Abstract
Adipose tissue undergoes thermogenic remodeling in response to thermal stress and metabolic cues, playing a crucial role in regulating energy expenditure and metabolic homeostasis. Endoplasmic reticulum (ER) stress is associated with adipose dysfunction in obesity and metabolic disease. It remains unclear, however, if ER stress-signaling in adipocytes mechanistically mediates dysregulation of thermogenic fat. Here we show that inositol-requiring enzyme 1α (IRE1α), a key ER stress sensor and signal transducer, acts in both white and beige adipocytes to impede beige fat activation. Ablation of adipocyte IRE1α promotes browning/beiging of subcutaneous white adipose tissue following cold exposure or β3-adrenergic stimulation. Loss of IRE1α alleviates diet-induced obesity and augments the anti-obesity effect of pharmacologic β3-adrenergic stimulation. Notably, IRE1α suppresses stimulated lipolysis and degrades Ppargc1a messenger RNA through its RNase activity to downregulate the thermogenic gene program. Hence, blocking IRE1α bears therapeutic potential in unlocking adipocytes’ thermogenic capacity to combat obesity and metabolic disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
There are no restrictions as to the availability of materials reported in the manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request. The RNA-seq data generated in this study have been deposited in the NCBI Gene Expression Omnibus and are accessible through accession number GSE180524. Source data are provided with this paper.
References
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Cohen, P. & Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-021-00350-0 (2021).
Morigny, P., Boucher, J., Arner, P. & Langin, D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 17, 276–295 (2021).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27, 226–236 (2018).
Wang, W. & Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17, 691–702 (2016).
Kajimura, S., Spiegelman, Bruce, M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Kahn, C. R., Wang, G. & Lee, K. Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 129, 3990–4000 (2019).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Bartelt, A. & Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36 (2014).
Sun, W., Modica, S., Dong, H. & Wolfrum, C. Plasticity and heterogeneity of thermogenic adipose tissue. Nat. Metab. 3, 751–761 (2021).
Chouchani, E. T. & Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189–200 (2019).
Rosenwald, M., Perdikari, A., Rülicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Roh, H. C. et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab. 27, 1121–1137 (2018).
Shao, M. et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Inagaki, T., Sakai, J. & Kajimura, S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 17, 480–495 (2016).
Lin, J., Handschin, C. & Spiegelman, B. M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370 (2005).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Kong, X. et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell 158, 69–83 (2014).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Mori, K., Ma, W., Gething, M. J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993).
Tirasophon, W., Welihinda, A. A. & Kaufman, R. J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812–1824 (1998).
Hetz, C., Martinon, F., Rodriguez, D. & Glimcher, L. H. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 91, 1219–1243 (2011).
Sidrauski, C. & Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031–1039 (1997).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Hollien, J. et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331 (2009).
Maurel, M., Chevet, E., Tavernier, J. & Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
So, J. S. et al. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 16, 487–499 (2012).
Osorio, F. et al. The unfolded-protein-response sensor IRE-1α regulates the function of CD8α+ dendritic cells. Nat. Immunol. 15, 248–257 (2014).
He, S. et al. IRE1α regulates skeletal muscle regeneration through Myostatin mRNA decay. J. Clin. Invest. https://doi.org/10.1172/jci143737 (2021).
Fu, S., Watkins, S. M. & Hotamisligil, G. S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 15, 623–634 (2012).
Huang, S., Xing, Y. & Liu, Y. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. J. Biol. Chem. 294, 18726–18741 (2019).
Boden, G. et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 (2008).
Gregor, M. F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009).
Shan, B. et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 18, 519–529 (2017).
Asada, R. et al. IRE1α-XBP1 is a novel branch in the transcriptional regulation of Ucp1 in brown adipocytes. Sci. Rep. 5, 16580 (2015).
Shao, M. et al. Hepatic IRE1α regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARα axis signalling. Nat. Commun. 5, 3528 (2014).
Shin, H. et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab. 26, 764–777 (2017).
Altshuler-Keylin, S. et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402–419 (2016).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genes 45, 593–605 (2007).
Schreiber, R. et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab. 26, 753–763 (2017).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465 (2021).
Foley, K. P. et al. Inflammation promotes adipocyte lipolysis via IRE1 kinase. J. Biol. Chem. 296, 100440 (2021).
