Abstract
Combined use of cannabis and alcohol results in greater psychoactive toxicity than either substance alone, but the underlying central mechanisms behind this worsened outcome remain unclear. Here we show that the synergistic effect of Δ9-tetrahydrocannabinol (THC) and ethanol on motor incoordination in mice is achieved by activating presynaptic type 1 cannabinoid receptors (CB1R) and potentiating extrasynaptic glycine receptors (GlyR) within cerebellar Purkinje cells (PCs). The combination of ethanol and THC significantly reduces miniature excitatory postsynaptic current frequency in a CB1R-dependent manner, while increasing the extrasynaptic GlyR-mediated chronic chloride current, both leading to decreased PC activity. Ethanol enhances THC actions by boosting the blood–brain-barrier permeability of THC and enriching THC in the cell membrane. Di-desoxy-THC, a designed compound that specifically disrupts THC–GlyR interaction without affecting the basic functions of CB1R and GlyR, is able to restore PC function and motor coordination in mice. Our findings provide potential therapeutic strategies for overcoming the synergistic toxicity caused by combining cannabis and alcohol use.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Abrahao, K. P., Salinas, A. G. & Lovinger, D. M. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron 96, 1223–1238 (2017).
Hall, W. & Solowij, N. Adverse effects of cannabis. Lancet 352, 1611–1616 (1998).
Yurasek, A. M., Aston, E. R. & Metrik, J. Co-use of alcohol and cannabis: a review. Curr. Addict. Rep. 4, 184–193 (2017).
Ramaekers, J. G. Driving under the influence of cannabis: an increasing public health concern. JAMA 319, 1433–1434 (2018).
Cole, T. B. & Saitz, R. Cannabis and impaired driving. JAMA 324, 2163–2164 (2020).
Dubois, S., Mullen, N., Weaver, B. & Bedard, M. The combined effects of alcohol and cannabis on driving: Impact on crash risk. Forensic Sci. Int. 248, 94–100 (2015).
Dar, M. S. Cerebellar CB(1) receptor mediation of Delta(9)-THC-induced motor incoordination and its potentiation by ethanol and modulation by the cerebellar adenosinergic A(1) receptor in the mouse. Brain Res. 864, 186–194 (2000).
Funada, M., Takebayashi-Ohsawa, M. & Tomiyama, K. I. Synthetic cannabinoids enhanced ethanol-induced motor impairments through reduction of central glutamate neurotransmission. Toxicol. Appl. Pharmacol. 408, 115283 (2020).
Singh, A., Stredny, C. M. & Loddenkemper, T. Pharmacotherapy for pediatric convulsive status epilepticus. CNS Drugs 34, 47–63 (2020).
Hirvonen, J. et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol. Psychiatr. 18, 916–921 (2013).
Kumar, R. N., Chambers, W. A. & Pertwee, R. G. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia 56, 1059–1068 (2001).
Pertwee, R. G. Pharmacological actions of cannabinoids. Handb. Exp. Pharmacol. https://doi.org/10.1007/3-540-26573-2_1 (2005).
Wick, M. J. et al. Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proc. Natl Acad. Sci. USA 95, 6504–6509 (1998).
Burgos, C. F., Munoz, B., Guzman, L. & Aguayo, L. G. Ethanol effects on glycinergic transmission: from molecular pharmacology to behavior responses. Pharmacol. Res. 101, 18–29 (2015).
Trudell, J. R., Messing, R. O., Mayfield, J. & Harris, R. A. Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol. Sci. 35, 317–323 (2014).
Lynch, J. W., Zhang, Y., Talwar, S. & Estrada-Mondragon, A. Glycine receptor drug discovery. Adv. Pharmacol. 79, 225–253 (2017).
Yevenes, G. E. & Zeilhofer, H. U. Allosteric modulation of glycine receptors. Br. J. Pharmacol. 164, 224–236 (2011).
Avila, A., Nguyen, L. & Rigo, J. M. Glycine receptors and brain development. Front. Cell Neurosci. 7, 184 (2013).
