Abstract
As the principal means of acquiring nutrients, feeding behavior is indispensable to the survival and well-being of animals. In response to energy or nutrient deficits, animals seek and consume food to maintain energy homeostasis. On the other hand, even when animals are calorically replete, non-homeostatic factors, such as the sight, smell, and taste of palatable food, or environmental cues that predict food, can stimulate feeding behavior. These homeostatic and non-homeostatic factors have traditionally been investigated separately, but a growing body of literature highlights that these factors work synergistically to promote feeding behavior. Furthermore, recent breakthroughs in cell type-specific and circuit-specific labeling, recording, and manipulation techniques have markedly accelerated the discovery of well-defined neural populations underlying homeostatic and non-homeostatic appetite control, as well as overlapping circuits that contribute to both types of appetite. This review aims to provide an update on our understanding of the neural circuit mechanisms for promoting homeostatic and non-homeostatic appetites, focusing on the function of recently identified, genetically defined cell types.
Similar content being viewed by others
Introduction
Food intake is essential to the survival of animals as a critical contributor to energy homeostasis1,2. While self-evident in our daily lives, the importance of this vital function is further emphasized by the fact that inappropriate regulation of feeding behavior is a hallmark of serious metabolic and psychiatric conditions, such as obesity and anorexia3,4,5. As such, decades of research have delved into the neural circuit mechanisms for appetite—the motivation to eat.
In response to caloric deficiency, animals seek and consume food to defend energy homeostasis1,2,6,7,8,9. Information on energy stores and nutrient availability is thought to be primarily conveyed by peripherally produced hormones to certain central neurons1,2,4,7,8,9. These interoceptive hormones can directly reach central neurons behind the blood–brain barrier (BBB) via several mechanisms within circumventricular organs (CVOs; e.g., the median eminence in the hypothalamus and area postrema in the hindbrain) that permit passive transport of hormones to these neurons2,10. In addition, these hormones can indirectly signal the energy state to hindbrain neurons by acting on vagal afferent neurons2,5,8. Upon direct and indirect exposure to peripheral signals of energy or nutrient deficit, central neurons that support homeostatic appetite are activated and drive feeding1,2,11.
Feeding behavior can also be initiated in the absence of apparent homeostatic needs1,2,9,11,12,13. For example, the sight, smell, and taste of palatable foods, as well as contextual cues that predict food reward, can all stimulate appetite even when animals are calorically replete1,2,11,12. Diverse neural populations mediating this non-homeostatic appetite have been identified and characterized thus far1,2,12,13. Some of these neurons drive feeding behavior specific to palatable foods regardless of the internal state2,4,9,11,12,13, while some populations are shown to promote food intake in response to conditioned sensory cues even in a sated state12,13. This mode of appetite is thought to encourage overconsumption beyond current physiological needs, possibly as a preventive mechanism against anticipated energy deficit1,2,12. However, in the modern obesogenic environment where we face an abundance, rather than a scarcity of food, this non-homeostatic appetite is considered to underlie the etiology of obesity, highlighting its clinical relevance4,11.
Much research has described these different appetites in isolation, with the predominant assumption that homeostatic and non-homeostatic appetites are mutually exclusive entities1,6. However, it has continuously been noted that these appetites frequently interact with each other to promote feeding behavior; for one example, energy deficits increase the incentive salience of food-predictive cues1,4,9,11,13. Accordingly, recent studies have found that the activity of neural populations that are well established to promote non-homeostatic appetite is also modulated by the hunger state and that the neurons essential to homeostatic appetite also respond to non-homeostatic factors1,11,14. In addition, many neural populations that play a pivotal role in both homeostatic and non-homeostatic appetite regulation have been identified9,11.
Recent advancements in techniques enabling anatomical and functional interrogation of genetically defined neuron types have markedly facilitated the disentanglement of well-defined neural circuit components mediating the control of homeostatic and non-homeostatic appetites14,15,16. Here, we review the body of knowledge on the neural mechanisms promoting feeding, focusing on the latest progress under the following three sections: (1) Brain circuits for homeostatic appetite, (2) Brain circuits for non-homeostatic appetite, and (3) Interactions between homeostatic and non-homeostatic appetites. We also discuss future directions that would provide further insights into a comprehensive understanding of the neural mechanisms underlying the generation of appetite and feeding behavior.
Brain circuits for homeostatic appetite
Several hormones produced from peripheral metabolic organs primarily transmit information on energy stores and nutrient availability8. A representative example is leptin, which is an anorexigenic hormone released by adipocytes into the blood circulation17. Plasma levels of leptin largely correlate with the body’s fat stores17. Systemic administration of leptin decreases food intake and induces weight loss, whereas in animals lacking the leptin gene, food consumption and body mass are dramatically increased, and this phenotype is fully reversed by leptin treatment17. Another example is ghrelin, which is an orexigenic hormone secreted by enteroendocrine cells in the stomach1. The levels of ghrelin peak before a meal and drop to baseline levels in less than an hour after eating18. The levels of plasma ghrelin increase following prolonged caloric restriction, whereas the extent to which ghrelin levels drop after a meal correlates with caloric load18. Curiously, while ghrelin administration sufficiently increases food intake, ghrelin-deficient mice exhibit no significant differences in standard chow intake18, perhaps due to other orexigenic mechanisms that prevent malnutrition in animals.
Central neurons that respond to these hormones have been found proximal to or within CVOs10. Most prominently, neurons expressing the gene encoding agouti-related peptide (AgRP; encoded by the Agrp gene) in the arcuate nucleus (ARCAgrp neurons), which reside near the BBB-lacking median eminence, detect changes in both leptin and ghrelin levels17,18,19 (Fig. 1). Increases in leptin lead to decreased activity of ARCAgrp neurons in leptin-deficient and leptin haplo-insufficient mice20, and application of leptin ex vivo decreases the firing rate of ARCAgrp neurons from fasted wild-type mice21. Conversely, ghrelin potently increases the activity of ARCAgrp neurons both in vivo and ex vivo22,23. Consistent with their responses to ghrelin and leptin, the overall activity of ARCAgrp neurons encodes the hunger state, as these neurons are highly active in the fasted state but inactive during the fed state22,24.
