Abstract
Increased expression of branched-chain amino acid transaminase 1 or 2 (BCAT1 and BCAT2) has been associated with aggressive phenotypes of different cancers. Here we identify a gain of function of BCAT1 glutamic acid to alanine mutation at codon 61 (BCAT1E61A) enriched around 2.8% in clinical gastric cancer samples. We found that BCAT1E61A confers higher enzymatic activity to boost branched-chain amino acid (BCAA) catabolism, accelerate cell growth and motility and contribute to tumor development. BCAT1 directly interacts with RhoC, leading to elevation of RhoC activity. Notably, the BCAA-derived metabolite, branched-chain α-keto acid directly binds to the small GTPase protein RhoC and promotes its activity. BCAT1 knockout-suppressed cell motility could be rescued by expressing BCAT1E61A or adding branched-chain α-keto acid. We also identified that candesartan acts as an inhibitor of BCAT1E61A, thus repressing RhoC activity and cancer cell motility in vitro and preventing peritoneal metastasis in vivo. Our study reveals a link between BCAA metabolism and cell motility and proliferation through regulating RhoC activation, with potential therapeutic implications for cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated and supporting the findings of this study are available. The MS proteomics data have been deposited to the ProteomeXchange Consortium with the dataset identifier PXD041556. Source data are provided with this paper.
References
Ericksen, R. E. et al. Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 29, 1151–1165 (2019).
Raffel, S. et al. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature 551, 384–388 (2017).
Wang, Y. et al. Branched-chain amino acid metabolic reprogramming orchestrates drug resistance to EGFR tyrosine kinase inhibitors. Cell Rep. 28, 512–525 (2019).
Tonjes, M. et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med. 19, 901–908 (2013).
Li, J. T. et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat. Cell Biol. 22, 167–174 (2020).
Zhu, Z. et al. Tumour-reprogrammed stromal BCAT1 fuels branched-chain ketoacid dependency in stromal-rich PDAC tumours. Nat. Metab. 2, 775–792 (2020).
Xu, Y. et al. Overexpression of BCAT1 is a prognostic marker in gastric cancer. Hum. Pathol. 75, 41–46 (2018).
Kakiuchi, M. et al. Recurrent gain-of-function mutations of RhoA in diffuse-type gastric carcinoma. Nat. Genet. 46, 583–587 (2014).
Zhang, H. et al. Gain-of-function RhoA mutations promote focal adhesion kinase activation and dependency in diffuse gastric cancer. Cancer Discov. 10, 288–305 (2020).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Liu, N. et al. RhoC is essential for the metastasis of gastric cancer. J. Mol. Med. 85, 1149–1156 (2007).
Hakem, A. et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 (2005).
Soshnev, A. A. et al. Genome-wide studies of the multi-zinc finger Drosophila suppressor of hairy-wing protein in the ovary. Nucleic Acids Res. 40, 5415–5431 (2012).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Cooper, A. J., Conway, M. & Hutson, S. M. A continuous 96-well plate spectrophotometric assay for branched-chain amino acid aminotransferases. Anal. Biochem. 308, 100–105 (2002).
Prohl, C., Kispal, G. & Lill, R. Branched-chain-amino-acid transaminases of yeast Saccharomyces cerevisiae. Methods Enzymol. 324, 365–375 (2000).
Hattori, A. et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 545, 500–504 (2017).
Solon-Biet, S. M. et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 1, 532–545 (2019).
Lucey, M., Unger, H. & van Golen, K. L. RhoC GTPase activation assay. J. Vis. Exp. https://doi.org/10.3791/2083 (2010).
Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science 357, eaan2507 (2017).
Wynn, M. L. et al. RhoC GTPase is a potent regulator of glutamine metabolism and N-acetylaspartate production in inflammatory breast cancer cells. J. Biol. Chem. 291, 13715–13729 (2016).
Szklarczyk, D. et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Szklarczyk, D. et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Oughtred, R. et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 (2021).
Guilluy, C. et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat. Med. 16, 183–190 (2010).
Guilluy, C., Dubash, A. D. & Garcia-Mata, R. Analysis of RhoA and Rho GEF activity in whole cells and the cell nucleus. Nat. Protoc. 6, 2050–2060 (2011).
