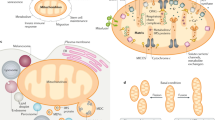
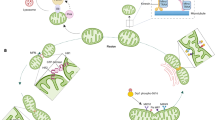
Abstract
Mitochondria have cell-type specific phenotypes, perform dozens of interconnected functions and undergo dynamic and often reversible physiological recalibrations. Given their multifunctional and malleable nature, the frequently used terms ‘mitochondrial function’ and ‘mitochondrial dysfunction’ are misleading misnomers that fail to capture the complexity of mitochondrial biology. To increase the conceptual and experimental specificity in mitochondrial science, we propose a terminology system that distinguishes between (1) cell-dependent properties, (2) molecular features, (3) activities, (4) functions and (5) behaviours. A hierarchical terminology system that accurately captures the multifaceted nature of mitochondria will achieve three important outcomes. It will convey a more holistic picture of mitochondria as we teach the next generations of mitochondrial biologists, maximize progress in the rapidly expanding field of mitochondrial science, and also facilitate synergy with other disciplines. Improving specificity in the language around mitochondrial science is a step towards refining our understanding of the mechanisms by which this unique family of organelles contributes to cellular and organismal health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marieb, E. & Hoehn, K. Essentials of Human Anatomy & Physiology (Pearson Education, 2022).
Picard, M. & Shirihai, O. S. Mitochondrial signal transduction. Cell Metab. 34, 1620–1653 (2022).
Siekevitz, P. Powerhouse of the Cell. Sci. Am. 197, 131–144 (1957).
Harper, M. E. & Patti, M. E. Metabolic terminology: what’s in a name? Nat. Metab. 2, 476–477 (2020).
Schmidt, C. A. Prescription drugs and mitochondrial metabolism. Biosci. Rep. 42, BSR20211813 (2022).
Eisner, V., Picard, M. & Hajnoczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20, 755–765 (2018).
Brand, M. D. & Nicholls, D. G. Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312 (2011).
Mracek, T., Drahota, Z. & Houstek, J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta 1827, 401–410 (2013).
Graves, S. M. et al. Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat. Neurosci. 23, 15–20 (2020).
Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim. Biophys. Acta 1807, 1507–1538 (2011).
Wiedemann, N. & Pfanner, N. Mitochondrial machineries for protein import and assembly. Annu. Rev. Biochem. 86, 685–714 (2017).
Braymer, J. J. & Lill, R. Iron–sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 292, 12754–12763 (2017).
Hinton, T. V., Batelu, S., Gleason, N. & Stemmler, T. L. Molecular characteristics of proteins within the mitochondrial Fe–S cluster assembly complex. Micron 153, 103181 (2022).
Read, A. D., Bentley, R. E., Archer, S. L. & Dunham-Snary, K. J. Mitochondrial iron–sulfur clusters: structure, function, and an emerging role in vascular biology. Redox Biol. 47, 102164 (2021).
Rydstrom, J. Mitochondrial NADPH, transhydrogenase and disease. Biochim. Biophys. Acta 1757, 721–726 (2006).
Bahat, A., MacVicar, T. & Langer, T. Metabolism and innate immunity meet at the mitochondria. Front. Cell Dev. Biol. 9, 720490 (2021).
Elesela, S. & Lukacs, N. W. Role of mitochondria in viral infections. Life. 11, 232 (2021).
McWhirter, S. M., Tenoever, B. R. & Maniatis, T. Connecting mitochondria and innate immunity. Cell 122, 645–647 (2005).
Moore, C. B. & Ting, J. P. Regulation of mitochondrial antiviral signaling pathways. Immunity 28, 735–739 (2008).
Walker, J. E. The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans. 41, 1–16 (2013).
Bernardi, P., Carraro, M. & Lippe, G. The mitochondrial permeability transition: recent progress and open questions. FEBS J. 289, 7051–7074 (2021).
MacVicar, T. & Langer, T. OPA1 processing in cell death and disease—the long and short of it. J. Cell Sci. 129, 2297–2306 (2016).
