Abstract
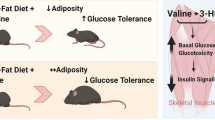
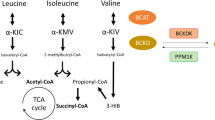
Elevated levels of plasma branched-chain amino acids (BCAAs) have been associated with insulin resistance and type 2 diabetes since the 1960s. Pharmacological activation of branched-chain α-ketoacid dehydrogenase (BCKDH), the rate-limiting enzyme of BCAA oxidation, lowers plasma BCAAs and improves insulin sensitivity. Here we show that modulation of BCKDH in skeletal muscle, but not liver, affects fasting plasma BCAAs in male mice. However, despite lowering BCAAs, increased BCAA oxidation in skeletal muscle does not improve insulin sensitivity. Our data indicate that skeletal muscle controls plasma BCAAs, that lowering fasting plasma BCAAs is insufficient to improve insulin sensitivity and that neither skeletal muscle nor liver account for the improved insulin sensitivity seen with pharmacological activation of BCKDH. These findings suggest potential concerted contributions of multiple tissues in the modulation of BCAA metabolism to alter insulin sensitivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report (CDC, 2020).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 281, 785–789 (1963).
McGarry, J. D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258, 766–770 (1992).
Corkey, B. E. Banting Lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61, 4–13 (2012).
Shulman, G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 371, 2236–2238 (2014).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083 (2014).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223 (2018).
Fazakerley, D. J., Krycer, J. R., Kearney, A. L., Hocking, S. L. & James, D. E. Muscle and adipose tissue insulin resistance: malady without mechanism? J. Lipid Res. 60, 1720–1732 (2019).
Davis, T. A., Fiorotto, M. L. & Reeds, P. J. Amino acid compositions of body and milk protein change during the suckling period in rats. J. Nutr. 123, 947–956 (1993).
Moura, A., Savageau, M. A. & Alves, R. Relative amino acid composition signatures of organisms and environments. PLoS ONE 8, e77319 (2013).
Schweigert, B. S., Bennett, B. A. & Guthneck, B. T. Amino acid composition of organ meats. J. Food Sci. 19, 219–223 (1954).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Ichihara, A. Isozyme patterns of branched-chain amino acid transaminase during cellular differentiation and carcinogenesis. Ann. N. Y. Acad. Sci. 259, 347–354 (1975).
Goto, M., Shinno, H. & Ichihara, A. Isozyme patterns of branched-chain amino acid transaminase in human tissues and tumors. Gan 68, 663–667 (1977).
Ichihara, A. & Koyama, E. Transaminase of branched-chain amino acids. J. Biochem. 59, 160–169 (1966).
Johnson, W. A. & Connelly, J. L. Cellular localization and characterization of bovine liver branched-chain α-keto acid dehydrogenases. Biochemistry 11, 1967–1973 (1972).
Aevarsson, A., Seger, K., Turley, S., Sokatch, J. R. & Hol, W. G. Crystal structure of 2-oxoisovalerate and dehydrogenase and the architecture of 2-oxo acid dehydrogenase multienzyme complexes. Nat. Struct. Biol. 6, 785–792 (1999).
Ævarsson, A. et al. Crystal structure of human branched-chain α-ketoacid dehydrogenase and the molecular basis of multienzyme complex deficiency in maple syrup urine disease. Structure 8, 277–291 (2000).
Harris, R. A. et al. Purification, characterization, regulation and molecular cloning of mitochondrial protein kinases. Adv. Enzyme Regul. 32, 267–284 (1992).
Damuni, Z. & Reed, L. J. Purification and properties of the catalytic subunit of the branched-chain α-keto acid dehydrogenase phosphatase from bovine kidney mitochondria. J. Biol. Chem. 262, 5129–5132 (1987).
Lu, G. et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Invest. 119, 1678–1687 (2009).
Neinast, M., Murashige, D. & Arany, Z. Branched-chain amino acids. Annu. Rev. Physiol. 81, 139–164 (2019).
Adibi, S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J. Appl. Physiol. 25, 52–57 (1968).