Leonard, D. A., Evans, T., Hart, M., Cerione, R. A. & Manor, D. Investigation of the GTP-binding/GTPase cycle of Cdc42Hs using fluorescence spectroscopy. Biochemistry 33, 12323–12328 (1994).
Pierce, B. G. et al. ZDOCK server: interactive docking prediction of protein–protein complexes and symmetric multimers. Bioinformatics 30, 1771–1773 (2014).
Grankvist, N., Lagerborg, K. A., Jain, M. & Nilsson, R. Gabapentin can suppress cell proliferation independent of the cytosolic branched-chain amino acid transferase 1 (BCAT1). Biochemistry 57, 6762–6766 (2018).
Dallakyan, S. & Olson, A. J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 1263, 243–250 (2015).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Bastiaenen, V. P. et al. A mouse model for peritoneal metastases of colorectal origin recapitulates patient heterogeneity. Lab. Invest. 100, 1465–1474 (2020).
Yun, J. et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325, 1555–1559 (2009).
Wen, W. et al. Genetic variations of DNA bindings of FOXA1 and co-factors in breast cancer susceptibility. Nat. Commun. 12, 5318 (2021).
Yang, G. et al. Dominant effects of an Msh6 missense mutation on DNA repair and cancer susceptibility. Cancer Cell. 6, 139–150 (2004).
Wu, X. et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat. Genet. 41, 991–995 (2009).
Sasaki, M. et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488, 656–659 (2012).
Khalaf, N. & Wolpin, B. M. Metabolic alterations as a signpost to early pancreatic cancer. Gastroenterology 156, 1560–1563 (2019).
Pothiwala, P., Jain, S. K. & Yaturu, S. Metabolic syndrome and cancer. Metab. Syndr. Relat. Disord. 7, 279–288 (2009).
Gu, Z. et al. Loss of EZH2 reprograms BCAA metabolism to drive leukemic transformation. Cancer Discov. 9, 1228–1247 (2019).
D’Antona, G. et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 12, 362–372 (2010).
Silva, L. S. et al. Branched-chain ketoacids secreted by glioblastoma cells via MCT1 modulate macrophage phenotype. EMBO Rep. 18, 2172–2185 (2017).
Bueno De Paiva, L. et al. Effects of RhoA and RhoC upon the sensitivity of prostate cancer cells to glutamine deprivation. Small GTPases 12, 20–26 (2021).
Honda, T. et al. Effects of liver failure on branched-chain α-keto acid dehydrogenase complex in rat liver and muscle: comparison between acute and chronic liver failure. J. Hepatol. 40, 439–445 (2004).
Ding, C. et al. A cell-type-resolved liver proteome. Mol. Cell Proteom. 15, 3190–3202 (2016).
Patrick, M. et al. Metabolon formation regulates branched-chain amino acid oxidation and homeostasis. Nat. Metab. 4, 1775–1791 (2022).
French, J. B. et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science 351, 733–737 (2016).
Pareek, V., Tian, H., Winograd, N. & Benkovic, S. J. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science 368, 283–290 (2020).
Chen, J. et al. SAR1B senses leucine levels to regulate mTORC1 signalling. Nature 596, 281–284 (2021).
Liu, Y. et al. Role for the endoplasmic reticulum stress sensor IRE1α in liver regenerative responses. J. Hepatol. 62, 590–598 (2015).
Scholten, D., Trebicka, J., Liedtke, C. & Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim. 49, 4–11 (2015).
Acknowledgements
We thank members of the Lei Laboratory for discussion throughout this study and the Biomedical Core Facility of Fudan University for technical support. This work was supported by the National Key R&D Program of China (no. 2020YFA0803402, 2019YFA0801703 to Q.-Y.L.), the Natural Science Foundation of China (no. 82121004, 91959202 to Q.-Y.L.; no. 81872240 to M.Y.) and the Innovation Program of Shanghai Municipal Education Commission (no. 2023ZKD11 to Q.-Y.L.).