Aguayo, L. G., van Zundert, B., Tapia, J. C., Carrasco, M. A. & Alvarez, F. J. Changes on the properties of glycine receptors during neuronal development. Brain Res. Rev. 47, 33–45 (2004).
Zou, G. C. et al. Cannabinoids Rescue cocaine-induced seizures by restoring brain glycine receptor dysfunction. Cell Rep. 30, 4209–4219 (2020).
Steiner, H., Bonner, T. I., Zimmer, A. M., Kitai, S. T. & Zimmer, A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc. Natl Acad. Sci. USA 96, 5786–5790 (1999).
Kishimoto, Y. & Kano, M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J. Neurosci. 26, 8829–8837 (2006).
Schaefer, N. et al. Disruption of a structurally important extracellular element in the glycine receptor leads to decreased synaptic integration and signaling resulting in severe startle disease. J. Neurosci. 37, 7948–7961 (2017).
Crisp, S. J. et al. Glycine receptor autoantibodies disrupt inhibitory neurotransmission. Brain: a J. Neurol. 142, 3398–3410 (2019).
Harvey, R. J., Topf, M., Harvey, K. & Rees, M. I. The genetics of hyperekplexia: more than startle! Trends Genet. 24, 439–447 (2008).
Jin, S. et al. Brain ethanol metabolism by astrocytic ALDH2 drives the behavioural effects of ethanol intoxication. Nat. Metab. 3, 337–351 (2021).
DeSanty, K. P. & Dar, M. S. Cannabinoid-induced motor incoordination through the cerebellar CB1 receptor in mice. Pharmacol. Biochem. Behav. 69, 251–259 (2001).
Sanes, J. N. Motor cortex rules for learning and memory. Curr. Biol. 10, R495–R497 (2000).
Bullitt, E. Expression of C-Fos-like protein as a marker for neuronal-activity following noxious-stimulation in the rat. J. Comp. Neurol. 296, 517–530 (1990).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Schiffmann, S. N. et al. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. PNAS 96, 5257–5262 (1999).
Shibasaki, K., Ishizaki, Y. & Mandadi, S. Astrocytes express functional TRPV2 ion channels. Biochem. Biophys. Res. Commun. 441, 327–332 (2013).
Herkenham, M. et al. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. USA 87, 1932–1936 (1990).
Arenas, Y. M., Cabrera-Pastor, A., Juciute, N., Mora-Navarro, E. & Felipo, V. Blocking glycine receptors reduces neuroinflammation and restores neurotransmission in cerebellum through ADAM17-TNFR1-NF-κβ pathway. J. Neuroinflammation 17, 269 (2020).
Xiong, W. et al. Cannabinoid potentiation of glycine receptors contributes to cannabis-induced analgesia. Nat. Chem. Biol. 7, 296–303 (2011).
Xiong, W. et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 209, 1121–1134 (2012).
Xiong, W. et al. A common molecular basis for exogenous and endogenous cannabinoid potentiation of glycine receptors (April, pg 5200, 2012). J. Neurosci. 32, 12979–12979 (2012).
Lu, J. et al. Involvement of glycine receptor α1 subunits in cannabinoid-induced analgesia. Neuropharmacology 133, 224–232 (2018).
Wells, M. M. et al. Ensemble-based virtual screening for cannabinoid-like potentiators of the human glycine receptor α1 for the treatment of pain. J. Med. Chem. 58, 2958–2966 (2015).
Molnar, E. Motor learning and long-term plasticity of parallel fibre-Purkinje cell synapses require post-synaptic Cdk5/p35. J. Neurochem. 131, 1–3 (2014).
Yao, L. et al. Membrane cholesterol dependence of cannabinoid modulation of glycine receptor. FASEB J. 34, 10920–10930 (2020).
Zou, G., Xia, J., Han, Q., Liu, D. & Xiong, W. The synthetic cannabinoid dehydroxylcannabidiol restores the function of a major GABAA receptor isoform in a cell model of hyperekplexia. J. Biol. Chem. 295, 138–145 (2020).
Scherma, M. et al. Brain activity of anandamide: a rewarding bliss. Acta Pharmacol. Sin. 40, 309–323 (2019).