Roger, A. J., Munoz-Gomez, S. A. & Kamikawa, R. The origin and diversification of mitochondria. Curr. Biol. 27, R1177–R1192 (2017).
Lill, R., Fekete, Z., Sipos, K. & Rotte, C. Is there an answer? Why are mitochondria essential for life? IUBMB Life 57, 701–703 (2005).
Inigo, M., Deja, S. & Burgess, S. C. Ins and outs of the TCA cycle: the central role of anaplerosis. Annu. Rev. Nutr. 41, 19–47 (2021).
Oates, E. H. & Antoniewicz, M. R. Coordinated reprogramming of metabolism and cell function in adipocytes from proliferation to differentiation. Metab. Eng. 69, 221–230 (2022).
Spinelli, J. B. & Haigis, M. C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754 (2018).
Chandel, N. S. Mitochondria as signaling organelles. BMC Biol. 12, 34 (2014).
Shen, K. et al. Mitochondria as cellular and organismal signaling hubs. Annu. Rev. Cell Dev. Biol. 38, 179–218 (2022).
Dai, Z., Ramesh, V. & Locasale, J. W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 21, 737–753 (2020).
Martinez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Murphy, M. P. & Chouchani, E. T. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol. 18, 461–469 (2022).
Bassi, G., Sidhu, S. K. & Mishra, S. The expanding role of mitochondria, autophagy and lipophagy in steroidogenesis. Cells 10, 1851 (2021).
Miller, W. L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 379, 62–73 (2013).
Beninca, C. et al. A new non-canonical pathway of Gαq protein regulating mitochondrial dynamics and bioenergetics. Cell. Signal. 26, 1135–1146 (2014).
Hebert-Chatelain, E. et al. A cannabinoid link between mitochondria and memory. Nature 539, 555–559 (2016).
Jimenez-Blasco, D. et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 583, 603–608 (2020).
Suofu, Y. et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl Acad. Sci. USA 114, E7997–E8006 (2017).
Torralba, D. et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 9, 2658 (2018).
Trumpff, C. et al. Stress and circulating cell-free mitochondrial DNA: a systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion 59, 225–245 (2021).
Marti Gutierrez, N. et al. Horizontal mtDNA transfer between cells is common during mouse development. iScience 25, 103901 (2022).
Brestoff, J. R. et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 33, 270–282 (2021).
Borcherding, N. et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 34, 1499–1513 (2022).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
Aharoni-Simon, M. et al. Oxidative stress facilitates exogenous mitochondria internalization and survival in retinal ganglion precursor-like cells. Sci. Rep. 12, 5122 (2022).
Nicolas-Avila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109 (2020).
Al Amir Dache, Z. et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. 34, 3616–3630 (2020).
Park, M. K., Lomax, R. B., Tepikin, A. V. & Petersen, O. H. Local uncaging of caged Ca2+ reveals distribution of Ca2+-activated Cl− channels in pancreatic acinar cells. Proc. Natl Acad. Sci. USA 98, 10948–10953 (2001).
Johnson, D. T., Harris, R. A., Blair, P. V. & Balaban, R. S. Functional consequences of mitochondrial proteome heterogeneity. Am. J. Physiol. Cell Physiol. 292, 698–707 (2007).
Johnson, D. T. et al. Tissue heterogeneity of the mammalian mitochondrial proteome. Am. J. Physiol. Cell. Physiol. 292, 689–697 (2007).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Morgenstern, M. et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metab. 33, 2464–2483 (2021).
Aryaman, J., Johnston, I. G. & Jones, N. S. Mitochondrial heterogeneity. Front. Genet. 9, 718 (2018).
Fecher, C. et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 22, 1731–1742 (2019).
Stewart, J. B. & Chinnery, P. F. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat. Rev. Genet. 16, 530–542 (2015).
Vincent, A. E. & Picard, M. Multilevel heterogeneity of mitochondrial respiratory chain deficiency. J. Pathol. 246, 261–265 (2018).
Pekkurnaz, G. & Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 4, 802–812 (2022).