Ohno, H., Shinoda, K., Spiegelman, B. M. & Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012).
Volkmann, K. et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J. Biol. Chem. 286, 12743–12755 (2011).
Cross, B. C. et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. PNAS 109, E869–E878 (2012).
Tirasophon, W., Lee, K., Callaghan, B., Welihinda, A. & Kaufman, R. J. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 14, 2725–2736 (2000).
Oikawa, D., Tokuda, M., Hosoda, A. & Iwawaki, T. Identification of a consensus element recognized and cleaved by IRE1α. Nucleic Acids Res. 38, 6265–6273 (2010).
Cypess, A. M. et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 130, 2209–2219 (2020).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Kleiner, S. et al. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. PNAS 109, 9635–9640 (2012).
Martínez-Redondo, V., Pettersson, A. T. & Ruas, J. L. The hitchhiker’s guide to PGC-1α isoform structure and biological functions. Diabetologia 58, 1969–1977 (2015).
Bartelt, A. et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 24, 292–303 (2018).
Zhou, Z. et al. Endoplasmic reticulum-associated degradation regulates mitochondrial dynamics in brown adipocytes. Science 368, 54–60 (2020).
Gregor, M. F. et al. The role of adipocyte XBP1 in metabolic regulation during lactation. Cell Rep. 3, 1430–1439 (2013).
Deng, Y. et al. Adipocyte Xbp1s overexpression drives uridine production and reduces obesity. Mol. Metab. 11, 1–17 (2018).
Shao, M. et al. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol. Metab. 6, 111–124 (2017).
Sha, H. et al. The IRE1α-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 9, 556–564 (2009).
Villarroya, F., Cereijo, R., Villarroya, J., Gavaldà-Navarro, A. & Giralt, M. Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab. 27, 954–961 (2018).
Eguchi, J. et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 (2011).
Li, L. et al. Brown adipocytes can display a mammary basal myoepithelial cell phenotype in vivo. Mol. Metab. 6, 1198–1211 (2017).
Müller, T. D., Klingenspor, M. & Tschöp, M. H. Revisiting energy expenditure: how to correct mouse metabolic rate for body mass. Nat. Metab. 3, 1134–1136 (2021).
Virtue, S. & Vidal-Puig, A. GTTs and ITTs in mice: simple tests, complex answers. Nat. Metab. 3, 883–886 (2021).
Shao, M. et al. Zfp423 maintains white adipocyte identity through suppression of the beige cell thermogenic gene program. Cell Metab. 23, 1167–1184 (2016).
Tang, J. et al. Sam50-Mic19-Mic60 axis determines mitochondrial cristae architecture by mediating mitochondrial outer and inner membrane contact. Cell Death Differ. 27, 146–160 (2020).
Song, A. et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Investig. 130, 247–257 (2020).
Wiseman, R. L. et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-rnase domain of IRE1. Mol. Cell 38, 291–304 (2010).
Dufey, E. et al. Genotoxic stress triggers the activation of IRE1α-dependent RNA decay to modulate the DNA damage response. Nat. Commun. 11, 2401 (2020).
Liu, L. et al. Coupling of COPII vesicle trafficking to nutrient availability by the IRE1α-XBP1s axis. Proc. Natl Acad. Sci. USA 116, 11776–11785 (2019).
Acknowledgements
We thank L. Qi and J. Lin from University of Michigan Medical School and Z. Meng from Zhejiang University for insightful discussions. This work was supported by grants from the National Natural Science Foundation of China (31690102, 91857204, 32021003, 31922033, 91857105, 82170891 and 91739303) to Y.L., Z.G., M. Shao and J.L.; from the Ministry of Science and Technology of China (National Key R&D Program of China 2018YFA0800700) to Y.L. and Z.G.; from Fundamental Research Funds for the Central Universities (021414380511 and 2042020kf1056) to Z.G. and Y.L.; and from Shanghai Pujiang Program (21PJ1414600) and Shanghai Municipal Science and Technology Major Project (2019SHZDZX02) to M. Shao.
