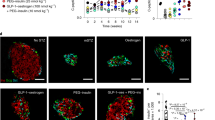
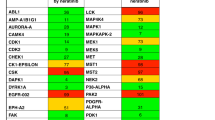
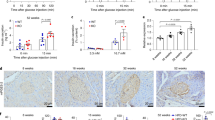
Abstract
Insulin is a life-saving drug for patients with type 1 diabetes; however, even today, no pharmacotherapy can prevent the loss or dysfunction of pancreatic insulin-producing β cells to stop or reverse disease progression. Thus, pancreatic β cells have been a main focus for cell-replacement and regenerative therapies as a curative treatment for diabetes. In this Review, we highlight recent advances toward the development of diabetes therapies that target β cells to enhance proliferation, redifferentiation and protection from cell death and/or enable selective killing of senescent β cells. We describe currently available therapies and their mode of action, as well as insufficiencies of glucagon-like peptide 1 (GLP-1) and insulin therapies. We discuss and summarize data collected over the last decades that support the notion that pharmacological targeting of β cell insulin signalling might protect and/or regenerate β cells as an improved treatment of patients with diabetes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banting, F. G. & Best, C. H. The internal secretion of the pancreas. Transl. Res. VII, 251–266 (1992).
Banting, F. G. The history of insulin. Edinb. Med. J. 36, 1–18 (1929).
Ashcroft, F. M. & Rorsman, P. Diabetes mellitus and the β cell: the last ten years. Cell 148, 1160–1171 (2012).
Vecchio, I., Tornali, C., Bragazzi, N. L. & Martini, M. The discovery of insulin: an important milestone in the history of medicine. Front. Endocrinol. 9, 613 (2018).
Florez, J. C. Newly identified loci highlight β cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia 51, 1100–1110 (2008).
McCarthy, M. I. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 363, 2339–2350 (2010).
Voight, B. F. et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 42, 579–589 (2010).
Nolan, C. J. & Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: time for a conceptual framework shift. Diab. Vasc. Dis. Res. 16, 118–127 (2019).
Kulkarni, R. N. et al. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96, 329–339 (1999).
Withers, D. J. et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904 (1998).
Ueki, K. et al. Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat. Genet. 38, 583–588 (2006).
Kulkarni, R. N. Receptors for insulin and insulin-like growth factor-1 and insulin receptor substrate-1 mediate pathways that regulate islet function. Biochem. Soc. Trans. 30, 317–322 (2002).
Leibiger, I. B., Leibiger, B. & Berggren, P.-O. Insulin signaling in the pancreatic β-cell. Annu. Rev. Nutr. 28, 233–251 (2008).
Brissova, M. et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53, 1087–1097 (2005).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103, 2334–2339 (2006).
Dolenšek, J., Rupnik, M. S. & Stožer, A. Structural similarities and differences between the human and the mouse pancreas. Islets 7, e1024405 (2015).
Gan, W. J. et al. Cell polarity defines three distinct domains in pancreatic β-cells. J. Cell Sci. 130, 143–151 (2017).
Bosco, D. et al. Unique arrangement of α- and β-cells in human islets of Langerhans. Diabetes 59, 1202–1210 (2010).
Stožer, A. et al. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLoS Comput. Biol. 9, e1002923 (2013).
Tritschler, S., Theis, F. J., Lickert, H. & Böttcher, A. Systematic single-cell analysis provides new insights into heterogeneity and plasticity of the pancreas. Mol. Metab. 6, 974–990 (2017).
Carrano, A. C., Mulas, F., Zeng, C. & Sander, M. Interrogating islets in health and disease with single-cell technologies. Mol. Metab. 6, 991–1001 (2017).
Nasteska, D. & Hodson, D. J. The role of β cell heterogeneity in islet function and insulin release. J. Mol. Endocrinol. 61, R43–R60 (2018).
Bader, E. et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535, 430–434 (2016).