Rausser, S. et al. Mitochondrial phenotypes in purified human immune cell subtypes and cell mixtures. eLife 10, e70899 (2021).
McLaughlin, K. L. et al. Novel approach to quantify mitochondrial content and intrinsic bioenergetic efficiency across organs. Sci. Rep. 10, 17599 (2020).
Lesner, N. P. et al. Differential requirements for mitochondrial electron transport chain components in the adult murine liver. eLife 11, e80919 (2022).
Jang, C. et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 30, 594–606 (2019).
De Kleer, I., Willems, F., Lambrecht, B. & Goriely, S. Ontogeny of myeloid cells. Front. Immunol. 5, 423 (2014).
Harridge, S. D. et al. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. 432, 913–920 (1996).
Picard, M., Hepple, R. T. & Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am. J. Physiol. Cell Physiol. 302, C629–641 (2012).
Picard, M., Ritchie, D., Thomas, M. M., Wright, K. J. & Hepple, R. T. Alterations in intrinsic mitochondrial function with aging are fiber-type-specific and do not explain differential atrophy between muscles. Aging Cell 10, 1047–1055 (2011).
Willingham, T. B., Ajayi, P. T. & Glancy, B. Subcellular specialization of mitochondrial form and function in skeletal muscle cells. Front. Cell Dev. Biol. 9, 757305 (2021).
Cogswell, A. M., Stevens, R. J. & Hood, D. A. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am. J. Physiol. 264, 383–389 (1993).
Koves, T. R. et al. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am. J. Physiol. Cell Physiol. 288, 1074–1082 (2005).
Ferreira, R. et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics 10, 3142–3154 (2010).
Benador, I. Y. et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885 (2018).
Benador, I. Y., Veliova, M., Liesa, M. & Shirihai, O. S. Mitochondria bound to lipid droplets: where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab. 29, 827–835 (2019).
Fawcett, D. W. The Cell Chapter 7: Mitochondria, 410–485 (W. B. Saunders Company, 1966).
Bulthuis, E. P., Adjobo-Hermans, M. J. W., Willems, P. & Koopman, W. J. H. Mitochondrial morphofunction in mammalian cells. Antioxid. Redox Signal. 30, 2066–2109 (2019).
Cogliati, S. et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155, 160–171 (2013).
Picard, M., Shirihai, O. S., Gentil, B. J. & Burelle, Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, 393–406 (2013).
Hong, X. et al. Mitochondrial dynamics maintains stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy Cell Stem Cell 29, 1298–1314 (2022).
Rambold, A. S., Kostelecky, B., Elia, N. & Lippincott-Schwartz, J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc. Natl Acad. Sci. USA 108, 10190–10195 (2011).
Liesa, M. & Shirihai, O. S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506 (2013).
Picard, M. & Turnbull, D. M. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62, 672–678 (2013).
Yu, T., Robotham, J. L. & Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl Acad. Sci. USA 103, 2653–2658 (2006).
Shenouda, S. M. et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124, 444–453 (2011).
Lewis, T. L. Jr., Kwon, S. K., Lee, A., Shaw, R. & Polleux, F. MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat. Commun. 9, 5008 (2018).
Rangaraju, V., Lauterbach, M. & Schuman, E. M. Spatially stable mitochondrial compartments fuel local translation during plasticity. Cell 176, 73–84 (2019).
Faitg, J. et al. 3D neuronal mitochondrial morphology in axons, dendrites, and somata of the aging mouse hippocampus. Cell Rep. 36, 109509 (2021).
Vincent, A. E. et al. Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep. 26, 996–1009(2019).
Zhang, R., Ogden, R. T., Picard, M. & Srivastava, A. Nonparametric k-sample test on shape spaces with applications to mitochondrial shape analysis. J. R. Stat. Soc. Ser. C Appl. Stat. 71, 51–69 (2022).
Justs, K. A. et al. Presynaptic mitochondrial volume and packing density scale with presynaptic power demand. J. Neurosci. 42, 954–967 (2022).