Calapai, F. et al. Cannabinoids, blood–brain barrier, and brain disposition. Pharmaceutics https://doi.org/10.3390/pharmaceutics12030265 (2020).
Fei, C., Lillico, D. M. E., Hall, B., Rieger, A. M. & Stafford, J. L. Connected component masking accurately identifies the ratio of phagocytosed and cell-bound particles in individual cells by imaging flow cytometry. Cytom. Part A 91, 372–381 (2017).
Xue, J. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 12, 692–700 (2017).
Li, W. et al. BBB pathophysiology-independent delivery of siRNA in traumatic brain injury. Sci Adv https://doi.org/10.1126/sciadv.abd6889 (2021).
Li, G. et al. Permeability of endothelial and astrocyte cocultures: in vitro blood–brain barrier models for drug delivery studies. Ann. Biomed. Eng. 38, 2499–2511 (2010).
Roth, J. G. et al. Advancing models of neural development with biomaterials. Nat. Rev. Neurosci. 22, 593–615 (2021).
Dono, L. M. & Currie, P. J. The cannabinoid receptor CB1 inverse agonist AM251 potentiates the anxiogenic activity of urocortin I in the basolateral amygdala. Neuropharmacology 62, 192–199 (2012).
Bogdanov, N. N., Poletaeva, I. I. & Popova, N. V. Pentylenetetrazol and strychnine convulsions in brain weight selected mice. Seizure 6, 135–138 (1997).
Metrik, J., Gunn, R. L., Jackson, K. M., Sokolovsky, A. W. & Borsari, B. Daily patterns of marijuana and alcohol co-use among individuals with alcohol and cannabis use disorders. Alcohol Clin. Exp. Res 42, 1096–1104 (2018).
Wolfe, S. A., Vozella, V. & Roberto, M. The synaptic interactions of alcohol and the endogenous cannabinoid system. Alcohol Res. 42, 03 (2022).
Xiong, W. et al. Presynaptic glycine receptors as a potential therapeutic target for hyperekplexia disease. Nat. Neurosci. 17, 232–239 (2014).
Dar, M. S. & Mustafa, S. J. Acute ethanol/cannabinoid-induced ataxia and its antagonism by oral/systemic/intracerebellar A1 adenosine receptor antisense in mice. Brain Res. 957, 53–60 (2002).
Smith, A. D. & Dar, M. S. Mouse cerebellar nicotinic-cholinergic receptor modulation of δ9-THC ataxia: role of the α4β2 subtype. Brain Res. 1115, 16–25 (2006).
Hanig, J. P., Morrison, J. M. Jr. & Krop, S. Ethanol enhancement of blood–brain barrier permeability to catecholamines in chicks. Eur. J. Pharmacol. 18, 79–82 (1972).
Pan, W., Barron, M., Hsuchou, H., Tu, H. & Kastin, A. J. Increased leptin permeation across the blood–brain barrier after chronic alcohol ingestion. Neuropsychopharmacology 33, 859–866 (2008).
Haorah, J. et al. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood–brain barrier compromise. Alcohol. Clin. Exp. Res. 29, 999–1009 (2005).
Haorah, J., Knipe, B., Leibhart, J., Ghorpade, A. & Persidsky, Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood–brain barrier dysfunction. J. Leukoc. Biol. 78, 1223–1232 (2005).
Lukas, S. E. & Orozco, S. Ethanol increases plasma δ(9)-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alcohol Depend. 64, 143–149 (2001).
Blazquez, C. et al. Inhibition of striatonigral autophagy as a link between cannabinoid intoxication and impairment of motor coordination. eLife 9, e56811 (2020).
Patra, M. et al. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys. J. 90, 1121–1135 (2006).
Goldstein, D. B. Effect of alcohol on cellular membranes. Ann. Emerg. Med. 15, 1013–1018 (1986).
Ingolfsson, H. I. & Andersen, O. S. Alcohol’s effects on lipid bilayer properties. Biophys. J. 101, 847–855 (2011).
Karila, L. et al. Acute and long-term effects of cannabis use: a review. Curr. Pharm. Des. 20, 4112–4118 (2014).