Roscioni, S. S., Migliorini, A., Gegg, M. & Lickert, H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 12, 695–709 (2016).
Bilekova, S., Sachs, S. & Lickert, H. Pharmacological targeting of endoplasmic reticulum stress in pancreatic β cells. Trends Pharmacol. Sci. 42, 85–95 (2021).
Iversen, J. & Miles, D. W. Evidence for a feedback inhibition of insulin on insulin secretion in the isolated, perfused canine pancreas. Diabetes 20, 1–9 (1971).
Rappaport, A. M. et al. Effects on insulin output and on pancreatic blood flow of exogenous insulin infusion into an in situ isolated portion of the pancreas. Endocrinology 91, 168–176 (1972).
Okada, T. et al. Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc. Natl Acad. Sci. USA 104, 8977–8982 (2007).
Otani, K. et al. Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am. J. Physiol. Endocrinol. Metab. 286, E41–E49 (2004).
Kulkarni, R. N. et al. β-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat. Genet. 31, 111–115 (2002).
George, M. et al. β cell expression of IGF-I leads to recovery from type 1 diabetes. J. Clin. Invest. 109, 1153–1163 (2002).
Fan, Y. et al. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 28, 2812–2824 (2009).
Johnson, J. D. A practical guide to genetic engineering of pancreatic β-cells in vivo: getting a grip on RIP and MIP. Islets 6, e944439 (2014).
Mehran, A. E. et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16, 723–737 (2012).
Wicksteed, B. et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59, 3090–3098 (2010).
Trinder, M., Zhou, L., Oakie, A., Riopel, M. & Wang, R. β-cell insulin receptor deficiency during in utero development induces an islet compensatory overgrowth response. Oncotarget 7, 44927–44940 (2016).
Skovsø, S. et al. β-cell specific Insr deletion promotes insulin hypersecretion and improves glucose tolerance prior to global insulin resistance. Nat. Commun. 13, 735 (2022).
Brouwers, B. et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 20, 979–990 (2014).
Thorens, B. et al. Ins1Cre knock-in mice for β cell-specific gene recombination. Diabetologia 58, 558–565 (2015).
Hashimoto, N. et al. Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β cell mass. Nat. Genet. 38, 589–593 (2006).
Bernal-Mizrachi, E. et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet β cells. J. Clin. Invest. 114, 928–936 (2004).
Nakae, J. et al. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 32, 245–253 (2002).
Rachdi, L. et al. Disruption of Tsc2 in pancreatic cells induces cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc. Natl Acad. Sci. USA 105, 9250–9255 (2008).
Rothenberg, P. L., Willison, L. D., Simon, J. & Wolf, B. A. Glucose-induced insulin receptor tyrosine phosphorylation in insulin-secreting β-cells. Diabetes 44, 802–809 (1995).
Velloso, L. A., Carneiro, E. M., Crepaldi, S. C., Boschero, A. C. & Saad, M. J. Glucose- and insulin-induced phosphorylation of the insulin receptor and its primary substrates IRS-1 and IRS-2 in rat pancreatic islets. FEBS Lett. 377, 353–357 (1995).
Rachdaoui, N. Insulin: the friend and the foe in the development of type 2 diabetes mellitus. Int. J. Mol. Sci. 21, 1770 (2020).
Leibiger, B. et al. Short-term regulation of insulin gene transcription by glucose. Proc. Natl Acad. Sci. USA 95, 9307–9312 (1998).
Leibiger, B., Wahlander, K., Berggren, P. O. & Leibiger, I. B. Glucose-stimulated insulin biosynthesis depends on insulin-stimulated insulin gene transcription. J. Biol. Chem. 275, 30153–30156 (2000).
Leibiger, B. et al. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic β cells. Mol. Cell 7, 559–570 (2001).
Leibiger, B., Moede, T., Uhles, S., Berggren, P. O. & Leibiger, I. B. Short-term regulation of insulin gene transcription. Biochem. Soc. Trans. 30, 312–317 (2002).