Meinild Lundby, A. K. et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta. Physiol. 222, e12976 (2018).
Wachsmuth, M., Hubner, A., Li, M., Madea, B. & Stoneking, M. Age-related and heteroplasmy-related variation in human mtDNA copy number. PLoS Genet. 12, e1005939 (2016).
Picard, M. Blood mitochondrial DNA copy number: what are we counting? Mitochondrion 60, 1–11 (2021).
Al-Mehdi, A. B. et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal 5, ra47 (2012).
Desai, R. et al. Mitochondria form contact sites with the nucleus to couple prosurvival retrograde response. Sci. Adv. 6, eabc9955 (2020).
Sun, T., Qiao, H., Pan, P. Y., Chen, Y. & Sheng, Z. H. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4, 413–419 (2013).
Segawa, M. et al. Quantification of cristae architecture reveals time-dependent characteristics of individual mitochondria. Life Sci. Alliance 3, e201900620 (2020).
Frazier, A. E., Vincent, A. E., Turnbull, D. M., Thorburn, D. R. & Taylor, R. W. Assessment of mitochondrial respiratory chain enzymes in cells and tissues. Methods Cell. Biol. 155, 121–156 (2020).
Bose, H. S., Lingappa, V. R. & Miller, W. L. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417, 87–91 (2002).
Friedman, J. R. & Nunnari, J. Mitochondrial form and function. Nature 505, 335–343 (2014).
Scarpulla, R. C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638 (2008).
Cagin, U. & Enriquez, J. A. The complex cross-talk between mitochondria and the nucleus: What goes in between. Int. J. Biochem. Cell Biol. 63, 10–15 (2015).
Giacomello, M., Pyakurel, A., Glytsou, C. & Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 (2020).
Kraus, F., Roy, K., Pucadyil, T. J. & Ryan, M. T. Function and regulation of the divisome for mitochondrial fission. Nature 590, 57–66 (2021).
Kleele, T. et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593, 435–439 (2021).
Zorova, L. D. et al. Mitochondrial membrane potential. Anal. Biochem. 552, 50–59 (2018).
Ramshesh, V. K. & Lemasters, J. J. Imaging of mitochondrial pH using SNARF-1. Methods Mol. Biol. 1782, 351–356 (2018).
Teodoro, J. S., Machado, I. F., Castela, A. C., Rolo, A. P. & Palmeira, C. M. The evaluation of mitochondrial membrane potential using fluorescent dyes or a membrane-permeable cation (TPP+) electrode in isolated mitochondria and intact cells. Methods Mol. Biol. 2184, 197–213 (2020).
Benevenga, N. J. & Blemings, K. P. Unique aspects of lysine nutrition and metabolism. J. Nutr. 137, 1610S–1615S (2007).
Borst, P. The malate–aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life 72, 2241–2259 (2020).
Ananieva, E. A. & Wilkinson, A. C. Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care 21, 64–70 (2018).
Kainulainen, H., Hulmi, J. J. & Kujala, U. M. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc. Sport Sci. Rev. 41, 194–200 (2013).
Ueland, P. M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 34, 3–15 (2011).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Blemings, K. P., Crenshaw, T. D. & Benevenga, N. J. Mitochondrial lysine uptake limits hepatic lysine oxidation in rats fed diets containing 5, 20 or 60% casein. J. Nutr. 128, 2427–2434 (1998).
Cardaci, S. et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol. 17, 1317–1326 (2015).
King, M. P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989).
LaNoue, K. F., Bryla, J. & Bassett, D. J. Energy-driven aspartate efflux from heart and liver mitochondria. J. Biol. Chem. 249, 7514–7521 (1974).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Goldberg, E. J. et al. Tissue-specific characterization of mitochondrial branched-chain keto acid oxidation using a multiplexed assay platform. Biochem. J. 476, 1521–1537 (2019).
Ko, J. H. et al. BCAT1 affects mitochondrial metabolism independently of leucine transamination in activated human macrophages. J. Cell Sci. 133, jcs247957 (2020).
