Abstract
Anti-programmed death-1 (PD-1) immunotherapy that aims to restore T cell activity in cancer patients frequently leads to immune-related adverse events such as colitis. However, the underlying mechanism is still elusive. Here, we find that Pdcd1-deficient mice exhibit disrupted gut microbiota and aggravated dextran sulfate sodium (DSS)-induced colitis. In addition to T cells, PD-1 is also substantially expressed in colonic lymphoid tissue inducer (LTi) cells. During DSS-induced colitis, LTi cell activation is accompanied by increased PD-1 expression, whereas PD-1 deficiency results in reduced interleukin-22 (IL-22) production by LTi cells and exacerbated inflammation. Mechanistically, activated LTi cells reprogram their metabolism toward carbohydrate metabolism and fatty acid synthesis, while fatty acid oxidation (FAO) is unchanged. However, PD-1 deficiency leads to significantly elevated FAO in LTi cells, which in turn attenuates their activation and IL-22 production. Consistently, FAO suppression efficiently restores IL-22 production in Pdcd1−/− LTi cells. Thus, our study provides unforeseen mechanistic insight into colitis occurrence during anti-PD-1 immunotherapy through LTi cell metabolic reconfiguration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-seq and scRNA-seq data generated in this study are publicly available in the NCBI Gene Expression Omnibus repository under the accession number GSE144687. This study did not generate any unique codes. All other data can be made available from the authors on reasonable request. Source data are provided with this paper.
References
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Haanen, J. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv119–iv142 (2017).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Macdonald, T. T. & Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 307, 1920–1925 (2005).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38, 769–781 (2013).
Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Barlow, J. L. et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 129, 191-8.e1-4 (2012).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Tang, Q. et al. Development of IL-22-producing NK lineage cells from umbilical cord blood hematopoietic stem cells in the absence of secondary lymphoid tissue. Blood 117, 4052–4055 (2011).
Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288 (2014).
Kim, H. Y. et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 20, 54–61 (2014).
Klose, C. S. N. et al. A T-bet gradient controls the fate and function of CCR6−RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Zhong, C., Zheng, M. & Zhu, J. Lymphoid tissue inducer—a divergent member of the ILC family. Cytokine Growth Factor Rev. 42, 5–12 (2018).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Zhong, C. et al. Differential expression of the transcription factor GATA3 specifies lineage and functions of innate lymphoid cells. Immunity 52, 83–95.e4 (2020).
Zhong, C. & Zhu, J. Transcriptional regulators dictate innate lymphoid cell fates. Protein Cell 8, 242–254 (2017).
Rankin, L. C. et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat. Immunol. 17, 179–186 (2016).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Sonnenberg, G. F., Monticelli, L. A., Elloso, M. M., Fouser, L. A. & Artis, D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134 (2011).
Yu, Y. et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 539, 102–106 (2016).
Taylor, S. et al. PD-1 regulates KLRG1+ group 2 innate lymphoid cells. J. Exp. Med. 214, 1663–1678 (2017).
Pentcheva-Hoang, T., Chen, L., Pardoll, D. M. & Allison, J. P. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc. Natl Acad. Sci. USA 104, 17765–17770 (2007).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Patel, C. H., Leone, R. D., Horton, M. R. & Powell, J. D. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat. Rev. Drug Discov. 18, 669–688 (2019).
Wilhelm, C. et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J. Exp. Med. 213, 1409–1418 (2016).
Karagiannis, F. et al. Lipid-droplet formation drives pathogenic group 2 innate lymphoid cells in airway inflammation. Immunity 52, 620–634.e6 (2020).
Helou, D. G. et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat. Commun. 11, 3998 (2020).
Patsoukis, N. et al. PD-1 alters T-cell metabolic reprogramming by inhibiting and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6, 6692 (2015).
Kamada, N., Seo, S.-U., Chen, G. Y. & Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Wang, T. et al. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front. Immunol. 10, 1235 (2019).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Song, M.-Y. et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut 64, 260–271 (2015).
Saleh, M. & Trinchieri, G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 11, 9–20 (2011).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Sawa, S. et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat. Immunol. 12, 320–326 (2011).
Mao, K. et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554, 255–259 (2018).
Rath, H. C. et al. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect. Immun. 69, 2277–2285 (2001).
Wlodarska, M. et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 79, 1536–1545 (2011).
Di Luccia, B., Gilfillan, S., Cella, M., Colonna, M. & Huang, S. C.-C. ILC3s integrate glycolysis and mitochondrial production of reactive oxygen species to fulfill activation demands. J. Exp. Med. 216, 2231–2241 (2019).
Wang, X. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514, 237–241 (2014).
Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39 (2014).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Seidel, J. A., Otsuka, A. & Kabashima, K. Treating tumors with immune checkpoint inhibitors: rationale and limitations. Trends Immunother. 1, 2–9 (2017).
Zhong, C. et al. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat. Immunol. 17, 169–178 (2016).
Acknowledgements
We thank Fuping You of Peking University (Beijing, China) for technique help. We thank Xiaohuan Guo of Tsinghua University (Beijing, China) for providing the C. rodentium strain and for assistance with the mixed bone marrow chimera experiments. We thank Huan Wang of Peking University for assistance with Seahorse assays. We thank all of the staff of the animal department for their help. This work is supported by the National Natural Science Foundation of China (no. 31770957, no. 91842102 and no. 32170896), and the Natural Science Foundation of Beijing (no. 18G10645).
Author information
Authors and Affiliations
Contributions
C.Z. conceived the project. D.W. performed all of the experiments. L.H. and Yime Zhang. helped with bioinformatic analyses. M.H. performed the scRNA-seq. Y.D. helped with cell sorting. G.R., X.Z., Z.L. P.L., Yinlian Zhang, S.C., J.L., Y.S. and P.W. helped in some experiments and data analysis. J.X. generated the critical mice tool. C.Z. and D.W. wrote the manuscript. C.Z. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Christoph Wilhelm and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary handling editor: Alfredo Gimenez-Cassina, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
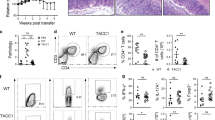
Extended Data Fig. 1 PD-1 deficiency results in disturbed microbiota and aggravated colitis.
a, Differences in fecal taxa in Pdcd1+/+ and Pdcd1-/- mice were shown in taxonomic cladogram from linear discriminant analysis of effect size (LEfSe) (green, most abundant in Pdcd1+/+ mice; red, most abundant in Pdcd1-/- mice). b, Proportions of IgA-coating fecal bacteria from Pdcd1-/- mice and Pdcd1+/- littermates were examined by flow cytometry (n = 5 per group; *p = 0.0467). c, gut barrier integrity in Pdcd1+/+ and Pdcd1-/- mice were assessed by FITC-dextran 4 (FD4, molecular mass, 4 kDa) gavage at 10ml/kg. FD4 in mice serum was examined after 4 hours by fluorescence intensity (n = 5 per group; ns). d, Colitis was induced in Pdcd1-/- mice and Pdcd1+/- littermates by 3% DSS. Body weight change was examined every day (n = 5 per group; *p = 0.0484). e, On day 7 of DSS-induced colitis, mice were sacrificed and colon length was measured (n = 5 per group; **p = 0.0029). Data are representative of two independent experiment (a) or at least three independent experiments (b-e). Data are presented as the mean ± s.d., and statistical significance was determined by two-sided unpaired t-test (b, c, e) and two-sided paired t-test (d). Ns, not significant, *p < 0.05, **p < 0.01.
Extended Data Fig. 2 PD-1 expression on colonic LTi is upregulated during activation.
a, PD-1 expression on colonic ILCs, γδT, NKT, monocytes, and DC was examined by flow cytometry. Percentages of PD-1+ cells and their PD-1 MFI were calculated (n = 4 per group; ns, ns, ***p < 0.0001, ***p < 0.0001; ***p = 0.0008, ***p < 0.0001, ***p < 0.0001, ***p < 0.0001). b, Gating strategy for ILC subsets, including LTi, T-bet-(CCR6-) ILC3, T-bet+(NKp46-) ILC3, NKp46+ ILC3, ILC1/cNK, and ILC2. c, On day 7 of DSS-induced colitis, proportion of IL-22-producing lymphocytes in colon was analyzed (mean ± s.d.; n = 6). d, PD-1 expression between colonic LTi of B6 (Rag2+/+) and Rag2-/- mice were compared. Proportion of PD-1+ LTi and their PD-1 MFI were calculated (n = 5 per group; ***p < 0.0001, **p = 0.0027). e, IL-22 production by colonic LTi of mice in steady state and DSS-induced colitis (on day 7) was examined. Proportion of IL-22+ LTi and their IL-22 MFI were compared (n = 4 per group; **p = 0.0028, *p = 0.0189). f, On day 7 of DSS-induced colitis, forward and side scatters (FSC-A and SSC-A) of PD-1high and PD-1- colonic LTi were examined. Mean values of FSC-A and SSC-A were compared (n = 4 per group; ***p = 0.0001, ***p < 0.0001). g, On day 7 of DSS-induced colitis, IL-17A expression in PD-1high and PD-1- colonic LTi was examined. Proportion, IL-17A MFI and number of IL-17A+ LTi were compared (n = 5 per group; ***p = 0.0008, *p = 0.0454, **p = 0.0015). h, PD-1 expression on in vitro cultured LTi treated with heat-killed bacteria or PBS (left), or bacterial culture supernatant or culture medium (right) was examined. i, 7 days post C. rodentium infection, PD-1 expression on colonic LTi was examined. Proportion of PD-1+ LTi and their PD-1 MFI were calculated (n = 4 per group; ns, ns). Data are representative of at least three independent experiments. Data are presented as the mean ± s.d., and statistical significance was determined by two-sided unpaired t-test (a, d-g, i). Ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 3 Impact of PD-1 deficiency on colonic LTi activation.