Chattipakorn, S. C. & McMahon, L. L. Strychnine-sensitive glycine receptors depress hyperexcitability in rat dentate gyrus. J. Neurophysiol. 89, 1339–1342 (2003).
Sink, K. S. et al. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur. Neuropsychopharmacol. 20, 112–122 (2010).
Zhao, Q. C. et al. HDAC3 inhibition prevents blood–brain barrier permeability through Nrf2 activation in type 2 diabetes male mice. J. Neuroinflamm 16, 103 (2019).
Wilkinson, S. M., Price, J. & Kassiou, M. Improved accessibility to the desoxy analogues of δ(9)-tetrahydrocannabinol and cannabidiol. Tetrahedron Lett. 54, 52–54 (2013).
Acknowledgements
We thank T. Pan for the synthesis of THC, THC–Cy7 and DDT. We thank G.E. Homanics (University of Pittsburgh) for providing the GlyRα1S296A mice. This work was supported by National Key R&D Program of China (2021YFA0804900, W.X.), National Natural Science Foundation of China (grants 91849206, 91649121, 91942315, 92049304, 32121002, 32225020 to W.X.; 81901157 to G.Z.), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant XDB39050000, W.X.), Key Research Program of Frontier Science (CAS, grant ZDBS-LY-SM002, W.X.), CAS Interdisciplinary Innovation Team (JCTD-2018-20, W.X.), CAS Project for Young Scientists in Basic Research (YSBR-013, W.X.), the Fundamental Research Funds for the Central Universities, the Major Program of Development Foundation of Hefei Center for Physical Science and Technology (2017FXZY006, W.X.) and Users with Excellence Program/Project of Hefei Science Center CAS (2019HSC-UE006, W.X.), USTC Research Funds of the Double First-Class Initiative (YD9100002001, W.X.) and China Postdoctoral Science Foundation (2020TQ0314, G.Z.).
Author information
Authors and Affiliations
Contributions
W.X. initiated, designed and supervised the project; G.Z., C.M. and H.L. conducted electrophysiological recordings. G.Z., J.X. and X.Z. conducted behavior tests; G.Z. and D.X. conducted immunohistochemistry; Y.Y., Q.G. and J.X. conducted the BBB experiments. G.Z. and J.X. conducted the quantitative flow cytometry, in vivo small animal imaging and HPLC–MS experiments. G.Z. and W.X. analyzed data with assistance of T.X, Z.Z. and Y.Z. L.Z. contributed to experimental design, data interpretation and analysis. W.X. and G.Z wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Effects of THC and ethanol on motor coordination and the BEC in mice, Related to Fig. 1.
a, Schematic showing the timeline of drug administration and AR procedure. b, Effects of THC (i.p., 0.1, 0.3, 0.5, 1.0, 3.0 and 5.0 mg/kg) on AR latency of mice. c, Schematic showing the timeline of drug administration and AR procedure. d, Effects of ethanol (i.p., 0.1, 0.3, 0.5, 1.0 and 2.0 g/kg) on AR latency of mice. e, Schematic showing the timeline of drug administration and AR procedure. f, Effects of THC and ethanol in combination on AR latency of mice. g, Effects of ethanol (i.p., 1.0 and 2.0 g/kg) alone or in combination with THC (i.p., 1.0 mg/kg) on AR latency of mice. h, Effects of ethanol on AR latency of female mice. i, Compare of effects of ethanol on AR latency between male and female mice. Each data were normalized to its control group. j, Effects of ethanol (i.p., 0.5, 1.0 and 2.0 g/kg) alone or in combination with THC (i.p., 1.0 mg/kg) on AR latency in female mice. k,l, Normalized AR latency of female (k) and male (l) mice receiving THC (i.p., 1.0 mg/kg) and ethanol (i.p., 0.5, 1.0 and 2.0 g/kg) in combination. Each data were normalized to the related ethanol alone group. m-o, BEC of mice treated with i.p. ethanol at 0.5 g/kg (m), 1.0 g/kg (n) or 2.0 g/kg (o) alone with or without i.p. THC (1.0 mg/kg). n = 3 mice per group. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a one-way ANOVA followed by Tukey’s post hoc multiple-comparison test (b, d, f-h, j) or a two-way ANOVA followed by Tukey’s post hoc multiple-comparison test (i, k, l, m-o).