Smirnoff, N. l-ascorbic acid biosynthesis. Vitam. Horm. 61, 241–266 (2001).
May, J. M., Li, L., Qu, Z. C. & Cobb, C. E. Mitochondrial recycling of ascorbic acid as a mechanism for regenerating cellular ascorbate. Biofactors 30, 35–48 (2007).
Li, X., Cobb, C. E. & May, J. M. Mitochondrial recycling of ascorbic acid from dehydroascorbic acid: dependence on the electron transport chain. Arch. Biochem. Biophys. 403, 103–110 (2002).
Dodgson, S. J., Forster, R. E. 2nd, Storey, B. T. & Mela, L. Mitochondrial carbonic anhydrase. Proc. Natl Acad. Sci. USA 77, 5562–5566 (1980).
Palty, R. et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA 107, 436–441 (2010).
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Greotti, E. & De Stefani, D. Biosensors for detection of calcium. Methods Cell. Biol. 155, 337–368 (2020).
De Nadai, A., Vajente, N., Pendin, D. & Mattarei, A. Mt-fura-2, a ratiometric mitochondria-targeted Ca2+ sensor. Determination of spectroscopic properties and Ca2+ imaging assays. Methods Mol. Biol. 2275, 187–215 (2021).
Cecchini, G. Complexities of complex II: sulfide metabolism in vivo. J. Biol. Chem. 298, 101661 (2022).
Kumar, R. et al. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem. 298, 101435 (2022).
Landry, A. P. et al. A catalytic trisulfide in human sulfide quinone oxidoreductase catalyzes coenzyme A persulfide synthesis and inhibits butyrate oxidation. Cell Chem. Biol. 26, 1515–1525 (2019).
Zhang, X. et al. Sulfide-quinone oxidoreductase is required for cysteine synthesis and indispensable to mitochondrial health. Redox Biol. 47, 102169 (2021).
Libiad, M., Yadav, P. K., Vitvitsky, V., Martinov, M. & Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 289, 30901–30910 (2014).
Bertholet, A. M. et al. Mitochondrial uncouplers induce proton leak by activating AAC and UCP1. Nature 606, 180–187 (2022).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019).
Kazak, L. et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015).
Nicholls, D. G. & Locke, R. M. Thermogenic mechanisms in brown fat. Physiol. Rev. 64, 1–64 (1984).
Cannon, B. & Nedergaard, J. Respiratory and thermogenic capacities of cells and mitochondria from brown and white adipose tissue. Methods Mol. Biol. 155, 295–303 (2001).
Williams, N. C. & O’Neill, L. A. J. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 9, 141 (2018).
O’Neill, L. A. J. & Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 19, 273–281 (2019).
Cordes, T. et al. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol. Metab. 32, 122–135 (2020).
Ghandour, M. S., Parkkila, A. K., Parkkila, S., Waheed, A. & Sly, W. S. Mitochondrial carbonic anhydrase in the nervous system: expression in neuronal and glial cells. J. Neurochem. 75, 2212–2220 (2000).
Vanlander, A. V. & Van Coster, R. Clinical and genetic aspects of defects in the mitochondrial iron–sulfur cluster synthesis pathway. J. Biol. Inorg. Chem. 23, 495–506 (2018).
Ball, J. M., Chen, S. & Li, W. Mitochondria in cone photoreceptors act as microlenses to enhance photon delivery and confer directional sensitivity to light. Sci. Adv. 8, eabn2070 (2022).
Houten, S. M., Violante, S., Ventura, F. V. & Wanders, R. J. The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and Its genetic disorders. Annu. Rev. Physiol. 78, 23–44 (2016).
Bennett, M. J., Sheng, F. & Saada, A. Biochemical assays of TCA cycle and beta-oxidation metabolites. Methods Cell. Biol. 155, 83–120 (2020).
Schlame, M. & Greenberg, M. L. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1862, 3–7 (2017).
Eiyama, A., Aaltonen, M. J., Nolte, H., Tatsuta, T. & Langer, T. Disturbed intramitochondrial phosphatidic acid transport impairs cellular stress signaling. J. Biol. Chem. 296, 100335 (2021).