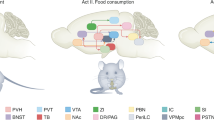
Each circle denotes a neural population that sends or receives signals involved in homeostatic appetite. Filled circles include genetically defined populations, whereas broken circles indicate projection targets of unknown identity. Solid and dashed lines indicate connections that are orexigenic and anorexigenic when artificially activated, respectively. Split terminals indicate activation of target neurons, and bar terminals indicate inhibition of target neurons. Specific marker genes are indicated by color-coded labels adjacent to regions that contain soma of each population. Note that not all connections depicted here have been confirmed to be monosynaptic. The listed genes encode Agrp, agouti-related peptide; Pomc, pro-opiomelanocortin; Pdyn, prodynorphin; Calca, calcitonin/calcitonin-related polypeptide, alpha; Th, tyrosine hydroxylase; Mc4r, melanocortin 4 receptor; Glp1r, glucagon-like peptide 1 receptor; Dbh, dopamine β-hydroxylase; Ghsr, growth hormone secretagogue receptor; Drd1, dopamine receptor D1; Sst, somatostatin; Oxtr, oxytocin receptor; Gpr65, G-protein-coupled receptor 65; Prkcd, protein kinase C delta. Abbreviations: BNST, bed nucleus of the stria terminalis; CeA, central amygdaloid nucleus; TN, tuberal nucleus of the hypothalamus; PVH, paraventricular hypothalamic nucleus; ARC, arcuate hypothalamic nucleus; PVT, paraventricular thalamic nucleus; LH, lateral hypothalamic area; NG, nodose ganglion; PB, parabrachial nucleus; NTS, nucleus of the solitary tract.
ARCAgrp neurons are well established to causally drive homeostatic appetite. These neurons are necessary for the intake of nonpalatable chow but dispensable for palatability-driven feeding25,26,27,28,29; specific ablation of ARCAgrp neurons leads to starvation when animals are fed standard chow25,26,27, but this does not significantly affect the intake of a palatable high-fat, high-sugar diet28,29. Artificial activation of ARCAgrp neurons reliably evokes feeding behavior30,31, likely by recruiting noncollateralizing inhibitory projections to the paraventricular hypothalamus (PVH), lateral hypothalamus (LH), bed nucleus of the stria terminalis (BNST), and paraventricular thalamus (PVT)32,33,34,35,36, but which of these projections are required for the orexigenic effect of ARCAgrp neuron stimulation remains to be determined. Furthermore, ARCAgrp neurons are critical for the orexigenic effect of ghrelin and the anorexigenic effect of leptin. Genetic ablation of leptin receptor (LepR) in ARCAgrp neurons via the CRISPR–Cas9 system increases food intake and body weight to a similar extent as mice lacking LepR completely37. These striking findings imply that leptin predominantly acts on ARCAgrp neurons—among its many potential targets—to maintain energy balance37. In addition, ARCAgrp neurons at least partially mediate ghrelin-evoked feeding. Ablating ARCAgrp neurons abrogates the increase in feeding that follows both oral administration of a ghrelin receptor agonist and subcutaneous injection of ghrelin29,38. In mice lacking Ghsr, the gene encoding growth hormone secretagogue receptor (GHSR), however, reinstating Ghsr specifically in ARCAgrp neurons only partially restores the orexigenic effects of subcutaneously injected ghrelin39. These results collectively suggest that ARCAgrp neurons are required but not sufficient for ghrelin-evoked feeding.
ARC neurons expressing the pro-opiomelanocortin-encoding gene (Pomc) (ARCPomc neurons) are another key population that responds to hormones signaling the energy state, and that plays an important role in homeostatic appetite40,41,42. In contrast to ARCAgrp neurons, the activity of ARCPomc neurons decreases upon fasting and ghrelin injection and increases upon food intake and leptin treatment22,41, although recent single-cell RNA sequencing and electrophysiological experiments have revealed substantial heterogeneity in the responses of ARCPomc neurons to leptin43. This antagonistic activity of ARCPomc and ARCAgrp neurons appears to be shaped by a mechanism analogous to the flip-flop model from digital electronics44. When mice are calorically replete, ARCPomc neurons are activated, which in turn decreases the activity of glutamatergic terminals presynaptic to ARCAgrp neurons by releasing β-endorphin, leading to reduced ARCAgrp neuronal activity (although the identity of the upstream glutamatergic neurons remains to be studied)44. Conversely, when mice are energy deficient, an increase in ghrelin levels activates Ghsr-expressing upstream glutamatergic terminals by activating an intracellular positive feedback loop involving AMP-activated protein kinase44. When the intracellular loop is initiated, ARCAgrp neurons are activated and maintain their elevated activity even after ghrelin is washed out, which inhibits ARCPomc neurons by the release of GABA44. This elevated activity remains persistent until ARCPomc neurons are activated—one suggested mechanism is via leptin, although this might not be physiologically relevant given that the leptin level does not acutely increase after food intake. This interesting study illustrates a neural mechanism by which ARCPomc and ARCAgrp neurons maintain their sustained activity in a bistable manner that is flipped by an energy excess and deficit, represented by leptin and ghrelin44.
In addition to their activity dynamics, the effects of ARCPomc neurons on appetite, as well as the effects of their neuropeptides on melanocortin receptors expressed in downstream neurons, also oppose those of ARCAgrp neurons1,30,42,45,46. Activation of ARCPomc neurons suppresses feeding30,45, whereas ablation of ARCPomc neurons induces massive obesity in mice45. α-Melanocyte stimulating hormone (α-MSH), a peptide product of the Pomc gene, acts as an agonist of melanocortin 3 and 4 receptors (MC3R and MC4R; melanocortin receptors known to be expressed in the brain47), whereas agouti-related peptide (AgRP) acts as an inverse agonist of these receptors47. Key convergent sites for these neurons are Mc4r-expressing neurons in the PVH (PVHMc4r neurons), of which the expression of MC4R is both necessary and sufficient for the control of feeding48. Application of α-MSH and AgRP to PVHMc4r neurons ex vivo increases and reduces their firing activity, respectively46.