Extended Data Fig. 2 The synergistic effect of THC and various doses of systemic ethanol on c-Fos immunohistochemistry in various brain regions and motor coordination, Related to Fig. 1.
a-c Representative images of immunohistochemistry c-Fos positive neurons in DMS, M1 and M2 of mice treated with Veh, Veh + AR or THC + ethanol + AR. Scale bar, 25 μm. Veh: vehicle, AR: accelerating rotarod. d, Effects of ethanol alone or in combination with THC (intra-4/5Cb., 5 μg/μl) on AR latency of mice. e, Normalized AR latency of mice receiving THC (intra-4/5Cb., 5 μg/μl) and ethanol in combination. Each data were normalized to the ethanol group with the same dose of ethanol. f, Effects of CNO (i.p., 1.0 mg/kg) on the number of c-Fos positive neurons in cerebellar 4/5Cb of mice transfected with AAV2/9-hSyn-hM4Di-mCitrine with or without AR tests. g, Effects of THC (i.p., 1.0 mg/kg) + ethanol (i.p., 0.5 g/kg), CNO (i.p., 1.0 mg/kg), or their combination on the number of c-Fos positive neurons in cerebellar 4/5Cb of mice expressing AAV2/9-hSyn-hM3Dq-mCitrine with or without AR tests. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a one-way ANOVA followed by Tukey’s post hoc multiple-comparison test (d) or a two-way ANOVA followed by Tukey’s post hoc multiple-comparison test (e-g).
Extended Data Fig. 3 Effects of chemogenetic activation of 4/5Cb on motor coordination in mice, Related to Fig. 1.
a, Schematic showing the timeline of chemogenetic experimental procedure. AAV2/9-hSyn-hM3Dq-mCitrine or the AAV2/9-hSyn-EGFP was injected into the 4/5Cb of mice. b, Representative images showing the EGFP or mCitrine signals in the injection sites of the 4/5Cb of mice. Scale bar, 1 mm. c, Schematic showing virus injection and chemogenetic experimental procedure. AAV2/9-hSyn-hM3Dq-mCitrine or the AAV2/9-hSyn-EGFP was injected into the 4/5Cb of mice. d, Representative images showing the EGFP or mCitrine signals in the injection sites of the 4/5Cb of mice. Scale bar, 1 mm. e, Effects of CNO (i.p., 1.0 mg/kg) on AR latency of mice expressing hM3Dq or EGFP as a control in the 4/5Cb. Values are represented as means ± s.e.m., n per group. Statistical differences were determined by a two-way ANOVA followed by Tukey’s post hoc multiple-comparison test.
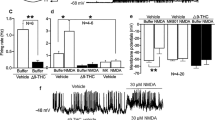
Extended Data Fig. 4 Effects of THC and ethanol combination on mIPSCs of PCs, Related to Fig. 2.
a, Trace records, average frequency and amplitude of GABAergic mIPSCs of PCs in 4/5Cb slices of mice. The neurons recorded were treated with THC (100 nM, 5 min) and ethanol (20 mM, 5 min), either alone or in combination. b,c, Cumulative probability plot for the inter-event interval (b) and amplitudes (c) for GABAergic mIPSCs of PCs in 4/5Cb slices of mice. Values are represented as means ± s.e.m., n per group. Statistical differences were determined by a one-way ANOVA followed by Tukey’s post hoc multiple-comparison test.
Extended Data Fig. 5 Effects of various antagonists on SETEM and motor coordination in mice, Related to Fig. 3.