Author information
Authors and Affiliations
Contributions
L.Q. and N.L. designed and performed the experiments and analyzed the data. X.-C.L. and M.X. performed animal and clinical studies, respectively. Y.L. helped with pathology analysis. K.L., Y.Z., K.H. and Y.-T.Q. helped with part of the experiments. W.W. and J.Y. helped with structure and mass spectrum analyses. L.Q., N.L. and M.Y co-wrote the manuscript. Y.-L.W. provided the US FDA-approved drug library. S.H. helped with WES analysis. Z.-J.C. gave advice on animal experiments. M.Y. provided intellectual discussion. Q.-Y.L. conceived the idea, designed and supervised the study, analyzed the data and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Jean-François Côté, Robert McGarrah and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling Editor: Alfredo Giménez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Screening the gene mutations from α-KG, glutamate and BCAA metabolism pathway.
a, List of genes in α-KG, glutamate and BCAA metabolism pathway. b, The results of gene mutations from Cancer Cell Line Encyclopedia (CCLE) database.
Extended Data Fig. 2 BCAT1E61A mutation information in database and determined the mutation by WES.
a, Mutation (E61A) of BCAT1 is occurred in multiple human cancer cell lines from CCLE database. b, The main mutation difference between the BCAT1 mutation and wild-type sample by whole exome sequencing (WES) sequencing of gastric cancer with 5% cutoff. c, The gene cope number in Adj and tumor tissues of gastric cancer patients.
Extended Data Fig. 3 BCAT1E61A has no effect on the dimer formation of BCAT1 but enhances the enzyme activity.
a, The model structure of BCAT1E61A shows mutation site locates on the surface and near the boundary of BCAT1 dimer. The data was created and downloaded from SWISS-MODEL (BCAT1E61A|Models (expasy.org)) under the CC BY-SA 4.0 (Creative Commons Attribution-ShareAlike 4.0) International License, without any changes or additions (https://doi.org/10.1093/nar/gky427). b, c, BCAT1E61A mutant does not affect the formation of BCAT1 dimers by Co-IP (b) and non-denaturation-PAGE (c) assays. Representative result from 3 independent experiments. d, Identification of the purification proteins of His-BCAT1WT and His-BCAT1E61A from E. coli (BL21) by Coomassie blue staining. Representative result from 3 independent experiments. e, Enzyme kinetic parameter table of His-BCAT1WT and His-BCAT1E61A. N = 3. Mean ± SD. two-tailed t-test. f, Flag-BCAT1WT and Flag-BCAT1E61A proteins expressed and purified in gastric cancer cell lines by Flag-IP. Representative result from 3 independent experiments. g, BCAT1E61A promotes its enzyme activity of both forward and reverse reactions by using eukaryotic expression proteins. N = 3, Mean ± SD. two-tailed t-test. h, i, BCAT1E61A increases the glutamate of forward reaction detected by GC-MS. The chromatography (h) and mass spectrum (i) of glutamate. j, k, BCAT1E61A mutant cell lines KATO lll and TE1 display stronger the overall metabolic level than BCAT1WT cell lines AGS and KYSE180. The metabolomics were established by employing LC–MS.
Extended Data Fig. 4 Tracing the metabolites in mouse dermal fibroblasts (MDFs).
a, The phylogenetic analysis of BCAT1 by MEGA-X. b, p61E residue of BCAT1 is conserved in mammals based MEGA-X analysis. The arrow indicates the E61 residue of BCAT1. c, A flowchart for the generation of Bcat1E61A knock-in mice using the CRISPR/Cas9 system. d, The verification of genome sequence of Bcat1E61A knock-in mice. e and h, The flux model of Valine-13C5 (e) and Leucine -13C6, 15N1 (h) tracing. f, g, The main flux of Valine-13C5 tracing. N = 3. Mean ± SD. Two-tailed t-test. k-p, The main flux of Leucine -13C6, 15N1 tracing. N = 3. Mean ± SD. Two-tailed t-test.
Extended Data Fig. 5 BCAT1E61A promotes cell growth.
a, The identification of BCAT1 knockout stable cells detected by Western blot. b, The identification of BCAT1 and BCAT1E61A overexpression stable cells detected by Western blot. c, BCAT1E61A increased colony-forming capacity in MDFs. Scale bar = 200 µm. a-c, Representative result from 3 independent experiments. d–g, The intracellular and extracellular concentrations of BCAA and BCKA of TE1 and AGS. N = 3. Mean ± SD. Two-tailed t-test. h, The model of BCAT1E61A promoting cell growth. Glutamate and BCKA produced by BCAT1 provide energy for tumor cell growth.
