein, M. & Eaton, J. W. in Handbook on the Toxicology of Metals 4th edn (eds. Nordberg, G. F. et al.) Vol. 2, 879–902 (Elsevier, 2015).
Hentze, M. W., Muckenthaler, M. U. & Andrews, N. C. Balancing acts: molecular control of mammalian iron metabolism. Cell 117, 285–297 (2004).
Weiss, G. & Goodnough, L. T. Anemia of chronic disease. N. Engl. J. Med. 352, 1011–1023 (2005).
Hentze, M. W., Muckenthaler, M. U., Galy, B. & Camaschella, C. Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 (2010).
Andrews, N. C. Disorders of iron metabolism. N. Engl. J. Med. 341, 1986–1995 (1999).
Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 460, 831–838 (2009).
Rouault, T. A. Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat. Rev. Mol. Cell Biol. 16, 45–55 (2015).
Jordan, A. & Reichard, P. Ribonucleotide reductases. Annu. Rev. Biochem. 67, 71–98 (1998).
Rudolf, J., Makrantoni, V., Ingledew, W. J., Stark, M. J. & White, M. F. The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol. Cell 23, 801–808 (2006).
Gray, N. K. & Hentze, M. W. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 13, 3882–3891 (1994).
Rouault, T. A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2, 406–414 (2006).
Kortman, G. A., Raffatellu, M., Swinkels, D. W. & Tjalsma, H. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol. Rev. 38, 1202–1234 (2014).
Nemeth, E. & Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 26, 323–342 (2006).
Drakesmith, H. & Prentice, A. M. Hepcidin and the iron-infection axis. Science 338, 768–772 (2012).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Donovan, A. et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 (2000).
Hellman, N. E. & Gitlin, J. D. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 22, 439–458 (2002).
Harris, Z. L., Durley, A. P., Man, T. K. & Gitlin, J. D. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl Acad. Sci. USA 96, 10812–10817 (1999).
Yu, Y. et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 136, 726–739 (2020).
Shayeghi, M. et al. Identification of an intestinal heme transporter. Cell 122, 789–801 (2005).
Conrad, M. E. & Umbreit, J. N. Iron absorption and transport-an update. Am. J. Hematol. 64, 287–298 (2000).
McKie, A. T. et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 (2000).
Gunshin, H. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 (1997).
Muckenthaler, M. U., Galy, B. & Hentze, M. W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev. Nutr. 28, 197–213 (2008).
Ohgami, R. S., Campagna, D. R., McDonald, A. & Fleming, M. D. The Steap proteins are metalloreductases. Blood 108, 1388–1394 (2006).
Arosio, P. & Levi, S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med 33, 457–463 (2002).
Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014).
Dowdle, W. E. et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079 (2014).
Diao, J. et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 520, 563–566 (2015).
Itakura, E., Kishi-Itakura, C. & Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 (2012).
Anandhan, A. et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 9, eade9585 (2023).
Pinnix, Z. K. et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2, 43ra56 (2010).
Zhang, D. L., Hughes, R. M., Ollivierre-Wilson, H., Ghosh, M. C. & Rouault, T. A. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 9, 461–473 (2009).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Doll, S. & Conrad, M. Iron and ferroptosis: a still ill-defined liaison. IUBMB Life 69, 423–434 (2017).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Nakamura, T. et al. Phase separation of FSP1 promotes ferroptosis. Nature 619, 371–377 (2023).
Mao, C., Liu, X., Yan, Y., Olszewski, K. & Gan, B. Reply to: DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E19–E23 (2023).
Mishima, E. et al. DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E9–E18 (2023).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3, e02523 (2014).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Torti, S. V. & Torti, F. M. Iron and cancer: 2020 vision. Cancer Res. 80, 5435–5448 (2020).
Xue, Q. et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy 19, 1982–1996 (2023).
Chiabrando, D., Vinchi, F., Fiorito, V., Mercurio, S. & Tolosano, E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharm. 5, 61 (2014).
Torti, S. V. & Torti, F. M. Iron and cancer: more ore to be mined. Nat. Rev. Cancer 13, 342–355 (2013).
Muckenthaler, M. U., Rivella, S., Hentze, M. W. & Galy, B. A red carpet for iron metabolism. Cell 168, 344–361 (2017).
Kumar, S. & Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 157, 175–188 (2005).
Papanikolaou, G. & Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 202, 199–211 (2005).
Bacon, B. R. et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 54, 328–343 (2011).
Anderson, G. J. & Frazer, D. M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 106, 1559S–1566S (2017).
Bridle, K. R. et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet 361, 669–673 (2003).
Kowdley, K. V. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology 127, S79–S86 (2004).
Fernandez-Real, J. M. et al. Blood letting in high-ferritin type 2 diabetes: effects on vascular reactivity. Diabetes Care 25, 2249–2255 (2002).
Wood, J. C. Cardiac iron across different transfusion-dependent diseases. Blood Rev. 22, S14–S21 (2008).
Fang, X. et al. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ. Res. 127, 486–501 (2020).
Fang, X. et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl Acad. Sci. USA 116, 2672–2680 (2019).
Dixon, S. J. & Stockwell, B. R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17 (2014).
Wang, H. et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66, 449–465 (2017).
Devos, D. et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 21, 195–210 (2014).
Ayton, S., Lei, P. & Bush, A. I. Metallostasis in Alzheimer’s disease. Free Radic. Biol. Med. 62, 76–89 (2013).
Conrad, M., Angeli, J. P., Vandenabeele, P. & Stockwell, B. R. Regulated necrosis: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 15, 348–366 (2016).
Kontoghiorghe, C. N. & Kontoghiorghes, G. J. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Devel. Ther. 10, 465–481 (2016).
Spangler, B. et al. A reactivity-based probe of the intracellular labile ferrous iron pool. Nat. Chem. Biol. 12, 680–685 (2016).
Muir, R. K. et al. Measuring dynamic changes in the labile iron pool in vivo with a reactivity-based probe for positron emission tomography. ACS Cent. Sci. 5, 727–736 (2019).
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R35ES031575).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, D.D. Ironing out the details of ferroptosis. Nat Cell Biol (2024). https://doi.org/10.1038/s41556-024-01361-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41556-024-01361-7