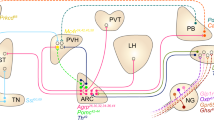
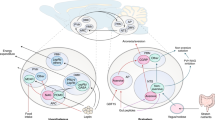
Abstract
The overconsumption of highly caloric and palatable foods has caused a surge in obesity rates in the past half century, thereby posing a healthcare challenge due to the array of comorbidities linked to heightened body fat accrual. Developing treatments to manage body weight requires a grasp of the neurobiological basis of appetite. In this Review, we discuss advances in neuroscience that have identified brain regions and neural circuits that coordinate distinct phases of eating: food procurement, food consumption, and meal termination. While pioneering work identified several hypothalamic nuclei to be involved in feeding, more recent studies have explored how neuronal populations beyond the hypothalamus, such as the mesolimbic pathway and nodes in the hindbrain, interconnect to modulate appetite. We also examine how long-term exposure to a calorically dense diet rewires feeding circuits and alters the response of motivational systems to food. Understanding how the nervous system regulates eating behaviour will bolster the development of medical strategies that will help individuals to maintain a healthy body weight.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Collaborators, G. B. D. O. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Sternson, S. M. & Eiselt, A. K. Three pillars for the neural control of appetite. Annu Rev. Physiol. 79, 401–423 (2017). This review served as inspiration for dividing up the discrete phases of feeding into three acts.
Anand, B. K., Dua, S. & Shoenberg, K. Hypothalamic control of food intake in cats and monkeys. J. Physiol. 127, 143–152 (1955).
Aravich, P. F. & Sclafani, A. Paraventricular hypothalamic lesions and medial hypothalamic knife cuts produce similar hyperphagia syndromes. Behav. Neurosci. 97, 970–983 (1983).
Bergen, H. T., Mizuno, T. M., Taylor, J. & Mobbs, C. V. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology 139, 4483–4488 (1998).
Gold, R. M., Quackenbush, P. M. & Kapatos, G. Obesity following combination of rostrolateral to VMH cut and contralateral mammillary area lesion. J. Comp. Physiol. Psychol. 79, 210–218 (1972).
Gold, R. M., Jones, A. P. & Sawchenko, P. E. Paraventricular area: critical focus of a longitudinal neurocircuitry mediating food intake. Physiol. Behav. 18, 1111–1119 (1977).
Holzwarth-McBride, M. A., Hurst, E. M. & Knigge, K. M. Monosodium glutamate induced lesions of the arcuate nucleus. I. Endocrine deficiency and ultrastructure of the median eminence. Anat. Rec. 186, 185–205 (1976).
Holzwarth-McBride, M. A., Sladek, J. R. Jr. & Knigge, K. M. Monosodium glutamate induced lesions of the arcurate nucleus. II. Fluorescence histochemistry of catecholamines. Anat. Rec. 186, 197–205 (1976).
Leibowitz, S. F. Reciprocal hunger-regulating circuits involving alpha- and beta-adrenergic receptors located, respectively, in the ventromedial and lateral hypothalamus. Proc. Natl Acad. Sci. USA 67, 1063–1070 (1970).
Leibowitz, S. F., Hammer, N. J. & Chang, K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 27, 1031–1040 (1981).
Teitelbaum, P. & Epstein, A. N. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol. Rev. 69, 74–90 (1962). This study was part of a revoultion of lesion experiments demonstrating the necessity of hypothalamic structures in the regulation of body weight homeostasis.
Boghossian, S., Park, M. & York, D. A. Melanocortin activity in the amygdala controls appetite for dietary fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R385–R393 (2010).
Booth, D. A. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J. Pharmacol. Exp. Ther. 160, 336–348 (1968).
Grill, H. J., Ginsberg, A. B., Seeley, R. J. & Kaplan, J. M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, 10128–10135 (1998).
Grossman, S. P. Eating or drinking elicited by direct adrenergic or cholinergic stimulation of hypothalamus. Science 132, 301–302 (1960).