Extended Data Fig. 6 BCAT1 interacts with RhoC.
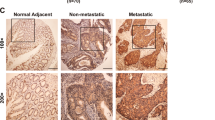
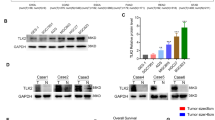
a, The activity of mTOR in lung, liver and stomach tissue was no different in liver and stomach tissue, but decreased in lung from Bcat1E61A knock-in mice than that of wild-type. b, The activity of mTOR was increased in MDFs. c, BCAT1 interacts with RhoC by Co-IP experiment. Identified the potential binding proteins with BCAT1 of IP–MS analysis by Flag-IP in 293T cells. d, RhoC interacts with BCAT1WT and BCAT1E61A as determined by Myc-IP in 293T cells. c, d, Representative result from 3 independent experiments. e, f, Both high levels of BCAT1 (e) and RhoC (f) expression are correlated with poorer prognosis of gastric cancer. Plots were generated online by using a Kaplan–Meier Plotter based on PROTEIN ATLAS. N = 353 cases (c) and n = 354 cases (d). Mean ± SD. Log-rank (Mantel-Cox) test. g, BCAT1WT or BCAT1E61A rescues the inhibition of cell migration and RhoC activity in BCAT1-knockout cells. N = 3. Cell migration data are five fields each, mean ± SD. Two-tailed t-test. h, F-actin staining in AGS and MGC803 cells with knockout BCAT1 and putbcak BCAT1WT and BCAT1E61A. N = 3, mean ± SD. Two-tailed t-test.
Extended Data Fig. 7 BCAT1E61A enhances the activity of RhoC by ARHGEF1.
a, b, Interacting proteins for RhoC in BioGRID (a) and STRING (b) database. a, The data was created and downloaded from BioGRID (RHOC (RP11-426L16.4) Result Summary | BioGRID (thebiogrid.org)) without any changes or additions. Permission is granted, free of charge, from TyersLab.com (https://doi.org/10.1002/pro.3978). b, The data was created and downloaded from STRING (RHOC protein (human) - STRING interaction network (string-db.org)) under ‘Creative Commons BY 4.0’ license, without any changes or additions (https://doi.org/10.1093/nar/gky1131). c, A schematic map of RhoC interaction with ARHGEF1. d, The standard GDP and GTP for HPLC. e, Molecular docking shows the bind sites of RhoC with BCKA, based on 3MTG (RSCB protein data bank, https://www.rcsb.org). The complex of BCAT1 and RhoC is generated by ZDOCK Server (https://zdock.umassmed.edu/). The enzyme activity domain is proximal to RhoC and affects its activity. f, BCAT1 mainly binds with the dominant active form of RhoC. WT, wild-type, DA, dominant active, DN, dominant negative, NF, nucleotide-free mutants of RhoC. g, BCAT1 pulls down with diminishes RhoC activity by using RBD-GST beads. h, i, BCAT1 depends on ARHGEFs to activate RhoC. g, Knockdown ARHGEF1 or ARHGEF11.Representative result from 3 independent experiments. i, The combined knockdown of GEFs genes of ARHGEF1, ARHGEF2, ARHGEF11, ARHGEF12 (LARG), ARHGEF17 (ECT2), ARHGEF27 (NGEF), ARHGEF28 (p190), and ARHGEF31 abolishes RhoC activity. f-i, Representative result from 3 independent experiments.