Within or proximal to the ARC, several other neural populations are found to be orexigenic and activated by hunger and ghrelin49,50. One example is the neurons marked by the expression of tyrosine hydroxylase (encoded by the Th gene) in the ARC, which are distinct from ARCAgrp and ARCPomc neurons. Optogenetic stimulation of this population robustly increases food intake, whereas tetanus toxin-mediated silencing of these neurons induces body weight loss49. In addition, these neurons show increased firing rates in the fasted state, as well as in response to ex vivo application of ghrelin49. These neurons inhibit ARCPomc neurons and neurons in the PVH, likely through the corelease of dopamine (DA) and GABA49; the genetic identity of PVH neurons postsynaptic to ARCTh neurons remains to be determined.
As another example, in the subregion of the hypothalamus residing lateral to the ARC called the tuberal nucleus, inhibitory neurons expressing the somatostatin gene (Sst) (TNSst neurons) are activated by ghrelin both ex vivo and in vivo50. When activated, these neurons promote food intake, as chemogenetic activation of this population dramatically stimulates food consumption (by increasing both the meal duration and frequency). In contrast, chemogenetic and optogenetic inhibition of these neurons reduces food consumption (only by decreasing meal frequency), and ablation of these neurons attenuates body weight gain over ten weeks compared with controls. Optogenetic stimulation of the projection from the TNSst neurons either to the BNST or PVH was sufficient to stimulate appetite50.
In addition to these key neural populations in the hypothalamus, catecholaminergic neurons in the nucleus of the solitary tract (NTS) that express dopamine β-hydroxylase or tyrosine hydroxylase (encoded by the Dbh or Th gene) have been implicated in promoting homeostatic appetite. These neurons are activated following food deprivation51, and the level of Dbh mRNA in the NTS is increased upon peripheral ghrelin injection52. Optogenetic and chemogenetic activation of Dbh-expressing NTS neurons potently increases food intake, while optogenetic inhibition of these neurons suppresses feeding51. Some of the Th-expressing NTS neurons project to the ARC, and activation of this projection stimulates appetite while inhibiting this pathway impairs feeding elicited by low blood glucose levels53. Moreover, ablating ARC-projecting, Dbh-expressing neurons throughout the brain, which is also found to ablate most of the catecholaminergic neurons in the NTS, abolishes feeding evoked by peripheral injection of ghrelin52. The appetite-promoting effect of ARC-projecting NTS catecholaminergic neuron stimulation is mediated by norepinephrinergic transmission in the ARC, as this effect is abolished by pharmacological blockade of α-adrenergic receptors53 (although NPY may also contribute to this orexigenic effect, as deletion of the gene encoding NPY specifically in catecholaminergic NTS neurons also attenuated the increase in food intake evoked by stimulation of these neurons51). Together with the observation that bath application of norepinephrine activates ARCAgrp neurons53,54, these results suggest that NTS catecholaminergic neurons induce feeding by recruiting ARCAgrp neurons. Given that ARCPomc neurons are also inhibited by norepinephrine in the presence of synaptic blockers54, it is likely that norepinephrine release in the ARC elicits a strong orexigenic effect via concurrent activation of ARCAgrp neurons and inhibition of ARCPomc neurons54.
Ghrelin also acts on vagal sensory neurons, many of which carry information about the nutrient composition of a meal or the mechanical distension of the gastrointestinal tract to central hindbrain neurons in the NTS1,55,56,57,58,59,60. Vagal sensory neurons are necessary for ghrelin-evoked food intake, as severing the vagal afferents that innervate visceral organs abolished the increase in food intake that follows systemic ghrelin administration61. Consistently, knockdown of Ghsr specifically in vagal sensory neurons blunted the response of these neurons to peripheral ghrelin61, indicating that ghrelin sensing in vagal sensory neurons requires the expression of GHSR.
Recent investigations have also identified specific circuits in the hypothalamus that drive nutrient-specific appetites in response to internal signals indicating the deficit of certain nutrients—thereby contributing to the maintenance of the levels of individual nutrients, in addition to the mechanisms for defending the homeostasis of the overall energy level62. For example, when animals are maintained on a high-carbohydrate and low-protein diet, the liver releases high levels of fibroblast growth factor 21 (FGF21) into the bloodstream63,64. Systemic administration of FGF21 decreases the preference for sugar against water65, and in mice presented with a choice of diets differing in protein composition, intracerebroventricular (i.c.v.) injection of FGF21 increases the preference for diets with a higher protein content66. Critically, the reduced carbohydrate preference upon increased FGF21 levels requires the expression of FGF21’s cognate receptor β-klotho in the brain66, specifically within the neurons in the ventromedial hypothalamus (expressing the region-specific marker Sf1), a region adjacent to the third ventricle and the ARC67.
Brain circuits for non-homeostatic appetite
Mounting evidence suggests that inhibitory neurons in the LH expressing vesicular GABA transporter (encoded by the Vgat gene) (LHVgat neurons) comprise a key circuit node for non-homeostatic appetite (Fig. 2). Optogenetic and chemogenetic activation of these neurons increases food consumption and the time spent in a food area, while acute inhibition of this population decreases food intake and the time spent in a food zone68. Moreover, ablation of LHVgat neurons reduces body weight68. However, in stark contrast to ARCAgrp neurons that are implicated in homeostatic appetite, these neurons, at least as a population, appear to not respond to energy deficit nor to serve in restoring caloric deficiency. Fos expression in these neurons was not increased after fasting69, implying that this population is not activated upon energy deficit. Activating the LHVgat population elicits consummatory behavior that is not specific to food but also directed toward a wide range of objects, including alcohol, nonnutritive sweeteners, water, and even a wooden stick70,71,72, which may not effectively resolve nutrient deficits. Instead, this population is crucial for non-homeostatic appetite driven by environmental cues69. Following chemogenetic activation of these neurons in a specific context, if mice were given chow food in the same context, sated mice consumed more food in the stimulation-paired context in the absence of chemogenetic activation69. Interestingly, this ‘contextual feeding conditioning’ driven by LHVgat neuron activation was not established when these neurons were activated without food or with a wooden stick69. In line with their role in contextual feeding conditioning, LHVgat neurons transmit positive valence signals, and stimulation of this population is reinforcing68. Although LHVgat neurons are sufficient to produce contextual feeding conditioning, inhibition of this population did not impair contextual feeding conditioning driven by high-fat food, wherein a standard chow diet and chemogenetic activation in sated mice during the conditioning phase was replaced with a high-fat diet but no chemogenetic stimulation in fasted mice (in contrast, inhibition of TNSst neurons did impair this conditioning; see below)69. Notably, activation of ARCAgrp neurons did not yield contextual feeding conditioning69.