Author information
Authors and Affiliations
Contributions
Y.C. and Y.L. conceived and designed the studies. Y.C. and Z. Wu performed most of the experiments and analyzed the data. S. Huang, X. Wang, Y. Hu, L.C., P.C., S.L. and S. He performed some of the animal and cell experiments. L.L. and Z.G. conducted the bioinformatics analysis. L.Z., S.D., B. Shan, Z. Song, L.J., Q. Wang, B. Song, J.L., L.R. and M. Shao provided essential reagents and assisted with the experimental design and data analysis. Y.C., M. Shao and Y.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Isabella Samuelson, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Metabolic phenotyping of Ern1AKO and Ern1BKO mice at thermoneutrality.
a, Immunoblot analysis of IRE1α protein in fractionated adipocytes and stromal vascular fraction (SVF) cells from interscapular brown adipose tissue (BAT), inguinal white adipose tissue (iWAT) and epididymal white adipose tissue (eWAT) of Ern1fl/fl littermates and Ern1AKO mice. VCL protein was used as the loading control. b-g, Male Ern1fl/fl and Ern1AKO (Ern1fl/fl;Adipoq-Cre) mice at 4 weeks of age were housed at 30oC for 6 weeks. (b) Body weight at the indicated time (n = 20 for Ern1fl/fl, n = 17 for Ern1AKO). (c) Averaged daily food intake (n = 20 for Ern1fl/fl, n = 17 for Ern1AKO). (d-g) Physical activity (d), oxygen consumption (VO2) (e), heat production (f) and respiratory exchange ratio (RER) (g) over a 24-hour period at 30oC. Shown also is regression-based analysis of absolute oxygen consumption (e) and heat production (f) against body weight (n = 10 per genotype). h, Immunoblot analysis of IRE1α in BAT, iWAT and eWAT from Ern1fl/fl and Ern1BKO (Ern1fl/fl;Ucp1-iCre) mice. i-n, Male Ern1fl/fl littermates and Ern1BKO mice were kept at room temperature (22oC) until 4 weeks of age, followed by 6 weeks of acclimation at thermoneutrality (30oC). (i,j) Body weight monitoring (i) and averaged daily food intake (j) (n = 19 per genotype). (k-n) Physical activity (k), oxygen consumption (VO2) (l), heat production (m) and RER (n) over a 24-hour period at 30oC. Shown also is regression-based analysis of absolute oxygen consumption (l) and heat production (m) against body weight (n = 8 per genotype). Quantitative results are shown as mean ± s.e.m.
Extended Data Fig. 2 Metabolic analyses of Ern1AKO and Ern1BKO mice in response to 3-day cold exposure.
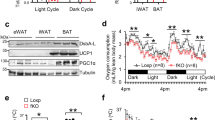
Male Ern1fl/fl littermates and Ern1AKO or Ern1BKO mice were kept at room temperature (22oC) until 4 weeks of age, followed by 6 weeks of acclimation at thermoneutrality (30oC). Mice were then maintained at 30oC or subjected to cold exposure at 8oC for 3 days. a,b, Oxygen consumption (VO2) (a) and heat production (b) of Ern1fl/fl and Ern1BKO mice were monitored over a 24-hour period on day 2 of cold exposure at 8oC. Shown also is regression-based analysis of absolute oxygen consumption (a) and heat production (b) against body weight (n = 10-11 per genotype). c, Physical activity of Ern1AKO (left) and Ern1BKO (right) mice over a 24-hour period on day 2 of cold exposure at 8oC. d, RER of Ern1AKO (left) and Ern1BKO (right) mice over a 24-hour period on day 2 of cold exposure at 8oC. e, Relative iWAT weight of Ern1fl/fl and Ern1BKO mice housed at 30oC or following 3 days of cold exposure (30oC, n = 13 for Ern1fl/fl, n = 10 for Ern1BKO; 8oC, n = 13 per genotype). f-i, Relative weight and representative H&E staining of eWAT (f,g) and BAT (h,i), respectively, from Ern1fl/fl and Ern1AKO mice housed at thermoneutrality (30oC) or after 3 days of cold exposure (n = 16 for Ern1fl/fl and n = 15 for Ern1AKO at 30oC, n = 18 for Ern1fl/fl and n = 18 for Ern1AKO at 8oC). j,k, Representative