Felig, P., Marliss, E. & Cahill, G. F. Jr Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 281, 811–816 (1969).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Würtz, P. et al. Branched-chain and aromatic amino aids are predictors of insulin resistance in young adults. Diabetes Care 36, 648–655 (2013).
Guasch-Ferré, M. et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Lotta, L. A. et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. 13, e1002179 (2016).
Krebs, M. et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 51, 599–605 (2002).
Krebs, M. et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans. Diabetologia 46, 917–925 (2003).
Tremblay, F. et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54, 2674–2684 (2005).
Harris, L.-A. L. S. et al. Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Diabetes 66, 1871–1878 (2017).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530 (2016).
White, P. J. et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 5, 538–551 (2016).
Neinast, M. D. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 29, 417–429 (2019).
White, P. J. et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 27, 1281–1293 (2018).
Zhou, M. et al. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 68, 1730–1746 (2019).
Tso, S.-C. et al. Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain α-ketoacid dehydrogenase kinase. J. Biol. Chem. 289, 20583–20593 (2014).
Vanweert, F. et al. A randomized placebo-controlled clinical trial for pharmacological activation of BCAA catabolism in patients with type 2 diabetes. Nat. Commun. 13, 3508 (2022).
Blair, M. C., Neinast, M. D. & Arany, Z. Whole-body metabolic fate of branched-chain amino acids. Biochem. J. 478, 765–776 (2021).
Coleman, D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148 (1978).
Savage, D. B., Petersen, K. F. & Shulman, G. I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 87, 507–520 (2007).
Brøns, C. & Vaag, A. Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes. J. Physiol. 587, 3977–3978 (2009).
Jang, C. et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 22, 421–426 (2016).
White, P. J. et al. Muscle-liver trafficking of BCAA-derived nitrogen underlies obesity-related glycine depletion. Cell Rep. 33, 108375 (2020).
Shimomura, Y. et al. Branched-chain amino acid catabolism in exercise and liver disease. J. Nutr. 136, 250S–253S (2006).
Cummings, N. E. et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 596, 623–645 (2018).
Ma, Q.-X. et al. BCAA–BCKA axis regulates WAT browning through acetylation of PRDM16. Nat. Metab. 4, 106–122 (2022).
Schadewaldt, P., Bodner-Leidecker, A., Hammen, H. W. & Wendel, U. Significance of L-alloisoleucine in plasma for diagnosis of maple syrup urine disease. Clin. Chem. 45, 1734–1740 (1999).
Podebrad, F. et al. 4,5-Dimethyl-3-hydroxy-2[5H]-furanone (sotolone)—the odour of maple syrup urine disease. J. Inherit. Metab. Dis. 22, 107–114 (1999).
Sewell, A. C., Mosandl, A. & Böhles, H. False diagnosis of maple syrup urine disease owing to ingestion of herbal tea. N. Engl. J. Med. 341, 769 (1999).
Yudkoff, M. Brain metabolism of branched-chain amino acids. Glia 21, 92–98 (1997).
Yudkoff, M. et al. [15 N] leucine as a source of [15 N] glutamate in organotypic cerebellar explants. Biochem. Biophys. Res. Commun. 115, 174–179 (1983).
Gambello, M. J. & Li, H. Current strategies for the treatment of inborn errors of metabolism. J. Genet. Genomics 45, 61–70 (2018).
Newgard, C. B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 (2012).
White, P. J. et al. Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol. Metab. 52, 101261 (2021).
Herman, M. A., She, P., Peroni, O. D., Lynch, C. J. & Kahn, B. B. Adipose tissue branched-chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 285, 11348–11356 (2010).
Wallace, M. et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 14, 1021–1031 (2018).
Sugiyama, M. G. & Agellon, L. B. Sex differences in lipid metabolism and metabolic disease risk. Biochem. Cell Biol. 90, 124–141 (2012).
Shi, H., Seeley, R. J. & Clegg, D. J. Sexual differences in the control of energy homeostasis. Front. Neuroendocrinol. 30, 396–404 (2009).
Grove, K. L., Fried, S. K., Greenberg, A. S., Xiao, X. Q. & Clegg, D. J. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int. J. Obes. 34, 989–1000 (2010).