Xu, G. G. & Rothenberg, P. L. Insulin receptor signaling in the beta-cell influences insulin gene expression and insulin content: evidence for autocrine β-cell regulation. Diabetes 47, 1243–1252 (1998).
Johnson, J. D. et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc. Natl Acad. Sci. USA 103, 19575–19580 (2006).
Movassat, J., Saulnier, C. & Portha, B. Insulin administration enhances growth of the β-cell mass in streptozotocin-treated newborn rats. Diabetes 46, 1445–1452 (1997).
Beith, J. L., Alejandro, E. U. & Johnson, J. D. Insulin stimulates primary β-cell proliferation via Raf-1 kinase. Endocrinology 149, 2251–2260 (2008).
Frerichs, H., Reich, U. & Creutzfeldt, W. Insulin secretion in vitro. I. Inhibition of glucose-induced insulin release by insulin. Klin. Wochenschr. 43, 136–140 (1965).
Ammon, H. P., Reiber, C. & Verspohl, E. J. Indirect evidence for short-loop negative feedback of insulin secretion in the rat. J. Endocrinol. 128, 27–34 (1991).
Jimenez-Feltstrom, J., Lundquist, I., Obermuller, S. & Salehi, A. Insulin feedback actions: complex effects involving isoforms of islet nitric oxide synthase. Regul. Pept. 122, 109–118 (2004).
Carpentier, J. L., Fehlmann, M., Van Obberghen, E., Gorden, P. & Orci, L. Insulin receptor internalization and recycling: mechanism and significance. Biochimie 67, 1143–1145 (1985).
Zick, Y. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE 2005, e4 (2005).
Guillen, C., Bartolomé, A., Nevado, C. & Benito, M. Biphasic effect of insulin on β cell apoptosis depending on glucose deprivation. FEBS Lett. 582, 3855–3860 (2008).
Bucris, E. et al. Prolonged insulin treatment sensitizes apoptosis pathways in pancreatic β cells. J. Endocrinol. 230, 291–307 (2016).
Rachdaoui, N., Polo-Parada, L. & Ismail-Beigi, F. Prolonged exposure to insulin inactivates Akt and Erk1/2 and increases pancreatic islet and INS1E β-cell apoptosis. J. Endocr. Soc. 3, 69–90 (2019).
Marchetti, P. et al. Insulin inhibits its own secretion from isolated, perifused human pancreatic islets. Acta Diabetol. 32, 75–77 (1995).
Song, S. H. et al. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 85, 4491–4499 (2000).
Wang, M., Li, J., Lim, G. E. & Johnson, J. D. Is dynamic autocrine insulin signaling possible? A mathematical model predicts picomolar concentrations of extracellular monomeric insulin within human pancreatic islets. PLoS ONE 8, e64860 (2013).
Ansarullah et al. Inceptor counteracts insulin signalling in β-cells to control glycaemia. Nature 590, 326–331 (2021).
Finegood, D. T., Scaglia, L. & Bonner-Weir, S. Dynamics of β-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 44, 249–256 (1995).
Weir, G. C. & Bonner‐Weir, S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann. N. Y. Acad. Sci. 1281, 92–105 (2013).
Meier, J. J. et al. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57, 1584–1594 (2008).
Kassem, S. A., Ariel, I., Thornton, P. S., Scheimberg, I. & Glaser, B. β-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49, 1325–1333 (2000).
Gregg, B. E. et al. Formation of a human β-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab. 97, 3197–3206 (2012).
Perl, S. et al. Significant human β-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 95, E234–E239 (2010).
Cnop, M. et al. The long lifespan and low turnover of human islet β cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 53, 321–330 (2010).
In’t Veld, P. et al. β-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes 59, 1702–1708 (2010).
Ritzel, R. A., Butler, A. E., Rizza, R. A., Veldhuis, J. D. & Butler, P. C. Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29, 717–718 (2006).