images of H&E staining (j) and UCP1 immunostaining (k) of full iWAT sections (left) with indicated amplified regions (right) from Ern1fl/fl and Ern1BKO mice housed at thermoneutrality (30oC) or after 3 days of cold exposure. Scale bar, 1 mm (left) or 200 μm (right). l, Representative images of DAPI and UCP1 immunostaining of iWAT sections from Ern1fl/fl and Ern1BKO mice housed at thermoneutrality (30oC) or after 3 days of cold exposure. Scale bar, 100 μm. m, Immunoblot analysis of IRE1α-Ser724 phosphorylation and IRE1α protein levels in iWAT (top) or BAT (bottom) from Ern1fl/fl and Ern1AKO mice housed at 30oC or after 1 hour or 3 days of cold exposure at 8oC. n,o, Immunoblot analysis of IRE1α and UCP1 protein (left) with quantification (right) of UCP1 protein levels in iWAT (n) or BAT (o) of Ern1fl/fl and Ern1BKO mice housed at 30oC or after 3 days of cold exposure at 8oC (n = 3 per genotype at 30oC; n = 5-6 per genotype at 8oC for quantification). p,q, Ern1fl/fl or Ern1BKO mice housed at 30oC for 6 weeks were cold exposed for 3 days at 8oC. (p) qRT–PCR analysis of exon 2-containing mRNA abundance of Ern1 in iWAT and BAT (n = 9-10 per genotype). (q) Agarose gel analysis of RT–PCR products corresponding to the region spanning the targeted exon 2 within Ern1 mRNA in iWAT and BAT (n = 3 per genotype). r-t, Immunostaining analysis of IRE1α protein in UCP1-expressing adipocytes using Rosa26mTmG reporter mice. (r) Representative confocal images of fixed iWAT (top) or BAT (bottom) sections from Ucp1-iCre;Rosa26mTmG mice housed for 7 days at 30oC or following 3 days of cold exposure at 8oC. Scale bar, 25 μm. Cellular membranes of UCP1-expressing beige or brown adipocytes are visualized by the membrane-targeted EGFP (mEGFP, green). (s,t) Ucp1-iCre;Rosa26mTmG or Ern1fl/fl;Ucp1-iCre;Rosa26mTmG mice maintained at 30oC for 6 weeks were subjected to cold exposure at 8oC for 3 days. (s) Representative immunostaining of IRE1α protein along with DAPI staining of fixed iWAT (top) and BAT (bottom) sections. Scale bar, 25 μm. (t) Quantification of the percentage of IRE1α-expressing adipocytes in mEGFP-positive adipocytes from iWAT and BAT sections (n = 15 images from 3 mice per genotype). Quantitative results are shown as mean ± s.e.m.; *P < 0.05, ***P < 0.001 by two-tailed Student’s t-test (p,t) or two-way ANOVA (h).
Extended Data Fig. 3 Energy expenditure analyses of Ern1AKO mice following CL316,243 stimulation.
Male Ern1fl/fl or Ern1AKO mice at 10 weeks of age were housed at room temperature and injected i.p. daily with saline or CL316,243 (1 mg/kg/day) for 3 days. (a) Heat production, (b) physical activity and (c) RER were monitored on day 3 in response to CL316,243 treatment. Shown also is the regression-based analysis (a) of CL316,243-stimulated absolute heat production against body weight. P = 0.009 when comparing the effect of genotype upon heat production (n = 15-16 per genotype). Data are shown as mean ± s.e.m.; *P < 0.05 by two-tailed Student’s t-test (a).
Extended Data Fig. 4 Stimulated lipolysis of iWAT from Ern1fl/fl and Ern1BKO mice.
iWAT explants from Ern1fl/fl and Ern1BKO mice at 10 weeks of age were treated with PBS or CL316,243 (1 μM). Glycerol (a) and FFA (b) levels were determined from the supernatant medium at the indicated stimulation intervals. Shown also is the release rate of glycerol (a) or FFA (b). Results represent 2 independent experiments (PBS, n = 24 explants from 2 mice per genotype; CL316,243, n = 48 explants from 2 mice per genotype). Data are presented as mean ± s.e.m.
Extended Data Fig. 5 Analysis of Adiponectin-Cre versus Ucp1-iCre-mediated IRE1α ablation during beige adipocyte differentiation from iWAT SVF cells.