Miliara, X. et al. Structural determinants of lipid specificity within Ups/PRELI lipid transfer proteins. Nat. Commun. 10, 1130 (2019).
Tatsuta, T. & Langer, T. Intramitochondrial phospholipid trafficking. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 1862, 81–89 (2017).
Lechuga-Vieco, A. V., Justo-Mendez, R. & Enriquez, J. A. Not all mitochondrial DNAs are made equal and the nucleus knows it. IUBMB Life 73, 511–529 (2021).
Rackham, O. & Filipovska, A. Organization and expression of the mammalian mitochondrial genome. Nat. Rev. Genet. 23, 606–623 (2022).
Fernandez-Silva, P., Acin-Perez, R., Fernandez-Vizarra, E., Perez-Martos, A. & Enriquez, J. A. In vivo and in organello analyses of mitochondrial translation. Methods Cell. Biol. 80, 571–588 (2007).
Fernandez-Vizarra, E. et al. Isolation of mitochondria for biogenetical studies: an update. Mitochondrion 10, 253–262 (2010).
Mitchell, P. & Moyle, J. Translocation of some anions cations and acids in rat liver mitochondria. Eur. J. Biochem. 9, 149–155 (1969).
Baron, S. et al. Role of mitochondrial Na+ concentration, measured by CoroNa red, in the protection of metabolically inhibited MDCK cells. J. Am. Soc. Nephrol. 16, 3490–3497 (2005).
Vasquez-Vivar, J., Shi, Z. & Tan, S. Tetrahydrobiopterin in cell function and death mechanisms. Antioxid. Redox Signal 37, 171–183 (2022).
Besse, A. et al. The GABA transaminase, ABAT, is essential for mitochondrial nucleoside metabolism. Cell Metab. 21, 417–427 (2015).
Cavalcanti-de-Albuquerque, J. P., de-Souza-Ferreira, E., de Carvalho, D. P. & Galina, A. Coupling of GABA metabolism to mitochondrial glucose phosphorylation. Neurochem. Res. 47, 470–480 (2022).
Bennett, C. F. et al. Peroxisomal-derived ether phospholipids link nucleotides to respirasome assembly. Nat. Chem. Biol. 17, 703–710 (2021).
Fang, J. et al. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci. Rep. 33, e00021 (2013).
Brosnan, M. E., MacMillan, L., Stevens, J. R. & Brosnan, J. T. Division of labour: how does folate metabolism partition between one-carbon metabolism and amino acid oxidation. Biochem. J. 472, 135–146 (2015).
Zheng, Y. & Cantley, L. C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 216, 253–266 (2019).
Zhao, L. N. & Kaldis, P. Pairing structural reconstruction with catalytic competence to evaluate the mechanisms of key enzymes in the folate-mediated one-carbon pathway. FEBS J. https://doi.org/10.1111/febs.16439 (2022).
Mitchell, P. & Moyle, J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 213, 137–139 (1967).
Morciano, G. et al. Measurement of ATP concentrations in mitochondria of living cells using luminescence and fluorescence approaches. Methods Cell. Biol. 155, 199–219 (2020).
Fernandez-Aguera, M. C. et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015).
Hernansanz-Agustin, P. et al. Na+ controls hypoxic signalling by the mitochondrial respiratory chain. Nature 586, 287–291 (2020).
Lopez-Barneo, J. & Ortega-Saenz, P. Mitochondrial acute oxygen sensing and signaling. Crit. Rev. Biochem. Mol. Biol. 57, 205–225 (2022).
Sommer, N. et al. Bypassing mitochondrial complex III using alternative oxidase inhibits acute pulmonary oxygen sensing. Sci. Adv. 6, eaba0694 (2020).
Carrer, A. et al. Defining the molecular mechanisms of the mitochondrial permeability transition through genetic manipulation of F-ATP synthase. Nat. Commun. 12, 4835 (2021).
Bonora, M., Giorgi, C. & Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 23, 266–285 (2022).