Wang, Y. J. et al. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab. 24, 616–626 (2016).
Kulkarni, R. N., Mizrachi, E.-B., Ocana, A. G. & Stewart, A. F. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61, 2205–2213 (2012).
Bernal-Mizrachi, E. et al. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes 63, 819–831 (2014).
Stewart, A. F. et al. Human β-cell proliferation and intracellular signaling: part 3. Diabetes 64, 1872–1885 (2015).
Alonso, L. C. et al. Glucose infusion in mice: a new model to induce β-cell replication. Diabetes 56, 1792–1801 (2007).
Levitt, H. E. et al. Glucose stimulates human β cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 54, 572–582 (2011).
Kondegowda, N. G. et al. Osteoprotegerin and denosumab stimulate human β cell proliferation through inhibition of the receptor activator of NF-κB ligand pathway. Cell Metab. 22, 77–85 (2015).
Robitaille, K. et al. High-throughput functional genomics identifies regulators of primary human β cell proliferation. J. Biol. Chem. 291, 4614–4625 (2016).
Dirice, E. et al. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes 65, 1660–1671 (2016).
Wang, P. et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic β cell replication. Nat. Med. 21, 383–388 (2015).
Shen, W. et al. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat. Commun. 6, 8372 (2015).
Shcheglova, E., Blaszczyk, K. & Borowiak, M. Mitogen synergy: an emerging route to boosting human β cell proliferation. Front. Cell Dev. Biol. 9, 734597 (2021).
Wang, P. et al. Human β cell regenerative drug therapy for diabetes: past achievements and future challenges. Front. Endocrinol. 12, 671946 (2021).
Robertson, R. P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J. Biol. Chem. 279, 42351–42354 (2004).
Tanaka, Y., Gleason, C. E., Tran, P. O., Harmon, J. S. & Robertson, R. P. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc. Natl Acad. Sci. USA 96, 10857–10862 (1999).
Kaneto, H. et al. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes 48, 2398–2406 (1999).
Leibowitz, G. et al. Glucose regulation of β-cell stress in type 2 diabetes. Diabetes Obes. Metab. 12, 66–75 (2010).
Kim, J.-W. & Yoon, K.-H. Glucolipotoxicity in pancreatic β-cells. Diabetes Metab. J. 35, 444–450 (2011).
Poitout, V. & Robertson, R. P. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr. Rev. 29, 351–366 (2008).
Prentki, M. & Nolan, C. J. Islet β cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812 (2006).
van Raalte, D. H. & Diamant, M. Glucolipotoxicity and β cells in type 2 diabetes mellitus: target for durable therapy? Diabetes Res. Clin. Pract. 93, S37–S46 (2011).
Eizirik, D. L., Cardozo, A. K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 (2008).
Oslowski, C. M. & Urano, F. A switch from life to death in endoplasmic reticulum stressed β-cells. Diabetes Obes. Metab. 12, 58–65 (2010).
Eguchi, N., Vaziri, N. D., Dafoe, D. C. & Ichii, H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int. J. Mol. Sci. 22, 1509 (2021).
Robertson, R. P., Harmon, J., Tran, P. O., Tanaka, Y. & Takahashi, H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 52, 581–587 (2003).
Hansen, J. B. et al. Glucolipotoxic conditions induce β-cell iron import, cytosolic ROS formation and apoptosis. J. Mol. Endocrinol. 61, 69–77 (2018).
Del Guerra, S. et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54, 727–735 (2005).
Maedler, K. et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 127, 1589 (2017).
Ehses, J. A., Böni-Schnetzler, M., Faulenbach, M. & Donath, M. Y. Macrophages, cytokines and β-cell death in type 2 diabetes. Biochem. Soc. Trans. 36, 340–342 (2008).
Welsh, N. et al. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 54, 3238–3244 (2005).