SVF cells isolated from iWAT of Ern1fl/fl, Ern1AKO or Ern1BKO mice were differentiated for 8 days into beige adipocytes in vitro. a-d, Oil-red O staining and quantification of Oil-red O in differentiated beige adipocytes from Ern1fl/fl and Ern1AKO (a,b) or Ern1fl/fl and Ern1BKO (c,d) mice at indicated time point. Scale bar (a,c), 200 μm. e, Immunoblot analysis of IRE1α, ATGL and C/EBPβ during Ern1fl/fl and Ern1AKO beige adipocyte differentiation. f, Immunoblot analysis of IRE1α, UCP1, ATGL and C/EBPβ during Ern1fl/fl and Ern1BKO beige adipocyte differentiation. g, Immunoblot analysis of the indicated proteins in differentiated Ern1fl/fl and Ern1BKO beige adipocytes after treatment with PBS or CL316,243 (1 μM, 6 hours). Quantitative results are shown as mean ± s.e.m.
Extended Data Fig. 6 Effects of adipocyte IRE1α abrogation upon iWAT transcriptome and BAT thermogenic program.
a-f, Temperature-dependent effects of adipocyte IRE1α abrogation upon iWAT transcriptome. Bulk RNA-seq analysis of iWAT RNAs was performed for Ern1fl/fl and Ern1AKO mice housed at 30oC or following 3 days of cold exposure at 8oC as described in Fig. 4a (n = 3 mice per group). (a) Volcano plot highlighting significantly upregulated and downregulated genes (fold change >1.5 and ANOVA p-value <0.05) in Ern1AKO iWAT relative to Ern1fl/fl iWAT when housed at 30oC. (b,c) Gene ontology (GO) biological process (BP) analysis of downregulated (b) and upregulated genes (c) resulting from IRE1α abrogation in mice at 30oC. (d) Volcano plot highlighting significantly upregulated and downregulated genes (fold change >1.5 and ANOVA p-value <0.05) in Ern1AKO iWAT relative to Ern1fl/fl iWAT after cold exposure at 8oC. (e,f) GO biological process analysis of downregulated (e) and upregulated genes (f) resulting from IRE1α abrogation after cold exposure. g-j, Adipocyte IRE1α deficiency does not significantly affect the adaptive thermogenic gene program in BAT. (g,h) qRT-PCR analysis of Xbp1 mRNA splicing (g) and mRNA abundance of the indicated thermogenic genes (h) in BAT of mice following 3 days of cold exposure or at thermoneutrality (n = 5 per genotype at 30oC; n = 10 per genotype at 8oC). (i,j) qRT-PCR analysis of Xbp1 mRNA splicing (i) and mRNA abundance of the indicated thermogenic genes (j) in BAT of mice following 3 days of daily saline or CL316,243 injection (saline control, n = 6 each genotype; CL316,243 treatment, n = 9 for each genotype). Data are presented as mean ± s.e.m.; *P < 0.05, **P < 0.01 by two-way ANOVA (g,i).
Extended Data Fig. 7 Adipocyte IRE1α suppresses PGC1α through its RIDD activity.
a-c, Immortalized iWAT SVF cells from C57BL/6 J mice were transduced with retroviruses expressing the indicated transgenes and were then differentiated for 8 days into beige adipocytes in vitro. (a) Representative Oil-red O staining of differentiated beige adipocytes with relative absorbance at OD510 shown from 3 independent experiments. (b) qRT-PCR analysis of the mRNA abundance of Ppargc1a and the indicated PGC1α-activated thermogenic genes (n = 3 independent experiments). (c) Immunoblot analysis (left) and quantification (right) of the indicated proteins. d-f, Immortalized iWAT SVF cells from C57BL/6 J mice were transduced with retrovirus expressing IRE1α WT or its RNase-defective mutant (K907A) and were then differentiated into beige adipocytes. (d) Representative Oil-red O staining of differentiated beige adipocytes with relative absorbance at OD510 shown from 3 independent experiments. (e) qRT-PCR analysis of Xbp1 mRNA splicing and the mRNA abundance of the indicated thermogenic genes, RIDD target genes and transcription factors (n = 4 independent experiments). (f) Immunoblot analysis of p-IRE1α, IRE1α, XBP1s and UCP1 proteins in differentiated beige adipocytes. g, Fluorescence-based analysis of IRE1α cleavage of mouse Ppargc1a (mPpargc1a) mRNA in vitro. Synthetic wild-type (WT) or G-to-C mutant (mut) mPpargc1a mRNA substrates possessing the indicated 5’-FAM and 3’-BQH1 chemical groups were incubated for 2.5 hours with purified recombinant human IRE1α protein (18 nM) that were pre-mixed for 1 hour with DMSO or 4μ8C (20 μM). Relative fluorescence unit (RFU) was monitored. h, Gel analysis of Ppargc1a mRNA cleavage. In vitro transcription-derived mRNA fragments of human Ppargc1a (1-1000 nt) were incubated with recombinant human IRE1α protein (37oC, 60 min) in the presence of DMSO or 4μ8C (20 μM), followed by 6% TBE-Urea PAGE gel analysis. Human Xbp1 mRNA (full coding region) were used as a positive control. Arrows indicate the probable major RNA cleavage products. Results represent 2 independent experiments. Data are shown as mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA (b,c,e).