Carraro, M. & Bernardi, P. Measurement of membrane permeability and the mitochondrial permeability transition. Methods Cell. Biol. 155, 369–379 (2020).
Hsu, Y. -T. et al. Apoptosis Techniques and Protocols 2nd edn, vol. 37 (Humana Press, 2002).
Bryant, J. D., Lei, Y., VanPortfliet, J. J., Winters, A. D. & West, A. P. Assessing mitochondrial DNA release into the cytosol and subsequent activation of innate immune-related pathways in mammalian cells. Curr. Protoc. 2, e372 (2022).
Pfanner, N., Warscheid, B. & Wiedemann, N. Mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 20, 267–284 (2019).
Hernansanz-Agustin, P. & Enriquez, J. A. Functional segmentation of CoQ and cyt c pools by respiratory complex superassembly. Free Radic. Biol. Med. 167, 232–242 (2021).
Nesci, S., Trombetti, F. & Pagliarani, A. Nicotinamide nucleotide transhydrogenase as a sensor of mitochondrial biology. Trends Cell Biol. 30, 1–3 (2020).
Keilin, D. The History of Cell Respiration and Cytochrome (Cambridge University Press, 1966).
Chance, B. & Williams, G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 217, 383–393 (1955).
Schmidt, C. A., Fisher-Wellman, K. H. & Neufer, P. D. From OCR and ECAR to energy: perspectives on the design and interpretation of bioenergetics studies. J. Biol. Chem. 297, 101140 (2021).
Hernansanz-Agustin, P. & Enriquez, J. A. Generation of reactive oxygen species by mitochondria. Antioxidants 10, 415 (2021).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Murphy, M. P. et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4, 651–662 (2022).
Esteves, F., Rueff, J. & Kranendonk, M. The central role of cytochrome P450 in xenobiotic metabolism-a brief review on a fascinating enzyme family. J. Xenobiot. 11, 94–114 (2021).
Omura, T. & Gotoh, O. Evolutionary origin of mitochondrial cytochrome P450. J. Biochem. 161, 399–407 (2017).
Lambeth, J. D. & Stevens, V. L. Cytochrome P450scc: enzymology, and the regulation of intramitochondrial cholesterol delivery to the enzyme. Endocr. Res. 10, 283–309 (1984).
Koshiba, T., Yasukawa, K., Yanagi, Y. & Kawabata, S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal 4, ra7 (2011).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, 6378 (2018).
Riley, J. S. et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 (2018).
Cogliati, S., Enriquez, J. A. & Scorrano, L. Mitochondrial cristae: where beauty meets functionality. Trends Biochem. Sci. 41, 261–273 (2016).
Xian, H. et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55, 1370–1385 (2022).
Wang, C. & Youle, R. J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 43, 95–118 (2009).
Santos, J. H. Mitochondria signaling to the epigenome: a novel role for an old organelle. Free Radic. Biol. Med. 170, 59–69 (2021).
Csordas, G., Weaver, D. & Hajnoczky, G. Endoplasmic reticulum–mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 28, 523–540 (2018).
Scorrano, L. et al. Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019).
Booth, D. M., Varnai, P., Joseph, S. K. & Hajnoczky, G. Oxidative bursts of single mitochondria mediate retrograde signaling toward the ER. Mol. Cell 81, 3866–3876 (2021).
Shai, N. et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome–mitochondria contact. Nat. Commun. 9, 1761 (2018).
Sabouny, R. & Shutt, T. E. Reciprocal regulation of mitochondrial fission and fusion. Trends Biochem. Sci. 45, 564–577 (2020).
Twig, G. et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 (2008).
Pernas, L. & Scorrano, L. Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 78, 505–531 (2016).
Simula, L. & Campello, S. Monitoring the mitochondrial dynamics in mammalian cells. Methods Mol. Biol. 1782, 267–285 (2018).
Santo-Domingo, J., Giacomello, M., Poburko, D., Scorrano, L. & Demaurex, N. OPA1 promotes pH flashes that spread between contiguous mitochondria without matrix protein exchange. EMBO J. 32, 1927–1940 (2013).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 (2014).