Williams, D. L., Kaplan, J. M. & Grill, H. J. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141, 1332–1337 (2000).
Hoebel, B. G. & Teitelbaum, P. Hypothalamic control of feeding and self-stimulation. Science 135, 375–377 (1962).
Tenen, S. S. & Miller, N. E. Strength of electrical stimulation of lateral hypothalamus, food deprivation, and tolerance for quinine in food. J. Comp. Physiol. Psychol. 58, 55–62 (1964).
Schultz, W. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol. 7, 191–197 (1997).
Salamone, J. D., Correa, M., Mingote, S. & Weber, S. M. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Ther. 305, 1–8 (2003).
Wise, R. A. The parsing of food reward. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1234–R1235 (2006).
Palmiter, R. D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 30, 375–381 (2007).
Zhou, Q. Y. & Palmiter, R. D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995).
Cohen, P. et al. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest. 108, 1113–1121 (2001).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This study identified that mutations in the ob gene encoding leptin resulted in profound obsesity and type II diabetes.
Butler, A. A. et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997). This is one of three studies that demonstrated the essential role of the melanocortin system on the control of energy balance.
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997). This is one of three studies that demonstrated the essential role of the melanocortin system on the control of energy balance.
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997). This is one of three studies that demonstrated the essential role of the melanocortin system on the control of energy balance.
Yaswen, L., Diehl, N., Brennan, M. B. & Hochgeschwender, U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 5, 1066–1070 (1999).
Scrocchi, L. A. et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 2, 1254–1258 (1996).
Scrocchi, L. A., Marshall, B. A., Cook, S. M., Brubaker, P. L. & Drucker, D. J. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes 47, 632–639 (1998).
Hansotia, T. et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 53, 1326–1335 (2004).
Gropp, E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 8, 1289–1291 (2005).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Wu, Q., Whiddon, B. B. & Palmiter, R. D. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc. Natl Acad. Sci. USA 109, 3155–3160 (2012).
Xi, D., Gandhi, N., Lai, M. & Kublaoui, B. M. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE 7, e36453 (2012).
Zhan, C. et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33, 3624–3632 (2013).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Muller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Tschop, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Drazen, D. L., Vahl, T. P., D’Alessio, D. A., Seeley, R. J. & Woods, S. C. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147, 23–30 (2006).
Wren, A. M. et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141, 4325–4328 (2000).
Wren, A. M. et al. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 86, 5992 (2001).
Nakazato, M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999). This study successfully translated rodent findings to a patient with congenital leptin deficiency.
Farr, O. M., Gavrieli, A. & Mantzoros, C. S. Leptin applications in 2015: what have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 22, 353–359 (2015).
Schaeffer, M. et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl Acad. Sci. USA 110, 1512–1517 (2013).
Baskin, D. G. et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 848, 114–123 (1999).
Willesen, M. G., Kristensen, P. & Romer, J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 70, 306–316 (1999).
Wilson, B. D. et al. Physiological and anatomical circuitry between Agouti-related protein and leptin signaling. Endocrinology 140, 2387–2397 (1999).
Takahashi, K. A. & Cone, R. D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146, 1043–1047 (2005). This study demonstrated that internal state of an animal drives activity of appetite-regulating neurons using acute brain slice electrophysiology.
Yang, R. et al. Restoring leptin signaling reduces hyperlipidemia and improves vascular stiffness induced by chronic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 300, H1467–H1476 (2011).
Liu, T. et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73, 511–522 (2012).
Mandelblat-Cerf, Y. et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4, e07122 (2015). This is one of three studies that demonstrated that activity of hypothalamic neurons are rapidly and robustly altered via anticipation of food consumption using in vivo electrophysiology.