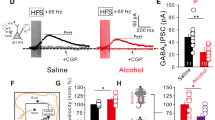
a,b, Effects of AM251 (i.p., 5.0 mg/kg) on the SETEM caused by THC (i.p., 1.0 mg/kg) and ethanol (i.p., 0.5, 1.0 and 2.0 g/kg) combination in male (a) and female (b) mice. c-e, Effects of strychnine (i.p., 1.0 mg/kg) (c), AM630 (i.p., 5.0 mg/kg) (d) and tranilast (i.p., 300 mg/kg) (e) on the SETEM caused by THC (i.p., 1.0 mg/kg) and ethanol (i.p., 1.0 and 2.0 g/kg) combination in mice. Stry: strychnine; Trani: Tranilast. f-i, Effects of AM251(f), strychnine (g), AM630(h) and tranilast (i) on AR latency of mice. j, Effects of AM251 (intra-4/5Cb., 5 μg /μl) on the SETEM caused by THC (i.p., 1.0 mg/kg) and ethanol (i.p., 1.0 and 2.0 g/kg) combination in mice. k, Effects of THC (intra-4/5Cb., 5 μg/μl) and ethanol (i.p., 1.0 g/kg) combination on AR latency in GlyRα1WT and GlyRα1S296A mice. l, Effects of THC (intra-4/5Cb., 5 μg/μl) and ethanol (i.p., 2.0 g/kg) combination on AR latency in GlyRα1WT and GlyRα1S296A mice. m, The AR latency of GlyRα1WT and GlyRα1S296A female mice receiving THC (i.p., 1.0 mg/kg) and ethanol (i.p., 0.5, 1.0 and 2.0 g/kg) combination. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a one-way ANOVA followed by Tukey’s post hoc multiple-comparison test (a-j) or a two-way ANOVA followed by Tukey’s post hoc multiple-comparison test (k-m).
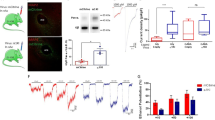
Extended Data Fig. 6 Effects of ethanol on Cy7 level in HEK-293 cells and N2A cells measured by standard flow cytometry, Related to Fig. 6.
a-d, Gating strategy to obtain HEK-293 cells with similar size (FSC-A) and granularity (SSC-A). The HEK-293 cells have been incubated with Cy7 (a), Cy7 + ethanol (b), THC-Cy7 (c) and THC-Cy7 + ethanol (d) for 60 min, respectively. Cy7 at 10 μM, THC-Cy7 at 10 μM and ethanol at 20 mM were used in this experiment. FSC-A: Forward Scatter Area; SSC-A: Side Scatter Area. e-h, Gating strategy to obtain N2A cells with similar size (FSC-A) and granularity (SSC-A). The N2A cells have been incubated with Cy7 (e), Cy7 + ethanol (f), THC-Cy7 (g) and THC-Cy7 + ethanol (h) for 60 min, respectively. i,j Histograms (i) and bar graph (j) of MFI (Median Fluorescence Intensity) obtained from standard flow cytometry analysis of HEK-293 cells treated with Cy7 or Cy7 plus ethanol in combination. k,l Histograms (k) and bar graph (l) of MFI obtained from standard flow cytometry analysis of N2A cells treated with Cy7 or Cy7 plus ethanol in combination. m, HPLC calibration curve of HEK-293 cell buffer spiked with known amounts of THC. The calibration curve: y = 2180.40x − 1662.02. n, HPLC calibration curve of N2A cell buffer spiked with known amounts of THC. The calibration curve: y = 2495.71x + 418.599. o,p Representative mass spectra of THC obtained from HEK-293 cell (o) and N2A cell (p) buffer. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a a two-sided unpaired t-test (j and l) or a linear regression test (m and n).
Extended Data Fig. 7 Effects of ethanol on Cy7 level in HEK-293 cells and N2A cells measured by imaging flow cytometr, Related to Fig. 6.
a, b Representative histogram used to discriminate between unfocused and focused images of HEK-293 cells (a) and N2A cells (b) incubated with Cy7, Cy7 + ethanol, THC-Cy7 and THC-Cy7 + ethanol. Cy7 at 10 μM, THC-Cy7 at 10 μM and ethanol at 20 mM were used. These experiments were done using the standard IDEAS® software platform. Upon this image, Gradient RMS values between 40–100 (R1: Region 1) were included for further analysis. Gradient RMS: Root Mean Square. c,d Representative graph of area versus aspect ratio used to gating focused images of HEK-293 cells (c) and N2A cells (d). Cells in the frame (R2: Region 2) were used for further membrane intensity analysis. Area: size of the focused cells; Aspect ratio: the minor axis divided by the major axis of the focused cells. e-g, Representative cell images (e), histograms (f) and bar graphs (g) of MFI obtained from IFC (Imaging Flow Cytometry) of HEK-293 cells treated with Cy7 or Cy7 and ethanol in combination. h-j, Representative cell images (h), histograms (i) and bar graphs (j) of MFI obtained from IFC of N2A cells treated with Cy7 or Cy7 and ethanol in combination. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a two-sided unpaired t-test.