Each circle denotes a neural population that sends or receives signals involved in non-homeostatic appetite. Filled circles include genetically defined populations, whereas broken circles indicate projection targets of unknown identity. Solid lines and dashed lines indicate connections that are orexigenic and anorexigenic when artificially activated, respectively. Split terminals indicate activation of target neurons, and bar terminals indicate inhibition of target neurons. Specific marker genes are indicated by color-coded labels adjacent to regions that contain soma of each population. Note that not all connections depicted here have been confirmed to be monosynaptic. The listed genes encode Vgat, vesicular GABA transporter; Vglut2, vesicular glutamate transporter 2; Vglut1, vesicular glutamate transporter 1; Pomc, pro-opiomelanocortin; Dat, dopamine active transporter; Pnoc, prepronociceptin; Drd1, dopamine receptor D1; Sst, somatostatin; Prkcd, protein kinase c delta; Nos1, nitric oxide synthase 1; and Chat, choline acetyltransferase. Abbreviations: DS, dorsal striatum; NAc, nucleus accumbens; CeA, central amygdaloid nucleus; InsCtx, insular cortex; BNST, bed nucleus of the stria terminalis; TN, tuberal nucleus of the hypothalamus; PVH, paraventricular hypothalamic nucleus; PVT, paraventricular thalamic nucleus; vBF, ventral subdivision of the basal forebrain; DBB, diagonal band of Broca; LH, lateral hypothalamic area; VTA, ventral tegmental area; peri-LC, peri-locus coeruleus; vSub, ventral subiculum; SNc, substantia nigra pars compacta; PB, parabrachial nucleus; NTS, nucleus of the solitary tract; NG, nodose ganglion.
Multiple projection arms of LHVgat neurons extending to the PVH, diagonal band of Broca (DBB) of the basal forebrain, ventral tegmental area (VTA), and peri-locus coeruleus (peri-LC) are all capable of driving feeding behavior73,74,75,76,77. Among these, fibers projecting to the VTA, a midbrain dopamine center critical to reward processing2,6, represent a crucial connection through which LHVgat neurons engage the mesolimbic dopamine system to support feeding behavior73,76. Activating projections from LHVgat neurons in the VTA increases feeding accompanied by maladaptive gnawing behavior; this aberrant behavior was largely absent during bulk stimulation of LH to VTA projections, which suggests that LHVgat neurons act in coordination with excitatory signals across the LH-VTA pathway to promote appropriate feeding behavior73. Bulk stimulation of LH neuronal projections terminating in the VTA increased food consumption even when mice were required to cross an electric shock grid to obtain the food reward, indicating that these projections potently drive compulsive feeding behavior that persists even in the face of punishment73. Notably, activation of LHVgat neurons increases dopamine levels in the nucleus accumbens (NAc)76, a forebrain region densely innervated by the VTA that shows increased dopamine activity during food-seeking behavior6,78. Activation of LHVgat axons innervating the VTA inhibits GABAergic neurons in the VTA, which then disinhibits dopaminergic VTA neurons projecting to the NAc, thereby increasing dopamine levels in the NAc76. This increase in dopamine levels in the NAc may play a key modulatory role in promoting the intake of palatable foods9.
Another significant population downstream of LHVgat neurons is Vgat-expressing neurons in the peri-locus coeruleus (peri-LCVgat neurons); these neurons medially appose the locus coeruleus (LC). Activation of the projections from LHVgat neurons to the peri-LC, as well as activation of peri-LCVgat neurons, evokes voracious feeding behavior77. Remarkably, lesions of peri-LCVgat neurons completely block feeding driven by the activation of axons projecting from LHVgat neurons to the peri-LC, indicating that the peri-LC neurons that LHVgat neurons recruit to promote feeding are GABAergic. In contrast, VTA GABAergic neuron lesions did not alter feeding stimulated by activation of LHVgat projections to the VTA77. Considering that axons from LHVgat neurons to the peri-LC bypass the VTA, it was suggested that feeding evoked by LHVgat-VTA projection stimulation is attributable to activation of LHVgat axon fibers that pass the VTA and terminate in the peri-LC77.
Activation of LHVgat-DBB projections also induces feeding. In particular, when mice are provided with both normal and high-fat chow, optogenetic stimulation of the projections evokes indiscriminate feeding toward both normal and high-fat diets74. In the DBB, the majority of projection target neurons innervated by LH neurons express the cholinergic marker gene encoding choline acetyltransferase (Chat)74. In line with the notion that LHVgat neurons inhibit DBBChat neurons to drive feeding, optogenetic activation of DBBChat neurons reduces food intake, whereas ablation of these neurons or conditional Chat knockout in the DBB increases feeding and body weight79. Intriguingly, optogenetic activation of the projections from DBBChat neurons to the ARC reduces feeding, and ablation of cholinergic neurons in the DBB lowers Pomc gene expression in ARCPomc neurons79. As ARCPomc neurons are activated by acetylcholine, it seems plausible that LHVgat neurons inhibit ARCPomc neurons through DBBChat neurons to promote overconsumption79.