Mazzone, C. M. et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat. Neurosci. 23, 1253–1266 (2020). This study used longitudinal recordings of hypothalamic neurons and mesolimbic dopamine to demonstrate that palatable food exposure diminishes the capacity of chow diets to alleviate the negative valence associated with hunger and the rewarding properties of food discovery.
Krashes, M. J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
Clark, J. T., Kalra, P. S., Crowley, W. R. & Kalra, S. P. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (1984).
Rossi, M. et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139, 4428–4431 (1998).
Semjonous, N. M. et al. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. Int. J. Obes. 33, 775–785 (2009).
Stratford, T. R. & Kelley, A. E. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J. Neurosci. 17, 4434–4440 (1997).
Krashes, M. J., Shah, B. P., Koda, S. & Lowell, B. B. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 18, 588–595 (2013).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Cavalcanti-de-Albuquerque, J. P., Bober, J., Zimmer, M. R. & Dietrich, M. O. Regulation of substrate utilization and adiposity by Agrp neurons. Nat. Commun. 10, 311 (2019).
Joly-Amado, A. et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31, 4276–4288 (2012).
Burke, L. K. et al. mTORC1 in AGRP neurons integrates exteroceptive and interoceptive food-related cues in the modulation of adaptive energy expenditure in mice. eLife 6, e22848 (2017).
Steculorum, S. M. et al. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165, 125–138 (2016).
Alhadeff, A. L. et al. Central leptin signaling transmits positive valence. Brain Res. 1724, 146441 (2019).
Reichenbach, A. et al. Metabolic sensing in AgRP neurons integrates homeostatic state with dopamine signalling in the striatum. eLife 11, e72668 (2022).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011). This study was the first to show that acute optogenetic activation of hypothalamic AgRP neurons emulated a fasted state driving food intake.
Burnett, C. J. et al. Hunger-driven motivational state competition. Neuron 92, 187–201 (2016).
Burnett, C. J. et al. Need-based prioritization of behavior. eLife 8, e44527 (2019)
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016).
Alhadeff, A. L. et al. A neural circuit for the suppression of pain by a competing need state. Cell 173, 140–152 e115 (2018).
Li, X. Y. et al. AGRP neurons project to the medial preoptic area and modulate maternal nest-building. J. Neurosci. 39, 456–471 (2019).
Dietrich, M. O., Zimmer, M. R., Bober, J. & Horvath, T. L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232 (2015).
Goldstein, N. et al. Hypothalamic neurons that regulate feeding can influence sleep/wake states based on homeostatic need. Curr. Biol. 28, 3736–3747 (2018). This study investigated the intersection between feeding circuits and sleep architecture finding reciprocal influences of these two essential behaviors.
Jikomes, N., Ramesh, R. N., Mandelblat-Cerf, Y. & Andermann, M. L. Preemptive stimulation of AgRP neurons in fed mice enables conditioned food seeking under threat. Curr. Biol. 26, 2500–2507 (2016).
Chen, Y., Lin, Y. C., Kuo, T. W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015). This is one of three studies that demonstrated that activity of hypothalamic neurons are rapidly and robustly altered via anticipation of food consumption using in vivo optical-fibre photometry.
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015). This is one of three studies that demonstrated that activity of hypothalamic neurons are rapidly and robustly altered via anticipation of food consumption using in vivo single-photon microendoscopy.
Su, Z., Alhadeff, A. L. & Betley, J. N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017).
Horio, N. & Liberles, S. D. Hunger enhances food-odour attraction through a neuropeptide Y spotlight. Nature 592, 262–266 (2021).
Garfield, A. S. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016).
Berrios, J. et al. Food cue regulation of AGRP hunger neurons guides learning. Nature 595, 695–700 (2021).
Beutler, L. R. et al. Dynamics of gut–brain communication underlying hunger. Neuron 96, 461–475 e465 (2017).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 e1123 (2019).
Campos, C. A., Bowen, A. J., Schwartz, M. W. & Palmiter, R. D. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016).