Stubbins, R. E., Holcomb, V. B., Hong, J. & Núñez, N. P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 51, 861–870 (2012).
Yang, Y., Smith, D. L., Keating, K. D., Allison, D. B. & Nagy, T. R. Variations in body weight, food intake and body composition after long-term high-fat-diet feeding in C57BL/6J mice. Obesity 22, 2147–2155 (2014).
Dorfman, M. D. et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 8, 14556 (2017).
Benz, V. et al. Sexual dimorphic regulation of body weight dynamics and adipose tissue lipolysis. PLoS ONE 7, e37794 (2012).
Gosis, B. S. et al. Inhibition of nonalcoholic fatty liver disease in mice by selective inhibition of mTORC1. Science 376, eabf8271 (2022).
Bollinger, E. et al. BDK inhibition acts as a catabolic switch to mimic fasting and improve metabolism in mice. Mol. Metab. 66, 101611 (2022).
Ibrahim, A. et al. Insulin-stimulated adipocytes secrete lactate to promote endothelial fatty acid uptake and transport. J. Cell Sci. 135, jcs258964 (2022).
Zeng, X. et al. Gut bacterial nutrient preferences quantified in vivo. Cell 185, 3441–3456 (2022).
Murashige, D. et al. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab. 34, 1749–1764 (2022).
Nakai, N., Kobayashi, R., Popov, K. M., Harris, R. A. & Shimomura, Y. Determination of branched-chain α-keto acid dehydrogenase activity state and branched-chain α-keto acid dehydrogenase kinase activity and protein in mammalian tissues. Methods Enzymol. 324, 48–62 (2000).
Webb, L. A. et al. Changes in tissue abundance and activity of enzymes related to branched-chain amino acid catabolism in dairy cows during early lactation. J. Dairy Sci. 102, 3556–3568 (2019).
Acknowledgements
We thank R. Miller and K. Filipski for their feedback. M.C.B. was supported by a National Institutes of Health (NIH) grant no. F31DK132839. Z.A. was supported by NIH grant nos. DK114103 and CA248315. This work was also supported in part by Pfizer. We also thank the Penn Vector Core at the University of Pennsylvania for producing the custom AAV8 vectors, the Comparative Pathology Core at the University of Pennsylvania School of Veterinary Medicine for preparing and staining the mouse muscle slides, and J. Davis at the Rodent Metabolic Phenotyping Core, supported in part by Penn Diabetes Research Center grant nos. P30-DK19525 and S10-OD025098, for the lean mass body weight measurements. Metabolic studies were in part supported by NIH Diabetes Research Center grant no. P30 DK019525.
Author information
Authors and Affiliations
Contributions
M.C.B. and Z.A. designed the project. M.C.B. generated most of the data, analysed all the data, created the figures and wrote the text. M.D.N. generated most of the LC–MS data, assisted in the generation of data for some of the figure panels and contributed substantially to the early stages of project design. C.J. generated some of the LC–MS data. Q.C. performed all catheterization surgeries. J.W.J. designed, optimized and assisted in performing the BCKDH activity assays. J.A., M.R.B., C.T. and K.L. each assisted with data collection. A.H. generated the BckdkloxP/loxP animals. S.Y. generated the DbtloxP/loxP animals. R.J.R.F. and B.B.Z. gave support and feedback throughout the data generation process. J.D.R. assisted, in collaboration with M.D.N. and C.J., with the use of the LC–MS instruments. Z.A. contributed substantial support to the project with regular feedback and idea generation, as well as editing the figures and text.