a, Percentage and number of colonic LTi in Pdcd1+/+ and Pdcd1-/- mice were calculated (n = 4 per group; ns, ns). b, On day 7 of DSS-induced colitis, proportion, IL-22 MFI and number of IL-22+ colonic LTi in Pdcd1-/- and Pdcd1+/- (littermate) mice were analyzed (n = 5 per group; ***p = 0.0006, *p = 0.0489, *p = 0.0425). c, On day 3 of DSS-induced colitis, proportion, IL-17A MFI and number of IL-17A+ colonic LTi in Pdcd1+/+Rag2-/- and Pdcd1-/-Rag2-/- mice were analyzed (n = 5 per group; ns, ns, ns). (d-h) Pdcd1+/+Rag2-/- and Pdcd1-/-Rag2-/- mice were subjected to C. rodentium infection. d, Body weight was measured daily (n = 5 per group; ***p < 0.0001). Colon length (e) (n = 5 per group; *p = 0.0436) and H&E staining indicated tissue damage (f) was assessed on day 3, g, The bacteria burdens in feces, spleen, and liver were assessed on day 3 and 7 (n = 5 per group; *p = 0.0235, *p = 0.0389; *p = 0.0242, *p = 0.0142; *p = 0.0104, *p = 0.0492). CFU, colony-forming unit. h, On day 3, proportion, IL-22 MFI and number of IL-22+ colonic LTi were analyzed (n = 5 per group; *p = 0.0182, **p = 0.0029, *p = 0.0331). i, Under different stimulations, proportion and IL-22 MFI of IL-22+ siLP LTi in Pdcd1+/+Rag2-/- and Pdcd1-/-Rag2-/- mice were examined (n = 4 per group; ***p < 0.0001, ***p < 0.0001, ***p < 0.0001; *p = 0.0267, *p = 0.0423, ***p = 0.0001). P/I, PMA+ionomycin. j, Construction strategy for Pdcd1h/h mice. k, On day 7 of DSS-induced colitis, proportion and hPD-1 MFI of hPD-1+ colonic LTi in Pdcd1h/+ and Pdcd1h/- mice were assessed (n = 4 per group; ns, ns). l, PD-1 expression on lineage-RORγt+ siLP lymphocytes of Pdcd1fl/-Rorc-Cre and Pdcd1fl/- (littermate) mice were compared. Data are representative of at least three independent experiments. Data are presented as the mean ± s.d., and statistical significance was determined by two-sided unpaired t-test (a-c, e, g-i, k) and two-sided paired t-test (d). Ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 4 PD-1 signaling represses STAT3 phosphorylation in activated LTi.
a, Sorted LTi were stimulated with IL-23 (0.5 ng/ml) for 25 min, in presence or absence of rPD-L1 (5 µg/ml). Afterwards, STAT3 phosphorylation was examined by flow cytometry. b, On day 7 of DSS-induced colitis, colonic lymphocytes were collected from Pdcd1+/+ and Pdcd1-/- mice. STAT3 phosphorylation (pSTAT3) in LTi was compared by flow cytometry. Isotype antibody staining was used as negative control. MFI of pSTAT3 was calculated (n = 5 per group; ns). Data are representative of at least three independent experiments. Data are presented as the mean ± s.d., and statistical significance was determined by two-sided unpaired t-test (b). Ns, not significant.