Chen, Y., Lin, Y. C., Zimmerman, C. A., Essner, R. A. & Knight, Z. A. Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife 5, e18640 (2016).
Chen, Y. et al. Sustained NPY signaling enables AgRP neurons to drive feeding. eLife 8, e46348 (2019).
Engstrom Ruud, L., Pereira, M. M. A., de Solis, A. J., Fenselau, H. & Bruning, J. C. NPY mediates the rapid feeding and glucose metabolism regulatory functions of AgRP neurons. Nat. Commun. 11, 442 (2020).
Tan, K., Knight, Z. A. & Friedman, J. M. Ablation of AgRP neurons impairs adaption to restricted feeding. Mol. Metab. 3, 694–704 (2014).
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Fu, O. et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun. 10, 4560 (2019).
Henry, F. E., Sugino, K., Tozer, A., Branco, T. & Sternson, S. M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 4, e09800 (2015).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Briggs, D. I., Lemus, M. B., Kua, E. & Andrews, Z. B. Diet-induced obesity attenuates fasting-induced hyperphagia. J. Neuroendocrinol. 23, 620–626 (2011).
Ueno, N., Asakawa, A. & Inui, A. Blunted metabolic response to fasting in obese mice. Endocrine 32, 192–196 (2007).
Beutler, L. R. et al. Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat. eLife 9, e55909 (2020).
Baver, S. B. et al. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 34, 5486–5496 (2014).
Wei, W. et al. Diet composition, not calorie intake, rapidly alters intrinsic excitability of hypothalamic AgRP/NPY neurons in mice. Sci. Rep. 5, 16810 (2015).
Zhu, C. et al. Profound and redundant functions of arcuate neurons in obesity development. Nat. Metab. 2, 763–774 (2020).
Zhang, X. & van den Pol, A. N. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat. Neurosci. 19, 1341–1347 (2016).
Luo, S. X. et al. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81 (2018).
Jais, A. et al. PNOC(ARC) neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106, 1009–1025 (2020). This study characterized a novel population of hypothalamic neurons activated upon palatable food consumption with the capacity to promote hyperphagia.
Wiesenfeld, Z., Halpern, B. P. & Tapper, D. N. Licking behavior: evidence of hypoglossal oscillator. Science 196, 1122–1124 (1977).
Mickelsen, L. E. et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019).
Rossi, M. A. et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364, 1271–1274 (2019). This study performed longitudinal two-photon neural recordings to demonstrate how diet disrupts the function of an endogenous feeding suppression system to promote overeating and obesity.
Rossi, M. A. et al. Transcriptional and functional divergence in lateral hypothalamic glutamate neurons projecting to the lateral habenula and ventral tegmental area. Neuron 109, 3823–3837 (2021).
Rada, P., Tucci, S., Murzi, E. & Hernández, L. Extracellular glutamate increases in the lateral hypothalamus and decreases in the nucleus accumbens during feeding. Brain Res. 768, 338–340 (1997).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Siemian, J. N., Arenivar, M. A., Sarsfield, S. & Aponte, Y. Hypothalamic control of interoceptive hunger. Curr. Biol. 31, 3797–3809 (2021).
Jennings, JoshuaH. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Leinninger, G. M. et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10, 89–98 (2009).
Schiffino, F. L. et al. Activation of a lateral hypothalamic-ventral tegmental circuit gates motivation. PLoS ONE 14, e0219522 (2019).
Siemian, J. N. et al. Lateral hypothalamic LEPR neurons drive appetitive but not consummatory behaviors. Cell Rep. 36, 109615 (2021).
Mickelsen, L. E. et al. Cellular taxonomy and spatial organization of the murine ventral posterior hypothalamus. eLife 9, e58901 (2020).
Qu, D. et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247 (1996).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
de Lecea, L. et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl Acad. Sci. USA 95, 322–327 (1998).