Extended Data Fig. 8 Effects of various doses of ethanol on plasma and brain intensity of THC and BBB permeability. Related to Fig. 7.
a, Whole body fluorescence imaging of mice treated with Cy7/Cy7-THC (i.p., 10 mg/kg) or Cy7/Cy7-THC (i.p., 10 mg/kg) and ethanol (i.p., 0.5 g/kg) in combination. b, Representative fluorescence images of mice treated with Cy7 alone or Cy7 with ethanol in combination. c, Brain fluorescence intensity of Cy7. n = 5 mice per group. d, Representative fluorescence images of mice treated with THC-Cy7 or THC-Cy7 with ethanol in combination. e, Brain fluorescence intensity of THC-Cy7. n = 5 mice per group. f, Schematic illustration of the in vitro BBB model. g, Relative level of FITC-Dextran (1.0 mg/ml, 1 h) crossing endothelial monolayer with or without ethanol (20 mM, 5 min) treatment. h,i, Representative images (h) and quantitative analysis (i) of FITC signal in the brain of mice treated with FITC-Dextran (i.p., 50 mg/kg, 1 h) with or without ethanol (i.p., 0.5 g/kg, 5 min) administration. Scale bars: 1 mm. j, Calibration curve of plasma spiked with known amounts of THC. The calibration curve: y = 889.307x – 1295.26. k, Representative mass spectra of THC obtained from plasma of C57BL/6 J mice administrated with THC (i.p., 10 mg/kg). l,m Quantitative analysis of plasma (l) and cerebellar (m) THC intensity in mice treated with THC or THC and ethanol in combination. n = 3 mice per group. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a two-sided unpaired t-test (g and i) or a two-way ANOVA followed by Tukey’s post hoc multiple-comparison test (c, e, l and m) or a linear regression test (j).
Extended Data Fig. 9 Effects of DDT on acute and chronic ethanol exposure associated SETEM and ethanol-induced motor incoordination, Related to Fig. 8.
a, Schematic illustration of synthesis of di-desoxy-THC (DDT). b, AR latency of mice receiving THC (i.p., 1.0 mg/kg), ethanol (i.p., 1.0 and 2.0 g/kg), DDT (i.p., 30 mg/kg), or their combination. c, Effects of DDT (i.p., 30 mg/kg) on motor incoordination caused by various doses of ethanol. d, Schematic illustration of the CEE. e, The effects of THC and DDT on the CEE-induced motor incoordination. f, The effects of THC and AM251 on the CEE-induced motor incoordination. g, Representative images showing a mouse walking on treadmill and its paw prints detected from a video underneath the treadmill. LF: left forelimb; RF: right forelimb; LH: left hindlimb; RH: right hindlimb. h-j, Effects of DDT (i.p., 30 mg/kg, 20 min before tests) on the stride length (h), stride duration (i) and stance width (j) of mice in the gait test. k-m, Effects of DDT (i.p., 30 mg/kg) on grip strength (k), body temperature (l) and food intake (m) of mice in the grip test. Values are represented as means ± s.e.m., n per group. Exact P values are shown. Statistical differences were determined by a one-way ANOVA followed by Tukey’s post hoc multiple-comparison test (b, c, e and f) or a two-way ANOVA followed by Tukey’s post hoc multiple-comparison test (h-j) or a two-sided unpaired t-tests (k–m).
Supplementary information
Supplementary Information
Supplementary Methods
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, G., Xia, J., Luo, H. et al. Combined alcohol and cannabinoid exposure leads to synergistic toxicity by affecting cerebellar Purkinje cells. Nat Metab 4, 1138–1149 (2022). https://doi.org/10.1038/s42255-022-00633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00633-6