Corresponding author
Ethics declarations
Competing interests
R.J.R.F. and B.B.Z. are employees of Pfizer. This work was in part supported by Pfizer. The other authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Yibin Wang, Owen McGuinness and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Isabella Samuelson, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 BT2 treatment, acutely or chronically, does not affect body weight.
a–c, Western quantification of the ratio p-BCKDH/BCKDH in fasted tissues; n = 6 vehicle- and n = 6 BT2-treated. d, Plasma BCAA concentration in HFD-fed mice fasted for 5-hr or re-fed for 2-hr after an overnight fast; n = 10 vehicle- and n = 10 BT2-treated. Mice were gavaged with 50 mg/kg/day of either vehicle or BT2 for 4 weeks prior to plasma collection. e, Body weights of mice used for acute BT2 experiment; n = 10 vehicle- and n = 10 BT2-treated. f, Body weights of mice used for chronic BT2 experiment; n = 10 vehicle- and n = 10 BT2-treated. g, Body weights of mice used for chronic BT2 GTT (at treatment day 28); n = 10 vehicle- and n = 10 BT2-treated. Mice used for these experiments were male C57BL/6 J diet-induced obesity (DIO) mice ordered from the Jackson Laboratory. They were age 10–12 weeks and on HFD for 4–6 weeks. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: ***p < 0.001. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: a, p = 0.000097. c, p = 0.000556. d, valine p = 0.000002, leucine p = 0.000014, isoleucine p = 0.000557.
Extended Data Fig. 2 BCKDK SM knockout is specific and does not affect body weight or muscle physiology on chow diet.
a–d, Gene expression data via qPCR of Bckdk, Bckdk exon 6 & 7 (deleted region), Bckdha, and Bckdhb, respectively; n = 3 Bckdkl/l (WT) and n = 5 Bckdkl/l-HSA-CreER (Cre). e, Body weights; n = 8 WT and n = 8 Cre. f-j, Western quantification of BCKDK in fasted tissues normalized to loading control; n = 3 WT and n = 3 Cre. k-o, Western quantification of the ratio pBCKDH/BCKDH in fasted tissues; n = 3 WT and n = 3 Cre. p, Western blotting for p-ACLY and ACLY in fasted livers; n = 6 WT and n = 6 Cre. 14-3-3 is the loading control. q, Western quantification of the ratio p-ACLY/ACLY from the blot in p. r, H&E staining of tibialis anterior (TA) muscle. Representative images from n = 4 WT and n = 3 Cre mice. s, Ratio of 3-HIB/valine, and t, Concentration of 3-HIB in the plasma from mice fasted for 5-hr (n = 10 WT and n = 8 Cre) or re-fed for 2-hr after an overnight fast (n = 10 WT and n = 8 Cre). Mice used for these experiments were male, aged 8–10 weeks and fed normal chow. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05, **p < 0.01, ***p < 0.001. Exact p-values: a, p = 0.000060. b, p = 0.000020. g, p = 0.008871. h, p = 0.031431. l, p = 0.039537. m, p = 0.015917. s, fasted p = 0.002253, re-fed p = 0.029746. t, re-fed p = 0.023789.
Extended Data Fig. 3 Increased SM BCAA oxidation is specific in a fasted state and does not affect body weight or glucose tolerance on Western diet.
a, Body weights of mice; n = 8 Bckdkl/l (WT) and n = 9 Bckdkl/l-HSA-CreER (Cre). b, Plasma BCAA levels, c, Ratio of 3-HIB/valine, and d, Relative amount of 3-HIB in mice after a 5-hr fast (fasted) and in a fed state collected at 10 pm (fed); n = 12 WT and n = 17 Cre. e, Fasting plasma pool size of various amino acids, and f, Steady-state plasma labeling of BCAAs by U13C-BCAAs (fraction) during steady-state infusion of U13C-BCAAs before (basal, 5-hr fasted) and during (clamp, 7-hr fasted) HIEC in n = 6 WT and n = 6 Cre mice. g, Normalized labeling of TCA cycle intermediates by U13C-BCAAs in liver, h, iBAT, and i, gWAT at the end of the infusion and HIEC (7-hr fasted); n = 6 WT and n = 6 Cre. j, BCKDH complex activity measured in fasted quad samples fed HFD for 4 weeks; n = 4 WT and n = 3 Cre. k, 2 g/kg glucose IP-GTT in 5-hr fasted mice fed Western diet for 5 weeks; n = 8 WT and n = 9 Cre. l, Area-under-the-curve (AUC) for the GTT in k. Mice used in these experiments were male, aged 18–24 weeks and fed Western diet for 5–12 weeks. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05, **p < 0.01, ***p < 0.001. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: b, valine fasted p < 0.000001, leucine fasted p < 0.000001, isoleucine fasted p < 0.000001, isoleucine fed p = 0.029124. c, fasted p = 0.013891. f, valine basal p = 0.030476, isoleucine basal p = 0.046680. g, glutamate p = 0.004926. h, malate p = 0.034456. I, succinate p = 0.039056.