Rossi, M. et al. Investigation of the feeding effects of melanin concentrating hormone on food intake — action independent of galanin and the melanocortin receptors. Brain Res. 846, 164–170 (1999).
Della-Zuana, O. et al. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague–Dawley rats. Int. J. Obes. 26, 1289–1295 (2002).
Gomori, A. et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Am. J. Physiol. Endocrinol. Metab. 284, E583–E588 (2003).
Noble, E. E. et al. Hypothalamus–hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat. Commun. 10, 4923 (2019).
Ludwig, D. S. et al. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am. J. Physiol. Endocrinol. Metab. 274, E627–E633 (1998).
Edwards, C. M. et al. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J. Endocrinol. 160, R7–R12 (1999).
Jeon, J. Y. et al. MCH–/– mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes 55, 428–434 (2006).
Inutsuka, A. et al. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85, 451–460 (2014).
Hara, J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001).
González, J. A. et al. Inhibitory interplay between orexin neurons and eating. Curr. Biol. 26, 2486–2491 (2016).
Dilsiz, P. et al. MCH neuron activity is sufficient for reward and reinforces feeding. Neuroendocrinology 110, 258–270 (2020).
Delgado, J. M. & Anand, B. K. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am. J. Physiol. 172, 162–168, (1953). This study was the first to show that excitation of the lateral hypothalamus via electrical stimulation augmented food intake.
Mogenson, G. J. & Stevenson, J. A. Drinking induced by electrical stimulation of the lateral hypothalamus. Exp. Neurol. 17, 119–127 (1967).
Woodworth, C. H. Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol. Behav. 6, 345–353 (1971).
Valenstein, E. S., Cox, V. C. & Kakolewski, J. W. Modification of motivated behavior elicited by electrical stimulation of the hypothalamus. Science 159, 1119–1121 (1968).
Vaughan, E. & Fisher, A. E. Male sexual behavior induced by intracranial electrical stimulation. Science 137, 758–760 (1962).
Navarro, M. et al. Lateral hypothalamus GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology 41, 1505–1512 (2016). This study demonstrated that artificial activation of feeding centers can elicit consummatory responses to inedible, non-caloric objects.
Nieh, E. H. et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015).
Margules, D. L. & Olds, J. Identical ‘feeding’ and ‘rewarding’ systems in the lateral hypothalamus of rats. Science 135, 374–375 (1962).
Nieh, EdwardH. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
O’Connor, EoinC. et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015).
Thoeni, S., Loureiro, M., O’Connor, E. C. & Lüscher, C. Depression of accumbal to lateral hypothalamic synapses gates overeating. Neuron 107, 158–172.e154 (2020).
Broberger, C., Johansen, J., Johansson, C., Schalling, M. & Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl Acad. Sci. USA 95, 15043–15048 (1998).
Tolson, K. P. et al. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J. Neurosci. 30, 3803–3812 (2010).
Baltasar, A. et al. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes. Surg. 15, 1124–1128 (2005).
Michaud, J. L., Rosenquist, T., May, N. R. & Fan, C. M. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 12, 3264–3275 (1998).
Xi, B., Chandak, G. R., Shen, Y., Wang, Q. & Zhou, D. Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. PLoS ONE 7, e45731 (2012).
Li, M. M. et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667 e656 (2019).
Shah, B. P. et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl Acad. Sci. USA 111, 13193–13198 (2014).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Sutton, A. K. et al. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J. Neurosci. 34, 15306–15318 (2014).
Sutton, A. K. et al. Paraventricular, subparaventricular and periventricular hypothalamic IRS4-expressing neurons are required for normal energy balance. Sci. Rep. 10, 5546 (2020).
An, J. J., Liao, G. Y., Kinney, C. E., Sahibzada, N. & Xu, B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 22, 175–188 (2015).
Kim, J. et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci. 22, 576–585 (2019).
Sweeney, P., Chen, C., Rajapakse, I. & Cone, R. D. Network dynamics of hypothalamic feeding neurons. Proc. Natl Acad. Sci. USA 118, e2011140118 (2021).