In addition to LHVgat neurons and their downstream pathways, several populations in the forebrain and hindbrain regions have also been implicated in non-homeostatic appetite, as activation of these populations selectively increases the intake of palatable foods without affecting that of ordinary food or drives feeding even in a sated state. For example, the projection from the anterior PVT (aPVT) to the NAc preferentially drives the intake of palatable high-fat food over standard chow food80. Activation or inhibition of aPVT-NAc projections promotes or suppresses consumption of a high-fat diet, respectively, without affecting standard chow feeding80. Within the ventral subdivision of the basal forebrain (vBF), defined as the region including the DBB, the substantia innominata, and magnocellular preoptic nucleus, Sst-expressing neurons (vBFSst neurons) promote the intake of palatable food81. Activating these neurons in mice specifically increases high-fat chow intake and sucrose preference without affecting the consumption of standard chow81. Interestingly, activating vBFSst neuronal projections to the LH significantly increases the intake of high-fat chow but does not affect sucrose preference, revealing a putative dissociation of preference for fat and sugar within the category of palatable food81.
Some hypothalamic neurons within and proximal to the ARC also drive palatable feeding. Inhibitory neurons in the ARC expressing the Pnoc gene (which encodes prepronociceptin) (ARCPnoc neurons) are also important in driving palatable food intake82. These neurons are distinct from ARCAgrp, ARCTh, and ARCPomc neurons, and these neurons are activated upon high-fat food intake but not upon standard chow intake82. Activation of these neurons or their projections to the BNST increased standard chow intake in animals maintained on a standard chow diet, and stimulating these neurons in high-fat-fed animals increased consumption of a high-fat diet. In addition, activating these neurons elicited direct GABAergic inhibition of neighboring ARCPomc neurons82. Conversely, selective ablation of these cells reduces high-fat feeding and body weight gain in mice maintained on a high-fat diet for three days, but it does not affect food intake or body weight under standard chow consumption82. On the other hand, activation of TNSst neurons in sated mice and inhibition of these neurons in hungry mice increased and decreased the intake of palatable food, respectively, without affecting standard chow consumption69. Chemogenetic activation of these neurons elicits a preference for the stimulation-paired chamber50.
Recently, neurons expressing vesicular glutamate transporter 2 (Vglut2) in the hindbrain peri-LC (peri-LCVglut2 neurons) have been implicated in palatability-driven feeding83 (the authors of this work have defined the peri-LC as the pontine region medial to the anterior locus coeruleus, lateral to the laterodorsal tegmental area, and ventral to the fourth ventricle83; this definition largely overlaps with the medial ‘peri-LC’ densely populated by peri-LCVgat neurons noted above but also includes regions dorsally apposing the LC77,83). Recording of peri-LCVglut2 neurons in vivo revealed that the majority of these neurons are inhibited during food consumption, and this inhibition was stronger during the consumption of more palatable substances83. Inhibition of these neurons promotes feeding and conditions place preference83. Interestingly, optogenetic inhibition of peri-LCVglut2 neurons increases food intake by sustaining food consumption only when light delivery is temporally coincident with spontaneously initiated bouts, rather than by triggering a bout. It was concluded that this circuit node supports a palatability-guided double-negative feedback model, where consumption of palatable substances inhibits these neurons, which in turn serves to prolong consummatory action83.
Notably, palatability-driven feeding can occur in the absence of ARCAgrp neurons28,29. Selective ablation of ARCAgrp neurons dramatically impaired standard chow intake but spared the consumption of several different kinds of palatable diets28,29. In line with this, body weight loss observed in mice lacking ARCAgrp neurons is prevented when these mice are fed palatable food28.
As illustrated above, many neuronal populations promote feeding specific to palatable food. However, it is noteworthy that food with palatable flavor, in most cases, is also dense in nutrients. Indeed, animals can acquire preferences for specific flavors that are paired with nutrients13. Unsurprisingly, a series of studies have investigated neural circuit mechanisms by which nutrient signals from the gut engage the central dopaminergic system to establish preferences for nutrient-rich food and its associated cues, which may later trigger non-homeostatic appetite12,13.
In the ventral striatum, dopamine levels rise upon sucralose (a nonnutritive sweetener) licking alone but not upon licking of sucralose mixed with bitter tastants84. In contrast, dopamine levels in the dorsal striatum are not increased by sucralose licking per se but are increased when sucralose licking is paired with nutrient infusion into the gut84. When sucralose licking is associated with noncaloric sugar, the dorsal striatal dopamine level is not altered84. These results indicate that the ventral striatal dopamine level relates to oral sweetness, while dorsal striatal dopamine release represents gut nutrients. Moreover, ablation of dopamine D1 receptor-expressing dorsal striatum neurons (DSD1r neurons) revealed that these neurons are required for the preference of nutrient-paired flavor84. When bitter-sweet mixture solution was paired with intragastric infusion of nutrients and sweet solution was paired with the infusion of sucralose, mice with ablated DSD1r neurons preferred sweet solution over bitter-sweet mixture, whereas neither wild-type mice nor mice with ablated dopamine D1 receptor-expressing ventral striatum neurons (VSD1r neurons) preferred sweet solution84. Conversely, optogenetic activation of DSD1r neurons, but not VSD1r neurons, robustly increased the intake of both sweet and bitter solutions, as well as another nonnutritive sweetener that was previously associated with visceral malaise84. Taken together, these results indicate that dopamine in the dorsal striatum is a key signal that underlies flavor-nutrient conditioning.
Furthermore, a remarkable recent study proposed the pathway from the gut to the dorsal striatum that signals the presence of fat in the gut85. Anterograde and retrograde transsynaptic tracing experiments have revealed an anatomical polysynaptic pathway originating from the gut and spanning across the right vagus nerve to the NTS, PB, substantia nigra pars compacta (SNc), and dorsal striatum85. Interestingly, between the right and left vagus nerve, the right one appears to carry fat signals that have positive valence and are reinforcing; the stimulation of the right, but not the left, nodose ganglion neurons that project to the NTS elicited a preference for place paired or conditioned with stimulation, sustained self-stimulation behavior and induced dopamine release upon intragastric fat infusion in the dorsal striatum85. Consistent with the anatomical tracing results, activating SNc-projecting PB neurons also recapitulated these effects85. Ablation of either ascending fibers from the right nodose ganglion or SNc-projecting PB neurons impaired the preference for flavor paired with intragastric fat infusion and abolished the increase in dopamine evoked by intragastric fat infusion85.