Extended Data Fig. 4 Increased SM BCAA oxidation is specific in a fed state and does not affect body weight or glucose tolerance on HFD.
a, Ratio of 3-HIB/valine, and b, Concentration of 3-HIB in mice fasted for 5-hr or re-fed for 2-hr after an overnight fast; n = 10 Bckdkl/l (WT) and n = 8 Bckdkl/l-HSA-CreER (Cre). c, 1.5 g/kg IP-GTT in 5-hr fasted mice; n = 11 WT and n = 14 Cre. d, Area-under-the-curve (AUC) for GTT in c. e, Insulin concentration at t = 0 and t = 20 min (n = 11 WT and n = 14 Cre) during GTT from c. f, Weights; n = 10 WT and n = 8 Cre. g, Lean mass; n = 7 WT and n = 9 Cre. h, Weights; n = 11 WT and n = 14 Cre. i-k, Quantification of BCKDK in re-fed tissues normalized to loading control. l-n Quantification of the ratio pBCKDH/BCKDH in re-fed tissues. o, Western blotting for p-ACLY&ACLY in re-fed livers. 14-3-3 is the loading control. p, Quantification of the ratio p-ACLY/ACLY. n = 6 WT and n = 6 Cre for i-p. q, Labeling fraction of BCAAs in plasma before (t = 0) and 10 min after gavage (t = 10) of U13C-BCAAs in mice re-fed for 2-hr, and labeling fraction of TCA cycle intermediates 10 min after gavage of U13C-BCAAs in re-fed r, liver, s, iBAT, and t, gWAT; n = 8 WT and n = 8 Cre. u, BCKDH activity in re-fed quads; n = 4 WT and n = 3 Cre. v, Plasma BCKAs in Bckdkl/l-HSA-CreER mice treated with 100 mg/kg of vehicle (Veh; n = 6) or BT2 (n = 7) the night before and morning of collection. w, Weights of Bckdkl/l-HSA-CreER mice treated with Veh (n = 6) or BT2 (n = 7). Mice in a, b, & f were male, aged 14–24 weeks and fed HFD for 4–5 weeks. Mice in c-e, & h were male, aged 20–26 weeks and fed HFD for 10 weeks. Mice in g & l-u were male, aged 16–17 weeks and fed HFD for 4 weeks. Mice in v & w were male, aged 12–20 and fed HFD for 4 weeks. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05, **p < 0.01, ***p < 0.001. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: a, fasted p = 0.024668. j, p = 0.000013. m, p = 0.000017. v, KIC/KMV p = 0.008925.
Extended Data Fig. 5 SM DBT knockout is specific and does not affect body weight, muscle physiology, or glucose tolerance on chow diet.
a-d, Gene expression data via qPCR of Dbt, Dbt exon 6 (deleted region), Bckdha, and Bckdhb, respectively; n = 3 Dbtl/l (WT) and n = 5 Dbtl/l-HSA-CreER (Cre). e-g, Western quantification of DBT in fasted tissues normalized to loading control; n = 3 WT and n = 3 Cre. h, H&E staining of tibialis anterior (TA) muscle. Representative images from n = 3 WT and n = 3 Cre. I, Ratio of 3-HIB/valine, and j, Concentration of 3-HIB from mice fasted for 5-hr (n = 10 WT and n = 8 Cre) or re-fed for 2-hr after an overnight fast (n = 11 WT and n = 9 Cre). k, Body weights; n = 10 WT and n = 8 Cre. l, 2 g/kg glucose IP-GTT in 5-hr fasted mice; n = 10 WT and n = 10 Cre. m, Area-under-the-curve (AUC) for the GTT in l. n, Insulin concentration at t = 0 and t = 20 min (n = 10 WT and n = 7 Cre) during GTT from l. Mice used in these experiments were male, aged 10–19 weeks fed normal chow. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05 and ***p < 0.001. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: a, p = 0.039254. b, p = 0.043912. f, p = 0.000749. g, p = 0.011125.