Extended Data Fig. 8 Ern1BKO mice exhibits enhanced thermogenic iWAT remodeling in response to chronic cold exposure of 15 days.
a,b, Ucp1-iCre;Rosa26mTmG or Ern1fl/fl;Ucp1-iCre;Rosa26mTmG mice were subjected to long-term cold exposure at 8oC for 15 days. (a) Representative immunostaining of IRE1α protein along with DAPI staining of fixed iWAT (top) and BAT (bottom) sections. Scale bar, 25 μm. (b) Quantification of the percentage of IRE1α-expressing adipocytes in mEGFP-positive adipocytes from iWAT and BAT sections (n = 15 images from 3 mice per group). c-o, Male Ern1fl/fl and Ern1BKO mice at 8 weeks of age were subjected to chronic cold exposure at 8oC for 15 days. (c) qRT-PCR analysis of exon 2-containing mRNA abundance of Ern1 in iWAT and BAT (n = 14 per genotype). (d) Agarose gel analysis of RT–PCR products corresponding to the region spanning the targeted exon 2 within Ern1 mRNA in iWAT and BAT (n = 3 per genotype). (e) Oxygen consumption, (f) heat production, (g) physical activity and (h) RER were monitored on day 13 of cold exposure at 8oC. Shown also are the regression-based analyses of absolute oxygen consumption (e) or heat production (f) against body weight. P = 0.036 or 0.044 when comparing the effect of genotype upon oxygen consumption or heat production (n = 14 per genotype). (i) Representative H&E staining and (j) UCP1 immunostaining of full iWAT sections (left) with indicated amplified regions (right). Scale bar, 1 mm (left) or 200 μm (right). (k) Immunoblot analysis (left) and quantification (right) of IRE1α and UCP1 proteins in iWAT of mice following 15 days of cold exposure (n = 6 per genotype). (l) qRT-PCR analysis of Xbp1 mRNA splicing and mRNA abundance of Ppargc1a and Ucp1 in iWAT of mice (n = 14 per genotype). (m) Representative H&E staining of BAT sections. Scale bar, 200 μm. (n) Immunoblot analysis (left) and quantification (right) of BAT IRE1α and UCP1 proteins (n = 6 per genotype). (o) qRT-PCR analysis of BAT Xbp1 mRNA splicing and mRNA abundance of Ppargc1a and Ucp1 (n = 14 per genotype). Quantitative results are shown as mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001 by two-tailed Student’s t-test (b,c,k,l,n,o).
Extended Data Fig. 9 Adipocyte IRE1α abrogation promotes energy expenditure and ameliorates dietary obesity and metabolic inflammation.