Wali, J. A. et al. Activation of the NLRP3 inflammasome complex is not required for stress-induced death of pancreatic islets. PLoS ONE 9, e113128 (2014).
Inoue, H. et al. Signaling between pancreatic β cells and macrophages via S100 calcium-binding protein A8 exacerbates β-cell apoptosis and islet inflammation. J. Biol. Chem. 293, 5934–5946 (2018).
Wajchenberg, B. L. β-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 28, 187–218 (2007).
DeFronzo, R. A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int. J. Clin. Pract. Suppl. https://doi.org/10.1111/j.1368-504x.2004.00389.x 9–21 (2004).
Lytrivi, M., Castell, A.-L., Poitout, V. & Cnop, M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J. Mol. Biol. 432, 1514–1534 (2020).
Prentki, M., Peyot, M.-L., Masiello, P. & Madiraju, S. R. M. Nutrient-induced metabolic stress, adaptation, detoxification, and toxicity in the pancreatic β-cell. Diabetes 69, 279–290 (2020).
Weir, G. C. Glucolipotoxicity, β-cells, and diabetes: the emperor has no clothes. Diabetes 69, 273–278 (2020).
Forouhi, N. G. et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case–cohort study. Lancet Diabetes Endocrinol. 2, 810–818 (2014).
Cnop, M. et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes 63, 1978–1993 (2014).
Mir, S. U. R. et al. Inhibition of autophagic turnover in β-cells by fatty acids and glucose leads to apoptotic cell death. J. Biol. Chem. 290, 6071–6085 (2015).
Trudeau, K. M. et al. Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J. Cell Biol. 214, 25–34 (2016).
Las, G., Serada, S. B., Wikstrom, J. D., Twig, G. & Shirihai, O. S. Fatty acids suppress autophagic turnover in β-cells. J. Biol. Chem. 286, 42534–42544 (2011).
Chen, Y.-Y. et al. Palmitate induces autophagy in pancreatic β-cells via endoplasmic reticulum stress and its downstream JNK pathway. Int. J. Mol. Med. 32, 1401–1406 (2013).
Bugliani, M. et al. Modulation of autophagy influences the function and survival of human pancreatic β cells under endoplasmic reticulum stress conditions and in type 2 diabetes. Front. Endocrinol. 10, 52 (2019).
Hong, S.-W. et al. Clusterin protects lipotoxicity-induced apoptosis via upregulation of autophagy in insulin-secreting cells. Endocrinol. Metab. 35, 943–953 (2020).
Thompson, P. J. et al. Targeted elimination of senescent β cells prevents type 1 diabetes. Cell Metab. 29, 1045–1060 (2019).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660 (2017).
Prata, L. G. P. L., Ovsyannikova, I. G., Tchkonia, T. & Kirkland, J. L. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin. Immunol. 40, 101275 (2018).
Midha, A. et al. Unique human and mouse β-cell senescence-associated secretory phenotype (SASP) reveal conserved signaling pathways and heterogeneous factors. Diabetes 70, 1098–1116 (2021).
Niedernhofer, L. J. et al. Nuclear genomic instability and aging. Annu. Rev. Biochem. 87, 295–322 (2018).
Ardestani, A. et al. MST1 is a key regulator of β cell apoptosis and dysfunction in diabetes. Nat. Med. 20, 385–397 (2014).
Thompson, P. J., Shah, A., Apostolopolou, H. & Bhushan, A. BET proteins are required for transcriptional activation of the senescent islet cell secretome in type 1 diabetes. Int. J. Mol. Sci. 20, 4776 (2019).
Aguayo-Mazzucato, C. et al. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 30, 129–142 (2019).
Talchai, C., Xuan, S., Lin, H. V., Sussel, L. & Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 (2012).
Wang, Z., York, N. W., Nichols, C. G. & Remedi, M. S. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19, 872–882 (2014).
Marselli, L. et al. Are we overestimating the loss of β cells in type 2 diabetes? Diabetologia 57, 362–365 (2014).