Extended Data Fig. 8 Candesartan inhibits the catalytic activity of BCAT1E61A.
a, Candidate is the potential inhibitor of BCAT1E61A by screening FDA proved drug library. b, The enzyme activity curve of BCAT1E61A by inhibitors treatment. c, Model of candesartan binding to BCAT1E61A from SWlSS MODEL. The BCAT1E61A data was created and downloaded from SWISS-MODEL (BCAT1E61A | Models (expasy.org)) under the CC BY-SA 4.0 (Creative Commons Attribution-ShareAlike 4.0) International License, without any changes or additions (https://doi.org/10.1093/nar/gky427). d, Candesartan has no effect on the protein level of BCAT1. e, Candesartan inhibits the activity of RhoC in a dose-dependent manner. f, Candesartan inhibits the activity of RhoC in BCAT1WT and BCAT1E61A overexpression cell lines. The cells were treated with Candesartan (250 µM) for 24 h. d-f, Representative result from 3 independent experiments. g, Candesartan inhibits the peritoneal metastasis in RhoC-dependent way while no effect on mice body weight as indicated. N = 5 biologically independent animals, mean ± SD. One or two-way ANOVA.
Extended Data Fig. 9 Phenotype of Bcat1E61A knock-in mouse.
a, Bcat1E61A mice are thinner than Bcat1WT mice at the age of 9 months. Representative pictures and body weight statistics of Bcat1WT mice and Bcat1E61A knock-in mice. Scale bar = 1 cm. N = 6 biologically independent animals, mean ± SD. One or two-way ANOVA. b–h, Representative H&E staining of multiple organs from Bcat1WT and Bcat1E61A knock-in mice at the age of 4 weeks as indicated. Scale bar = 50 µm. i, j, Representative Ki-67 staining and statistical analysis of lung, liver and stomach tissues from Bcat1WT and Bcat1E61A knock-in mice at the age of 4 weeks. Scale bar = 50 µm. b-j, N = 3 biologically independent samples, mean ± SD. Two-tailed t-test.
Extended Data Fig. 10 Bcat1E61A promotes tumorigenesis and development.
a–c, The body weight, food intake and water intake of gastric cancer. N = 14 biologically independent animals in Bcat1WT group. N = 37 biologically independent animals in Bcat1WT/KI group. N = 26 biologically independent animals in Bcat1KI/KI group. Mean ± SD. Two-tailed t-test. d, e. The TCA cycle and fat acid profiles of serum from gastric cancer mice. f, Representative workflow of KP lung tumor formation. g. Representative H&E staining of lung tissue from lung cancer mice. Mutation promotes the lung adenocarcinoma development. h, Representative workflow of DEN/CCl4 induced liver tumor formation as indicated for Bcat1WT and Bcat1KI/KI knock-in mice. i. Representative mice and organs. j-l. Detailed statistics of liver tumors. N = 10, biologically independent animals. Mean ± SD, two-tailed t-test for (k) and (i). m. The H&E staining and Ki-67 staining of liver cancer. n, A hepatic carcinoma at advanced stage was found in a 15-months old male Bcat1KI/KI knock-in mouse without chemical induced. Representative images of characterization a spontaneous hepatic carcinoma from Bcat1WT and Bcat1KI/KI knock-in mice. o, p, Representative H&E (o) and Ki-67 staining (p) of liver tissues from Bcat1WT and Bcat1KI/KI at the age of 15 months. g, m, o, p, Representative result from 3 independent experiments.
Supplementary information
Supplementary Table 1
Gene screening.
Supplementary Table 2
Clinical sample.
Supplementary Table 3
WES mutation.
Supplementary Table 4
IP–MS.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Unprocessed western blots and Statistical Source Data.
Source Data Fig. 4
Unprocessed western blots and Statistical Source Data.
Source Data Fig. 5
Unprocessed western blots and Statistical Source Data.
Source Data Fig. 6
Unprocessed western blots and Statistical Source Data.
Source Data Fig. 7
Unprocessed western blots and Statistical Source Data.
Source Data Extended Data Fig. 3
Unprocessed western blots and Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Unprocessed western blots and Statistical Source Data.
Source Data Extended Data Fig. 6
Unprocessed western blots and Statistical Source Data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots and Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qian, L., Li, N., Lu, XC. et al. Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression. Nat Metab 5, 1159–1173 (2023). https://doi.org/10.1038/s42255-023-00818-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00818-7
This article is cited by
-
HuR promotes castration-resistant prostate cancer progression by altering ERK5 activation via posttranscriptional regulation of BCAT1
Journal of Translational Medicine (2024)