Picard, M. et al. Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat. Commun. 6, 6259 (2015).
Koren, S. A. et al. All-optical spatiotemporal mapping of ROS dynamics across mitochondrial microdomains. Preprint at bioRxiv https://doi.org/10.1101/2023.01.07.523093 (2023).
Schauss, A. C. et al. A novel cell-free mitochondrial fusion assay amenable for high-throughput screenings of fusion modulators. BMC Biol. 8, 100 (2010).
Vincent, A. E., Turnbull, D. M., Eisner, V., Hajnoczky, G. & Picard, M. Mitochondrial Nanotunnels. Trends Cell Biol. 27, 787–799 (2017).
Molina, A. J. & Shirihai, O. S. Monitoring mitochondrial dynamics with photoactivatable green fluorescent protein. Methods Enzymol. 457, 289–304 (2009).
Nahacka, Z., Zobalova, R., Dubisova, M., Rohlena, J. & Neuzil, J. Miro proteins connect mitochondrial function and intercellular transport. Crit. Rev. Biochem. Mol. Biol. 56, 401–425 (2021).
Silva, C. A. et al. Activity-dependent regulation of mitochondrial motility in developing cortical dendrites. eLife 10, e62091 (2021).
Sugiura, A., McLelland, G. L., Fon, E. A. & McBride, H. M. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 33, 2142–2156 (2014).
Cadete, V. J. et al. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol. 594, 5343–5362 (2016).
Konig, T. et al. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat. Cell Biol. 23, 1271–1286 (2021).
Picard, M. et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl Acad. Sci. USA 111, 4033–4042 (2014).
Picard, M. & Sandi, C. The social nature of mitochondria: implications for human health. Neurosci. Biobehav. Rev. 120, 595–610 (2021).
Maslow, A. H. A theory of human motivation. Psychol. Rev. 50, 370–396 (1943).
Acknowledgements
We are grateful to investigators who have contributed to reveal tissue-type-specific and cell-type-specific mitochondrial phenotypes that served as the foundation and motivation for this Perspective. We apologize to authors whose work could not be included due to space constraints. We thank the Twitter mitochondrial community and C. Schmitt who contributed inputs to Table 1, and to members of the Mitochondrial Psychobiology Laboratory, and the Genoxphos Laboratory for stimulating discussions. Work of J.A.E. is supported by Ministerio de Ciencia, Innovación/Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER; RTI2018-099357-B-I00), the Biomedical Research Networking Center on Frailty and Healthy Ageing (CIBERFES-ISCiii-CB16/10/00289) and the Leducq TNE-17CVD. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades (MCNU) and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505). This work was supported by National Institutes of Health grants R01MH119336, R01MH122706, R01AG066828, R21MH123927 and RF1AG076821, the Wharton Fund and the Baszucki Brain Research Fund (to M.P.).
Author information
Authors and Affiliations
Contributions
M.P. drafted the figures. A.S.M., J.A.E. and M.P. contributed to the literature review and revised the final version of the figures and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Michael Murphy and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Monzel, A.S., Enríquez, J.A. & Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nat Metab 5, 546–562 (2023). https://doi.org/10.1038/s42255-023-00783-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-023-00783-1
This article is cited by
-
A prognostic model of idiopathic pulmonary fibrosis constructed based on macrophage and mitochondria-related genes
BMC Pulmonary Medicine (2024)
-
Kynurenine in IDO1high cancer cell-derived extracellular vesicles promotes angiogenesis by inducing endothelial mitophagy in ovarian cancer
Journal of Translational Medicine (2024)
-
The effects of restraint stress on ceramide metabolism disorders in the rat liver: the role of CerS6 in hepatocyte injury
Lipids in Health and Disease (2024)
-
Mitochondria as secretory organelles and therapeutic cargos
Experimental & Molecular Medicine (2024)
-
A break in mitochondrial endosymbiosis as a basis for inflammatory diseases
Nature (2024)