Extended Data Fig. 6 Decreased SM BCAA oxidation is specific and does not affect body weight or glucose tolerance on HFD.
a, Plasma labeling of BCAAs after U13C-BCAAs gavage (fraction) in 5-hr fasted mice; n = 8 Dbtl/l (WT) and n = 8 Dbtl/l-HSA-CreER (Cre). b, BCKDH complex activity measured in fasted quads from n = 4 WT and n = 4 Cre mice. Labeling fraction of TCA cycle intermediates 10 min after gavage of U13C-BCAAs in c, liver d, iBAT, & e, gWAT in 5-hr fasted mice; n = 8 WT and n = 8 Cre. f, Body weights; n = 10 WT and n = 14 Cre. g, Lean mass; n = 7 WT and n = 13 Cre. Fasting plasma pool size of various amino acids during steady-state infusion of U13C-BCAAs before (basal, 5-hr fasted) and during (clamp, 7-hr fasted) HIEC in n = 6 WT and n = 6 Cre mice. h, Plasma BCAA concentration in 5-hr fasted or 2-hr re-fed after an overnight fast in mice; n = 8 WT and n = 10 Cre. i, Steady-state plasma labeling of BCAAs by infusion of U13C-BCAAs before (basal) and during (clamp) HIEC; n = 6 WT and n = 6 Cre mice fed. j, 1.5 g/kg glucose IP-GTT in 5-hr fasted mice fed HFD for 4 weeks; n = 8 WT and n = 9 Cre. k, Area-under-the-curve (AUC) for the GTT in j. l, Insulin concentration at t = 0 and t = 20 min (n = 8 WT and n = 9 Cre) during the GTT from j. m, Plasma BCAA concentration in 5-hr fasted mice before (t = 0), n = 8 WT and n = 9 Cre, and 20 min after (t = 20), n = 6 WT and n = 7 Cre, a gavage of BCAAs. Mice used in these experiments were male, aged 10–20 weeks fed HFD for 4–8 weeks. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures.
Extended Data Fig. 7 Liver BCKDK knockout does not affect body weight or glucose tolerance at various time points on HFD or after a BCAA challenge.
a-c, Western quantification of BCKDK in tissues normalized to loading control; n = 3 Bckdkl/l-AAV8:GFP (GFP) and n = 3 Bckdkl/l-AAV8:Cre (Cre). d-f, Western quantification of the ratio pBCKDH/BCKDH; n = 3 GFP and n = 3 Cre. g, Body weights of n = 9 GFP and n = 10 Cre mice on HFD. h, Plasma labeling of BCAAs after U13C-BCAAs gavage (fraction) 5-hr fasted mice; n = 4 GFP and n = 5 Cre. Labeling fraction of TCA cycle intermediates 10 min post U13C-BCAA gavage in i, quad j, iBAT, & k, gWAT in 5-hr fasted mice; n = 4 GFP and n = 5 Cre. l, BCKDH complex activity assay measured in fasted livers from n = 4 GFP and n = 3 Cre mice. m, 1.5 g/kg glucose IP-GTT in 5-hr fasted mice fed HFD after a BCAA gavage given at t = 0; n = 8 GFP and n = 10 Cre. n, Area-under-the-curve (AUC) for the GTT in m. o, Plasma BCAA concentration of 5-hr fasted plasma before (t = 0) (n = 8 GFP and n = 9 Cre) and 20 min after (t = 20) (n = 8 GFP and n = 10 Cre) a BCAA gavage from GTT + BCAA gavage in m. Mice used in these experiments were male, aged 8–26 weeks and on HFD for 2–12 weeks. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05 and **p < 0.01. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: a, p = 0.029151. d, p = 0.005367.