Xu, S. et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science 370, eabb2494 (2020).
Stachniak, T. J., Ghosh, A. & Sternson, S. M. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014).
Gong, R., Xu, S., Hermundstad, A., Yu, Y. & Sternson, S. M. Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell 182, 1589–1605 (2020). This study highlights a population of hindbrain neurons that increase consumption by enhancing palatbility and prolonging ingestion duration.
Li, C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694 e685 (2019).
Travers, J. B., Dinardo, L. A. & Karimnamazi, H. Motor and premotor mechanisms of licking. Neurosci. Biobehav. Rev. 21, 631–647 (1997).
Mohammad, H. et al. A neural circuit for excessive feeding driven by environmental context in mice. Nat. Neurosci. 24, 1132–1141 (2021).
Zhang, X. & van den Pol, A. N. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859 (2017).
Herman, A. M. et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 538, 253–256 (2016).
Pinol, R. A. et al. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat. Neurosci. 21, 1530–1540 (2018).
Miller, G. D. Appetite regulation: hormones, peptides, and neurotransmitters and their role in obesity. Am. J. Lifestyle Med. 13, 586–601 (2019).
Schwartz, G. J., Salorio, C. F., Skoglund, C. & Moran, T. H. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am. J. Physiol. 276, R1623–R1629 (1999).
Ritter, S., Dinh, T. T. & Friedman, M. I. Induction of Fos-like immunoreactivity (Fos-li) and stimulation of feeding by 2,5-anhydro-d-mannitol (2,5-AM) require the vagus nerve. Brain Res. 646, 53–64 (1994).
Fan, W. et al. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci. 7, 335–336 (2004).
Kreisler, A. D., Davis, E. A. & Rinaman, L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol. Behav. 136, 47–54 (2014).
Blouet, C. & Schwartz, GaryJ. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 16, 579–587 (2012).
Chen, J. et al. A vagal–NTS neural pathway that stimulates feeding. Curr. Biol. 30, 3986–3998.e3985 (2020).
Browning, K. N. & Travagli, R. A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 4, 1339–1368 (2014).
Sutton, A. K. & Krashes, M. J. Integrating hunger with rival motivations. Trends Endocrinol. Metab. 31, 495–507 (2020).
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013). This study identified a neural circuit from the parabrachial nucleus to the amygdala that mediates appetite suppression in conditions when it is unfavorable to eat.
Carter, M. E., Han, S. & Palmiter, R. D. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J. Neurosci. 35, 4582–4586 (2015).
Campos, C. A., Bowen, A. J., Roman, C. W. & Palmiter, R. D. Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018).
Chen, J. Y., Campos, C. A., Jarvie, B. C. & Palmiter, R. D. Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100, 891–899 e895 (2018).
Reilly, S. The parabrachial nucleus and conditioned taste aversion. Brain Res. Bull. 48, 239–254 (1999).
Phua, S. C. et al. A distinct parabrachial-to-lateral hypothalamus circuit for motivational suppression of feeding by nociception. Sci. Adv. 7, eabe4323 (2021).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015).
Sabatini, P. V. et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl Acad. Sci. USA 118, e2021357118 (2021).
Alhadeff, A. L. & Grill, H. J. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R465–R470 (2014).
Richard, J. E. et al. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 155, 4356–4367 (2014).
Roman, C. W., Derkach, V. A. & Palmiter, R. D. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun. 7, 11905 (2016).
Roman, C. W., Sloat, S. R. & Palmiter, R. D. A tale of two circuits: CCK(NTS) neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience 358, 316–324 (2017).
Huang, D., Grady, F. S., Peltekian, L., Laing, J. J. & Geerling, J. C. Efferent projections of CGRP/Calca-expressing parabrachial neurons in mice. J. Comp. Neurol. 529, 2911–2957 (2021).