Cinti, F. et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J. Clin. Endocrinol. Metab. 101, 1044–1054 (2016).
Sachs, S. et al. Targeted pharmacological therapy restores β-cell function for diabetes remission. Nat. Metab. 2, 192–209 (2020).
Camunas-Soler, J. et al. Patch-seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 31, 1017–1031 (2020).
Kluth, O. et al. Dissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: role of forkhead box O1 (FOXO1) in glucose-induced β cell failure. Diabetologia 54, 605–616 (2011).
Sheng, C. et al. Reversibility of β-cell-specific transcript factors expression by long-term caloric restriction in db/db mouse. J. Diabetes Res. 2016, 6035046 (2016).
Casteels, T. et al. An inhibitor-mediated β-cell dedifferentiation model reveals distinct roles for FoxO1 in glucagon repression and insulin maturation. Mol. Metab. 54, 101329 (2021).
Oppenländer, L. et al. Vertical sleeve gastrectomy triggers fast β-cell recovery upon overt diabetes. Mol. Metab. 54, 101330 (2021).
Butler, A. E. et al. β-cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J. Clin. Endocrinol. Metab. 101, 523–532 (2016).
Amo-Shiinoki, K. et al. Islet cell dedifferentiation is a pathologic mechanism of long-standing progression of type 2 diabetes. JCI Insight 6, e143791 (2021).
Abd El Aziz, M. S., Kahle, M., Meier, J. J. & Nauck, M. A. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes. Metab. 19, 216–227 (2017).
Chaplin, S. Rybelsus: an oral formulation of the GLP‐1 agonist semaglutide. Prescriber 31, 32–33 (2020).
Danielsen, M. K., Bohsen, D. M., Svarrer, V. B., Rendbæk, A. S. & Root, M. J. Rybelsus® was more effective in achieving clinically relevant blood sugar and weight reductions in people with type 2 diabetes vs all active comparators. Ann Søndermølle Rendbæk 45, 2253 (2020).
Griffith, D. A. et al. A small-molecule oral agonist of the human glucagon-like peptide-1 receptor. J. Med. Chem. 65, 8208–8226 (2022).
Cornu, M. et al. Glucagon-like peptide-1 increases β-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J. Biol. Chem. 285, 10538–10545 (2010).
Szczerbinska, I. et al. Large-scale functional genomics screen to identify modulators of human β-cell insulin secretion. Biomedicines 10, 103 (2022).
Yang, Y. et al. Rheb1 promotes glucose-stimulated insulin secretion in human and mouse β-cells by upregulating GLUT expression. Metabolism 123, 154863 (2021).
Daziano, G. et al. Sortilin-derived peptides promote pancreatic β-cell survival through CREB signaling pathway. Pharmacol. Res. 167, 105539 (2021).
Ardestani, A. et al. Neratinib protects pancreatic β cells in diabetes. Nat. Commun. 10, 5015 (2019).
Home, P. D. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes. Metab. 14, 780–788 (2012).
Owens, D. R. & Bolli, G. B. The continuing quest for better subcutaneously administered prandial insulins: a review of recent developments and potential clinical implications. Diabetes Obes. Metab. 22, 743–754 (2020).
Marso, S. P. et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N. Engl. J. Med. 377, 723–732 (2017).
Najjar, S. M. & Perdomo, G. Hepatic insulin clearance: mechanism and physiology. Physiology 34, 198–215 (2019).
Caparrotta, T. M. & Evans, M. PEGylated insulin Lispro, (LY2605541)—a new basal insulin analogue. Diabetes Obes. Metab. 16, 388–395 (2013).
Geho, W. B., Geho, H. C., Lau, J. R. & Gana, T. J. Hepatic-directed vesicle insulin: a review of formulation development and preclinical evaluation. J. Diabetes Sci. Technol. 3, 1451–1459 (2009).