In the case of intragastric infusion of sucrose, VTA dopamine neurons were found to be activated86. This increased VTA dopamine neuron activity is impaired by transection of the hepatic branch of the vagus nerve (a branch that diverges from the left vagal trunk), whereas optogenetic stimulation of the neurons in the left nodose ganglion increases VTA dopamine activity86.
The presence of food-predictive environmental cues sufficiently triggers feeding behavior in the absence of hunger, and recent studies have identified genetically defined neural populations necessary for overconsumption in a conditioned context. Within the hypothalamus, activation of TNSst neurons as well as that of LHVgat neurons is sufficient for establishing contextual feeding conditioning69. However, only activation of TNSst neurons, but not LHVgat neurons, is necessary for context-driven overconsumption of palatable foods69. Both neural populations receive monosynaptic inputs from the ventral subiculum (vSub), a hippocampal region shown to transmit contextual information69. Interestingly, the amplitude of postsynaptic currents in TNSst neurons evoked by activation of vSub-TN axons was elevated in animals subjected to contextual conditioning for palatable food, whereas no conditioning-dependent synaptic plasticity was observed in LHVgat neurons69. Inhibition of TNSst neurons, but not ARCAgrp neurons or LHVgat neurons, reduces the intake of palatable food in a conditioned context, highlighting the necessity of establishing contextual feeding conditioning driven by palatable food69.
Context-dependent overconsumption also relies on a top-down circuit extending from the cerebral cortex. In the insular cortex (InsCtx), neurons expressing nitric oxide synthase 1 (encoded by the Nos1 gene) (InsCtxNos1 neurons) that project to the central amygdala (CeA) are required for overconsumption of chow food in a conditioned context87. Curiously, while InsCtxNos1 neurons projecting to the CeA are primarily glutamatergic, these neurons provide net inhibition of anorexigenic neurons expressing PKCδ (encoded by the Prkcd gene) in the CeA, possibly by recruiting inhibitory interneurons within the CeA87,88.
Interactions between homeostatic and non-homeostatic appetites
The current view on homeostatic and non-homeostatic appetites is that these engage overlapping neural circuits that jointly promote feeding behavior1,6. Recent studies have provided unprecedented insight into the circuit mechanisms that underlie the interactions between homeostatic and non-homeostatic appetite regulation1,6,11.
It is well known that hunger increases the reward value of food1,2,4,6,9,11,13. Consistently, ghrelin and leptin have been found to modulate dopamine reward circuits. Infusion (i.c.v.) of ghrelin potentiated food-evoked dopamine release in the NAc89, whereas leptin blunted the food cue-evoked activity observed in VTA dopamine neurons in hungry animals90,91. Moreover, chemogenetic activation of ARCAgrp neurons potentiates dopamine responses upon food presentation in the NAc92. Significantly, ARCAgrp neuron stimulation without food presentation did not change the baseline dopamine level in the NAc92.
Conversely, dopaminergic reward signaling plays a causal role in homeostatic appetite. Systemic injection of a D2R antagonist or genetic knockout of D2R blunts the hypophagic effect of systemic leptin injection93. In contrast, how dopamine signaling modulates the orexigenic effect of ghrelin is unclear, as both agonism and antagonism of central D1R or D2R/D3R receptors attenuated the orexigenic effects of i.c.v. ghrelin injections94, whereas the hyperphagic effects of peripheral ghrelin administration were not affected by systemic injection of D1R or D2R antagonists93. Remarkably, blockade of dopamine receptor signaling by systemic injection of D1R and D2R antagonists attenuates the response of ARCAgrp neurons to nutrients92. Moreover, systemic injection of a dopamine reuptake inhibitor in mice lacking ARCAgrp neurons can restore standard chow intake28, implying that dopamine signaling can override the absence of ARCAgrp neurons and promote standard chow intake.
As in the case of TNSst neurons, a single population can also be involved in homeostatic and non-homeostatic control of appetite50,69. These neurons are activated in the hunger state, as well as by ghrelin signaling an energy deficit, and activation of these neurons promotes chow food intake50. At the same time, these neurons are necessary for food consumption beyond the energy need that occurs in the context paired with high-fat food69. This notable case exemplifies that neural circuits for homeostatic and non-homeostatic appetites are not entirely dissociable.
Conclusion and future directions
Here, we reviewed studies on the neural circuit mechanisms driving homeostatic and non-homeostatic appetites, with an emphasis on recent findings. We also discussed that the neural systems supporting the two modes of appetite interact with each other and share common circuit nodes. A key objective in the study of neural circuit mechanisms underlying appetite regulation is identifying specific roles played by a particular neural population. Diverse experimental paradigms have illuminated how individual neural populations mediate particular aspects of feeding behavior. Employing the relevant methodologies to characterize neural populations affecting feeding behaviors would aid in the comprehensive elucidation of their functional significance. Among the numerous approaches, we summarize some notable examples and the possible interpretations that can result:
-
Recording the activity of neurons during energy deficit and surfeit, or in response to the changes in humoral signals such as hormones or nutrients, would reveal their possible role in sensing physiological energy states.
-
Assessing the effects of stimulating a neural population on the intake of diets of different nutritional compositions (e.g., high-fat, high-sugar, and high-protein diets) may uncover their causal role in nutrient-specific appetites.
-
Investigating the activity of a neural population during ingestion of nonpalatable and palatable foods will help determine its role in palatability-driven feeding.
-
Testing if these neurons are activated by food-predictive cues, if these cues are sufficient or necessary for the learning of food-cue associations, and if this neural population promotes overconsumption in the presence of learned cues can verify their involvement in cue-evoked conditioned overconsumption.
-
Detailed analysis of meal structure (e.g., bout number, bout duration, latency to the first bout) following perturbation of a neural population can help pinpoint where its orexigenic or anorexigenic effect is exerted along the sequence of feeding behavior (i.e., initiation and termination) and aid in the identification of their specific role in regulating ingestive behaviors (i.e., increase motivation to initiate feeding, decrease motivation to stop feeding).
-
Observing the long-term (i.e., days to weeks) effects of ablating or stimulating specific neurons would be informative in determining their necessity or sufficiency in promoting feeding behavior in the long term, which may bear higher clinical relevance.