Kim, J. H. et al. A discrete parasubthalamic nucleus subpopulation plays a critical role in appetite suppression. eLife 11, e75470 (2022).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-delta+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Cheng, W. et al. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non-aversive suppression of feeding. Cell Metab. 31, 301–312 e305 (2020).
Cheng, W. et al. NTS Prlh overcomes orexigenic stimuli and ameliorates dietary and genetic forms of obesity. Nat. Commun. 12, 5175 (2021).
Luskin, A. T. et al. Extended amygdala-parabrachial circuits alter threat assessment and regulate feeding. Sci. Adv. 7, eabd3666 (2021).
Flak, J. N. et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci. 17, 1744–1750 (2014).
Mumphrey, M. B. et al. Eating in mice with gastric bypass surgery causes exaggerated activation of brainstem anorexia circuit. Int J. Obes. 40, 921–928 (2016).
Evers, S. S., Sandoval, D. A. & Seeley, R. J. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev. Physiol. 79, 313–334 (2017).
Low, A. Y. T. et al. Reverse-translational identification of a cerebellar satiation network. Nature 600, 269–273 (2021).
Alhadeff, A. L., Rupprecht, L. E. & Hayes, M. R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153, 647–658 (2012).
Angulo, M. A., Butler, M. G. & Cataletto, M. E. Prader–Willi syndrome: a review of clinical, genetic, and endocrine findings. J. Endocrinol. Invest 38, 1249–1263 (2015).
Goldstein, N. et al. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 33, 676–687 (2021).
Corbett, D. & Wise, R. A. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 185, 1–15 (1980).
Yeomans, M. R. Taste, palatability and the control of appetite. Proc. Nutr. Soc. 57, 609–615 (1998).
Cabanac, M. Physiological role of pleasure. Science 173, 1103–1107 (1971).
Sorensen, L. B., Moller, P., Flint, A., Martens, M. & Raben, A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int. J. Obes. Relat. Metab. Disord. 27, 1152–1166 (2003).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Bowen, A. J. et al. Dissociable control of unconditioned responses and associative fear learning by parabrachial CGRP neurons. eLife 9, e59799 (2020).
Astrup, A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J. Obes. 36, 843–854 (2012).
Muller, T. D., Clemmensen, C., Finan, B., DiMarchi, R. D. & Tschop, M. H. Anti-obesity therapy: from rainbow pills to polyagonists. Pharm. Rev. 70, 712–746 (2018).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989 (2021).
Acknowledgements
The authors acknowledge with gratitude S. Sarsfield for comments on the manuscript. Y. A. is supported by the National Institute on Drug Abuse Intramural Research Program (NIDA IRP), US National Institutes of Health (NIH). M. K. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (NIDDK IRP), US National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
I.C.A, A.P.M.T, Y.A., and M.J.K. conceived the outline, elaborated on the concepts, and wrote the synopsis of the review. I.C.A. wrote the introduction, prologue and act I sections. A.P.M.T. and Y.A. wrote the act II section. M.J.K. wrote the introduction, act III, and epilogue sections and the boxes and figure legends. All authors read and gave feedback on all sections, and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alcantara, I.C., Tapia, A.P.M., Aponte, Y. et al. Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding. Nat Metab 4, 836–847 (2022). https://doi.org/10.1038/s42255-022-00611-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00611-y
This article is cited by
-
Reciprocal activity of AgRP and POMC neurons governs coordinated control of feeding and metabolism
Nature Metabolism (2024)
-
ANMCO (Italian Association of Hospital Cardiologists) scientific statement: obesity in adults—an approach for cardiologists
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2024)
-
Current perspectives on brain circuits involved in food addiction-like behaviors
Journal of Neural Transmission (2024)
-
Adrenergic modulation of melanocortin pathway by hunger signals
Nature Communications (2023)
-
Sequential appetite suppression by oral and visceral feedback to the brainstem
Nature (2023)