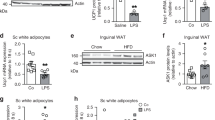
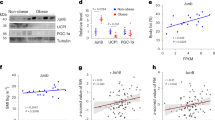
a-c, Hyperactivation of adipocyte IRE1α RNase is accompanied by decreased mRNA level of Ppargc1a and RIDD targets in HFD-fed mice. Male Ern1fl/fl mice were fed a normal chow (NC, 10% fat) or a high-fat diet (HFD, 60% fat) for 14 weeks, starting at 8 weeks of age. Total RNA was extracted from fractionated adipocytes prepared from iWAT, followed by qRT-PCR analysis (n = 10 for NC; n = 9 for HFD). (a) Relative levels of Ern1 mRNA and Xbp1 mRNA splicing. (b) Relative mRNA levels of two typical RIDD target genes. (c) Relative mRNA levels of the indicated thermogenic regulators. d-n, Metabolic effects of adipocyte IRE1α abrogation in HFD-fed mice. Male Ern1fl/fl and Ern1AKO mice at 8 weeks of age were maintained on a normal chow (NC, 10% fat) diet or fed a high-fat diet (HFD, 60% fat) for 14 weeks. (d-f) Body weight gain (d) (NC, n = 20 per genotype; HFD, n = 33 for Ern1fl/fl littermates and n = 31 for Ern1AKO), fat pad weight (e) (NC, n = 12 per genotype; HFD, n = 30 per genotype) and averaged daily food intake (f) (NC, n = 9 for Ern1fl/fl littermates and n = 12 for Ern1AKO; HFD, n = 23 for Ern1fl/fl and n = 21 for Ern1AKO). (g-j) Physical activity (g), oxygen consumption (VO2) (h), heat production (i) and RER (j) were monitored over a 24-hour period (NC, n = 12 per genotype; HFD, n = 19 for Ern1fl/fl and n = 18 for Ern1AKO). Shown also in (i) is the regression-based analysis of heat production against body weight, with P values representing the effect of genotype upon heat production. (k,l) Representative H&E staining (k) and F4/80 IHC staining (l) of eWAT sections. Scale bar, 100 μm. (m) Representative flow cytometry histograms for CD11b- and F4/80-expressing SVF cells from eWAT of HFD-fed animals. Bars represent percentages of CD11b+F4/80+ cells (n = 4 per genotype). (n) qRT-PCR analysis of mRNA abundance of the indicated inflammatory genes in SVF or adipocytes from eWAT (SVF, n = 4 for Ern1fl/fl and n = 3 for Ern1AKO; Adipocytes, n = 6 per genotype). Data are shown as mean ± s.e.m.; *P < 0.05, **P < 0.01, ***P < 0.001 by two-tailed Student’s t-test (a,b,c,e,m,n) or two-way ANOVA (d).
Extended Data Fig. 10 Pharmacological inhibition of IRE1α RNase activity ameliorates diet-induced obesity.
a, Experimental design. Male Ern1fl/fl were fed an HFD for 6 weeks prior to i.p. injection of vehicle (Veh) or 4μ8C every other day for 4 weeks. Male Ern1AKO littermates was likewise fed an HFD and treated with Veh to serve as a control cohort. b, Body weight monitoring every other day (n = 8 for Ern1fl/fl + Veh, n = 8 for Ern1fl/fl + 4μ8C, n = 9 for Ern1AKO + Veh). c, Body fat mass and lean mass (n = 8 for Ern1fl/fl + Veh, n = 8 for Ern1fl/fl + 4μ8C, n = 9 for Ern1AKO + Veh). d, Glucose tolerance test (n = 8 per group). e, Fat pad weight (n = 8 for Ern1fl/fl + Veh, n = 8 for Ern1fl/fl + 4μ8C, n = 9 for Ern1AKO + Veh). f,g, Representative H&E staining (f) and UCP1 immunostaining (g) of iWAT sections. Scale bar, 200 μm. h, qRT-PCR analysis of Xbp1 mRNA splicing and the mRNA abundance of thermogenic genes in iWAT from HFD-fed Ern1fl/fl mice following Veh or 4μ8C treatment (n = 8 per group). All results represent two independent 4μ8C treatment animal experiments, and data are shown as mean ± s.e.m.; #P < 0.05, ##P < 0.01, ###P < 0.001 (Ern1AKO + Veh versus Ern1fl/fl + Veh in d); *P < 0.05, **P < 0.01, ***P < 0.001 by two-tailed Student’s t-test (b,d,h) or one-way ANOVA (c,e).
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Supplementary Methods, Supplementary Tables 1–5 and Supplementary References.
Supplementary Table 6
Statistical data (exact P values and sample/cohort sizes for each dataset in the study).
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical Source Data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Wu, Z., Huang, S. et al. Adipocyte IRE1α promotes PGC1α mRNA decay and restrains adaptive thermogenesis. Nat Metab 4, 1166–1184 (2022). https://doi.org/10.1038/s42255-022-00631-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00631-8
This article is cited by
-
Pulling the trigger: Noncoding RNAs in white adipose tissue browning
Reviews in Endocrine and Metabolic Disorders (2024)
-
IRE1α: from the function to the potential therapeutic target in atherosclerosis
Molecular and Cellular Biochemistry (2023)