In addition, multiple neuropeptide systems (e.g., the melanocortin, neuropeptide Y, and opioid systems) are heavily involved in the control of appetite and should thus be taken into account in this research. Beyond studying each circuit one at a time, elucidating the cooperative workings of the many circuit components in a network context remains a salient future task for revealing a diagram of the full neurocircuitry that drives food intake. Cutting-edge techniques enabling interrogation of neural circuit elements in intact brain tissues would further accelerate this research endeavor14,15,16,95,96,97.
From a clinical perspective, it would be critical to examine how neural activity, synaptic connectivity, or transcriptomic profiles of each orexigenic population are affected in disease conditions such as obesity or anorexia nervosa. It is equally important to investigate how pharmacological (e.g., GLP1R agonists) or surgical interventions (e.g., gastric bypass surgery) for such diseases affect appetite-promoting circuit nodes, which would lead to crucial translational insights3,98,99,100,101. Furthermore, identification of the neural circuits responsible for such disorders and subsequent screening of druggable targets specific to these neurons by means of single-cell RNA sequencing holds great promise in opening novel avenues for the treatment of feeding pathologies3,4,15,102,103,104,105.
References
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Watts, A. G., Kanoski, S. E., Sanchez-Watts, G. & Langhans, W. The physiological control of eating: signals, neurons, and networks. Physiol. Rev. 102, 689–813 (2022).
Gautron, L., Elmquist, J. K. & Williams, K. W. Neural control of energy balance: translating circuits to therapies. Cell 161, 133–145 (2015).
Murray, S., Tulloch, A., Gold, M. S. & Avena, N. M. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 10, 540–552 (2014).
de Lartigue, G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 594, 5791–5815 (2016).
Rossi, M. A. & Stuber, G. D. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 27, 42–56 (2018).
Kim, K.-S., Seeley, R. J. & Sandoval, D. A. Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci. 19, 185–196 (2018).
Grill, H. J. & Hayes, M. R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16, 296–309 (2012).
Saper, C. B., Chou, T. C. & Elmquist, J. K. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211 (2002).
García-Cáceres, C. et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 22, 7–14 (2019).
Liu, C. M. & Kanoski, S. E. Homeostatic and non-homeostatic controls of feeding behavior: distinct vs. common neural systems. Physiol. Behav. 193, 223–231 (2018).
Johnson, A. W. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci. 36, 101–109 (2013).
de Araujo, I. E., Schatzker, M. & Small, D. M. Rethinking food reward. Annu. Rev. Psychol. 71, 139–164 (2020).
Alhadeff, A. L. Monitoring in vivo neural activity to understand gut–brain signaling. Endocrinology 162, bqab029 (2021).
Luo, L., Callaway, E. M. & Svoboda, K. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281 (2018).
Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546 (2017).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754–764 (2019).
Müller, T. D. et al. Ghrelin. Mol. Metab. 4, 437–460 (2015).
Liu, T., Wang, Q., Berglund, E. D. & Tong, Q. Action of neurotransmitter: a key to unlock the AgRP neuron feeding circuit. Front. Neurosci. 6, 200 (2013).
Beutler, L. R. et al. Dynamics of gut-brain communication underlying hunger. Neuron 96, 461–475.e5 (2017).
Baver, S. B. et al. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 34, 5486–5496 (2014).
Chen, Y., Lin, Y.-C., Kuo, T.-W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Cowley, M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Gropp, E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 8, 1289–1291 (2005).
Wu, Q., Boyle, M. P. & Palmiter, R. D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Denis, R. G. P. et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 22, 646–657 (2015).
Luquet, S., Phillips, C. T. & Palmiter, R. D. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 28, 214–225 (2007).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Betley, J. N., Cao, Z. F. H., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Kim, S.-R. & Kim, S.-Y. Functional dissection of glutamatergic and GABAergic neurons in the bed nucleus of the stria terminalis. Mol. Cells 44, 63–67 (2021).
Garfield, A. S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Essner, R. A. et al. AgRP neurons can increase food intake during conditions of appetite suppression and inhibit anorexigenic parabrachial neurons. J. Neurosci. 37, 8678–8687 (2017).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Xu, J. et al. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556, 505–509 (2018).
Joly-Amado, A. et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31, 4276–4288 (2012).
Wang, Q. et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 3, 64–72 (2014).
Quarta, C. et al. POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat. Metab. 3, 299–308 (2021).
Mandelblat-Cerf, Y. et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4, e07122 (2015).
Fenselau, H. et al. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci. 20, 42–51 (2017).
Biglari, N. et al. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat. Neurosci. 24, 913–929 (2021).
Yang, Y., Atasoy, D., Su, H. H. & Sternson, S. M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146, 992–1003 (2011).
Zhan, C. et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33, 3624–3632 (2013).
Ghamari-Langroudi, M. et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 520, 94–98 (2015).
Krashes, M. J., Lowell, B. B. & Garfield, A. S. Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci. 19, 206–219 (2016).
Shah, B. P. et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl Acad. Sci. USA 111, 13193–13198 (2014).
Zhang, X. & van den Pol, A. N. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat. Neurosci. 19, 1341–1347 (2016).
Luo, S. X. et al. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81 (2018).
Chen, J. et al. A Vagal-NTS neural pathway that stimulates feeding. Curr. Biol. 30, 3986–3998.e5 (2020).
Date, Y. et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 4, 323–331 (2006).
Aklan, I. et al. NTS catecholamine neurons mediate hypoglycemic hunger via medial hypothalamic feeding pathways. Cell Metab. 31, 313–326.e5 (2020).
Paeger, L. et al. Antagonistic modulation of NPY/AgRP and POMC neurons in the arcuate nucleus by noradrenalin. eLife 6, e25770 (2017).
de Lartigue, G., Singh, A., de Araujo, A., Maske, C. B. & Vergara, M. in Neuron Signaling in Metabolic Regulation 1st edn, Ch. 10 (CRC Press, 2021).
Kim, D.-Y. et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380 (2020).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143.e23 (2019).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Kim, M., Heo, G. & Kim, S.-Y. Neural signalling of gut mechanosensation in ingestive and digestive processes. Nat. Rev. Neurosci. 23, 135–156 (2022).