Extended Data Fig. 8 Liver DBT knockout does not affect body weight or glucose tolerance on multiple diets or after a BCAA challenge.
a-c, Western quantification of DBT in tissues normalized to loading control; n = 3 Dbtl/l-AAV8:GFP (GFP) and n = 3 Dbtl/l-AAV8:Cre (Cre). d, Body weights of n = 10 GFP and n = 10 Cre mice on chow and HFD. e, Plasma labeling of BCAAs after U13C-BCAA gavage (fraction); n = 4 GFP and n = 5 Cre. Labeling fraction of TCA cycle intermediates 10 min post U13C-BCAA gavage in f, quad g, iBAT, & h, gWAT in 5-hr fasted mice; n = 4 GFP and n = 5 Cre. i, BCKDH complex activity measured in fasted livers from n = 4 GFP and n = 4 Cre mice. j, 1.5 g/kg glucose IP-GTT in 5-hr fasted mice fed HFD for 4 weeks after a BCAA gavage given at t = 0; n = 8 GFP and n = 9 Cre. k, Area-under-the-curve (AUC) for the GTT in j. l, Insulin concentration at t = 0 and t = 20 min (n = 8 GFP and n = 9 Cre) during GTT from j. m, Plasma BCAA concentration in 5-hr fasted mice before (t = 0) and 20 min after (t = 20) a gavage of BCAAs from GTT + BCAA gavage in j; n = 8 GFP and n = 9 Cre. Mice used in these experiments were male, aged 16–24 weeks and fed normal chow for 4–5 weeks, followed by HFD for 4–6 weeks. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: b, p = 0.025494. m, valine t = 0 p = 0.031669, leucine t = 0 p = 0.033539.
Extended Data Fig. 9 Double knockout of BCKDK in both SM and liver is specific in fasted and re-fed states.
a–d, Western quantification of BCKDK in fasted tissues normalized to loading control collected from n = 3 WT and n = 3 BCKDK double KO (Double KO) mice. e-h, Western quantification of the ratio pBCKDH/BCKDH in fasted tissues; n = 3 WT and n = 3 Double KO. i, Western blotting for p-ACLY and ACLY in fasted livers. 14-3-3 is the loading control; n = 6 WT and n = 6 Double KO. j, Western quantification of the ratio p-ACLY/ACLY from the blot in i. k, Body weights of n = 10 WT and n = 10 Double KO mice on HFD. l, Lean mass of n = 8 WT and n = 8 Double KO mice on HFD. m, Ratio of 3-HIB/valine, and n, Concentration of 3-HIB in plasma in mice fasted for 5-hr or re-fed for 2-hr after an overnight fast; n = 10 WT and n = 10 Double KO mice fed HFD. o-r, Western quantification of BCKDK in re-fed tissues normalized to loading control; n = 3 WT and n = 3 Double KO. s-v, Western quantification of the ratio pBCKDH/BCKDH in re-fed tissues; n = 3 WT and n = 3 Double KO. w, Western blotting for p-ACLY and ACLY in re-fed livers. 14-3-3 is the loading control; n = 6 WT and n = 6 Double KO. x, Western quantification of the ratio p-ACLY/ACLY from the blot in w. y, Body weights of n = 8 BCKDK double KO + vehicle (Veh)-treated and n = 8 BCKDK double KO + BT2-treated mice fed HFD. Mice used for these experiments were aged 13–23 weeks and fed HFD for 6–7 weeks. Data are presented as means ± SEM. Comparisons of two groups use two-tailed Student’s t-test with significance defined as: *p < 0.05 and ***p < 0.001. Experiments with multiple comparisons at different time-points use two-way ANOVA with repeated measures. Exact p-values: a, p = 0.023348. b, p = 0.047025. e, p = 0.044708. f, p = 0.000955. m, fasted p = 0.018524. o, p = 0.000230. s, p = 0.044860. u, p = 0.031191.
Supplementary information
Source data
Source Data for Figs. 1–4 and 6–8.
Unprocessed western blots.
Source Data for Extended Data Figs. 2, 4 and 9.
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blair, M.C., Neinast, M.D., Jang, C. et al. Branched-chain amino acid catabolism in muscle affects systemic BCAA levels but not insulin resistance. Nat Metab 5, 589–606 (2023). https://doi.org/10.1038/s42255-023-00794-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00794-y