Zeng, Y., Wang, J., Gu, Z. & Gu, Z. Engineering glucose-responsive insulin. Med. Drug Discov. 3, 100010 (2019).
Wang, J. et al. Glucose-responsive insulin and delivery systems: innovation and translation. Adv Mater. 32, 1–35 (2020).
Zhou, X. et al. Oral delivery of insulin with intelligent glucose-responsive switch for blood glucose regulation. J. Nanobiotechnology 18, 96 (2020).
The Diabetes Control and Complications Trial Research Group, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
Cully, M. Findings from DCCT — glycaemic control prevents diabetes complications. Nat. Milestones, Diabetes https://go.nature.com/3wqnYKI (2021).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes: A patient- centered approach. Diabetes Care 35, 1364–1379 (2012).
Weng, J. et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371, 1753–1760 (2008).
Li, Y. et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic. Diabetes Care 27, 2597–2602 (2004).
Xu, W., Li, Y.-B., Deng, W.-P., Hao, Y.-T. & Weng, J.-P. Remission of hyperglycemia following intensive insulin therapy in newly diagnosed type 2 diabetic patients: a long-term follow-up study. Chin. Med. J. (Engl) 122, 2554–2559 (2009).
Hanefeld, M., Fleischmann, H., Landgraf, W. & Pistrosch, F. EARLY study: early basal insulin therapy under real-life conditions in type 2 diabetics. Diabetes Stoffw. Herz. 21, 91–97 (2012).
Kramer, C. K., Zinman, P. B. & Retnakaran, R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet, Diabetes Endocrinol. 1, 28–34 (2013).
Adeva-Andany, M. M., Martínez-Rodríguez, J., González-Lucán, M., Fernández-Fernández, C. & Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 13, 1449–1455 (2019).
Herman, M. E., O’Keefe, J. H., Bell, D. S. H. & Schwartz, S. S. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog. Cardiovasc. Dis. 60, 422–434 (2017).
Holden, S. E. et al. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes. Metab. 17, 350–362 (2015).
Gamble, J.-M. et al. Association of insulin dosage with mortality or major adverse cardiovascular events: a retrospective cohort study. Lancet Diabetes Endocrinol. 5, 43–52 (2017).
Yki-Jaarvinen, H. et al. Comparison of insulin regimens in patients with non-insulin dependent diabetes mellitus. Endocrinologist 3, 159 (1993).
Holman, R. R. et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361, 1736–1747 (2009).
Bonds, D. E. et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 340, b4909 (2010).
Skyler, J. S. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 32, e92–e93 (2009).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Author information
Authors and Affiliations
Contributions
C.J., A., S.B. and H.L. conceived the concepts described in this review. C.J., A., S.B. and H.L. wrote the manuscript. Figures were prepared by C.J., A. and S.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest. C.J. works as a fulltime employee at Genentech Inc., CA 94080, USA, and A. works as a fulltime employee at The Jackson Laboratory, Bar Harbor, ME 04609, USA.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Christoph Schmitt, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jain, C., Ansarullah, Bilekova, S. et al. Targeting pancreatic β cells for diabetes treatment. Nat Metab 4, 1097–1108 (2022). https://doi.org/10.1038/s42255-022-00618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00618-5
This article is cited by
-
Connections between body composition and dysregulation of islet α- and β-cells in type 2 diabetes
Diabetology & Metabolic Syndrome (2024)
-
ISR inhibition reverses pancreatic β-cell failure in Wolfram syndrome models
Cell Death & Differentiation (2024)
-
Delineating mouse β-cell identity during lifetime and in diabetes with a single cell atlas
Nature Metabolism (2023)
-
O304 ameliorates hyperglycemia in mice by dually promoting muscle glucose effectiveness and preserving β-cell function
Communications Biology (2023)
-
Talin-1 inhibits Smurf1-mediated Stat3 degradation to modulate β-cell proliferation and mass in mice
Cell Death & Disease (2023)