Davis, E. A. et al. Ghrelin signaling affects feeding behavior, metabolism, and memory through the vagus nerve. Curr. Biol. 30, 4510–4518.e6 (2020).
Münch, D., Ezra-Nevo, G., Francisco, A. P., Tastekin, I. & Ribeiro, C. Nutrient homeostasis—translating internal states to behavior. Curr. Opin. Neurobiol. 60, 67–75 (2020).
Solon-Biet, S. M. et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 24, 555–565 (2016).
Hill, C. M. et al. FGF21 and the physiological regulation of macronutrient preference. Endocrinology 161, bqaa019 (2020).
von Holstein-Rathlou, S. et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 23, 335–343 (2016).
Hill, C. M. et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 27, 2934–2947.e3 (2019).
Jensen-Cody, S. O. et al. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metab. 32, 273–286.e6 (2020).
Jennings, J. H. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Mohammad, H. et al. A neural circuit for excessive feeding driven by environmental context in mice. Nat. Neurosci. 24, 1132–1141 (2021).
Navarro, M. et al. Lateral hypothalamus GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology 41, 1505–1512 (2016).
Burnett, C. J. et al. Hunger-driven motivational state competition. Neuron 92, 187–201 (2016).
Jung, S. et al. A forebrain neural substrate for behavioral thermoregulation. Neuron 110, 266–279.e9 (2022).
Nieh, E. H. et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015).
Cassidy, R. M. et al. A lateral hypothalamus to basal forebrain neurocircuit promotes feeding by suppressing responses to anxiogenic environmental cues. Sci. Adv. 5, eaav1640 (2019).
Wu, Z. et al. GABAergic projections from lateral hypothalamus to paraventricular hypothalamic nucleus promote feeding. J. Neurosci. 35, 3312–3318 (2015).
Nieh, E. H. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
Marino, R. A. M. et al. Control of food approach and eating by a GABAergic projection from lateral hypothalamus to dorsal pons. Proc. Natl Acad. Sci. USA 117, 8611–8615 (2020).
Roitman, M. F., Stuber, G. D., Phillips, P. E. M., Wightman, R. M. & Carelli, R. M. Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 24, 1265–1271 (2004).
Herman, A. M. et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 538, 253–256 (2016).
Christoffel, D. J. et al. Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 12, 2135 (2021).
Zhu, C. et al. Somatostatin neurons in the basal forebrain promote high-calorie food intake. Cell Rep. 20, 112–123 (2017).
Jais, A. et al. PNOCARC neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106, 1009–1025.e10 (2020).
Gong, R., Xu, S., Hermundstad, A., Yu, Y. & Sternson, S. M. Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell 182, 1589–1605.e22 (2020).
Tellez, L. A. et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat. Neurosci. 19, 465–470 (2016).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678.e23 (2018).
Fernandes, A. B. et al. Postingestive modulation of food seeking depends on vagus-mediated dopamine. Neuron Act. Neuron 106, 778–788.e6 (2020).
Stern, S. A. et al. Top-down control of conditioned overconsumption is mediated by insular cortex Nos1 neurons. Cell Metab. 33, 1418–1432.e6 (2021).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Cone, J. J., McCutcheon, J. E. & Roitman, M. F. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J. Neurosci. 34, 4905–4913 (2014).
van der Plasse, G. et al. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int. J. Obes. 39, 1742–1749 (2015).
Domingos, A. I. et al. Leptin regulates the reward value of nutrient. Nat. Neurosci. 14, 1562–1568 (2011).
Alhadeff, A. L. et al. Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron 103, 891–908.e6 (2019).
Billes, S. K., Simonds, S. E. & Cowley, M. A. Leptin reduces food intake via a dopamine D2 receptor-dependent mechanism. Mol. Metab. 1, 86–93 (2012).
Romero-Picó, A. et al. Central manipulation of dopamine receptors attenuates the orexigenic action of ghrelin. Psychopharmacology 229, 275–283 (2013).
Seo, J., Choe, M. & Kim, S. Y. Clearing and labeling techniques for large-scale biological tissues. Mol. Cells 39, 439–446 (2016).
Park, H.-E. et al. Scalable and isotropic expansion of tissues with simply tunable expansion ratio. Adv. Sci. 6, 1901673 (2019).
Nectow, A. R. et al. Rapid molecular profiling of defined cell types using viral TRAP. Cell Rep. 19, 655–667 (2017).
Beutler, L. R. et al. Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat. eLife 9, e55909 (2020).
Payne, S. C., Furness, J. B. & Stebbing, M. J. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 16, 89–105 (2019).
Miras, A. D. & le Roux, C. W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 10, 575–584 (2013).
Gimeno, R. E., Briere, D. A. & Seeley, R. J. Leveraging the gut to treat metabolic disease. Cell Metab. 31, 679–698 (2020).
Nectow, A. R. et al. Identification of a brainstem circuit controlling feeding. Cell 170, 429–442.e11 (2017).
Tasic, B. Single cell transcriptomics in neuroscience: cell classification and beyond. Curr. Opin. Neurobiol. 50, 242–249 (2018).
Poulin, J.-F., Tasic, B., Hjerling-Leffler, J., Trimarchi, J. M. & Awatramani, R. Disentangling neural cell diversity using single-cell transcriptomics. Nat. Neurosci. 19, 1131–1141 (2016).
Armand, E. J., Li, J., Xie, F., Luo, C. & Mukamel, E. A. Single-cell sequencing of brain cell transcriptomes and epigenomes. Neuron 109, 11–26 (2021).
Acknowledgements
We are grateful to members of the S.-Y.K. laboratory for helpful discussions. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C3003208).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to the discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahn, B.H., Kim, M. & Kim, SY. Brain circuits for promoting homeostatic and non-homeostatic appetites. Exp Mol Med 54, 349–357 (2022). https://doi.org/10.1038/s12276-022-00758-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-022-00758-4
This article is cited by
-
The rostromedial tegmental nucleus gates fat overconsumption through ventral tegmental area output in male rats
Neuropsychopharmacology (2024)