Extended Data Fig. 5 scRNA-seq reveals metabolic reprogramming during ILC3 activation.
a, Lineage- CD127+ siLP ILCs sorted from B6 mice were subjected to single-cell RNA-sequencing. The cells were clustered into three subsets, ILC1, ILC2, and ILC3 (left), based on their Rorc, Gata3, and Ncr1 expression (right). b, Scorings of LTi (top) and Nkp46+ ILC3 (bottom) based on the ILC3 activation module was correlated with their Il22 expression in scatter plot. Pearson correlation was used to indicate the strength of linear relationship (95% confidence intervals by default). c, Differentially expressed genes in inactivated NKp46+ ILC3 and activated NKp46+ ILC3 clusters were shown with heatmap. d, Signature metabolic genes in inactivated NKp46+ ILC3 and activated NKp46+ ILC3 clusters were shown in violin plot. e, Enrichment of classical metabolic pathways (including carbohydrate metabolism, lipid metabolism, and respiration) in inactivated NKp46+ ILC3 and activated NKp46+ ILC3 was shown in radar chart. f, Respective enrichment of carbohydrate metabolism, lipid metabolism, and respiration pathways in inactivated NKp46+ ILC3 and activated NKp46+ ILC3, using the differentially expressed genes in (c), was compared in density plots. g, Enrichment of fatty acid oxidation and lipid biosynthesis in inactivated NKp46+ ILC3 and activated NKp46+ ILC3, using the differentially expressed genes in (c), was compared with density plot. ND, not detected, in the differentially expressed genes in (c).
Extended Data Fig. 6 Excessive FAO suppresses ILC3 activation.
a, Representative Seahorse palmitate-FAO assay result showed the impact of palmitate (PA) on FAO of LTi (n = 5 per group). b, Basal OCR, ATP-linked OCR, maximal OCR and SRC were quantified (n = 3 per group; **p = 0.0097, **p = 0.0082, *p = 0.0163, *p = 0.0462). c, After a 3-day PA (20 μM) or vehicle treatment, IL-22 expression in activated LTi was assessed . Proportion of IL-22+ LTi and their IL-22 MFI were calculated (n = 4 per group; **p = 0.001, *p = 0.0117). d, Viability of LTi after PA (100 μM) treatment was assessed (n = 5 per group; ns). e, CPT1A overexpression in LTi was measured by qRT-PCR (n = 5 per group; *p = 0.0297). f, Impact of CPT1A on IL-22 production in LTi were assessed. Proportion of IL-22+ LTi and their IL-22 MFI were calculated (n = 5 per group; *p = 0.0436, ***p < 0.0001). g, After a 3-day PA or vehicle treatment, IL-22 production in activated NKp46+ ILC3 was assessed. Proportion of IL-22+ NKp46+ ILC3 and their IL-22 MFI were calculated (n = 5 per group; ***p < 0.0001, ***p < 0.0001). (h and i) Rag2-/- mice were fed with HFD (high fat diet) or CD (chow diet) for 5 weeks. IL-22 expression in siLP NKp46+ ILC3 (h) and colonic LTi (i) was examined. Proportion of IL-22+ siLP NKp46+ ILC3 and their IL-22 MFI (n = 4 per group; *p = 0.0269, *p = 0.0151)(h), and proportion, IL-22 MFI and number of IL-22+ colonic LTi (n = 4 per group; *p = 0.0298, *p = 0.0477, *p = 0.0496)(i), were calculated. (j and k) Enriched features in PA untreated (j) and treated (k) LTi was analyzed using two-sided GSEA adopted permutation-test. Nominal p-value and NES were calculated. Data are representative of at least three independent experiments (a-i) or two independent experiments (j-k). Data are presented as the mean ± s.d., and statistical significance was determined by two-sided unpaired t-test (b-i). Ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
Extended Data Fig. 7 FAO inhibition restores IL-22 production in PD-1 deficient LTi.
a, Expression of CPT1A in colonic LTi from wild-type (Pdcd1+/+) and Pdcd1-/- mice in steady state was examined by flow cytometry. MFI of CPT1A was calculated (n = 4 per group; ns). b, FAO inhibitor Oxfenicine or vehicle (H2O) was added to siLP LTi sorted from Pdcd1+/+Rag2-/- and Pdcd1-/-Rag2-/- mice. After 4 days, the cells were harvested and stimulated by IL-23 (0.5 ng/ml) for 3 hours. Afterwards, IL-22 secretion was assessed by intracellular cytokine staining. c, Proportion and IL-22 MFI of IL-22+ LTi in (b) were calculated (n = 4 per group; **p = 0.0033, ns; **p = 0.0058, ns). d, Wild-type and Pdcd1-/- mice were injected (i.p.) with Oxfenicine or H2O during DSS-induced colitis. On day 7, mice were sacrificed, and IL-22 production in colonic LTi was examined by intracellular cytokine staining. Proportion, IL-22 MFI and cell number of IL-22+ LTi were calculated (n = 4 per group; **p = 0.0044, *p = 0.0119; **p = 0.0095, ***p = 0.0005; **p = 0.0033, ns). e, Cpt1a knockdown in LTi cells was assessed by qRT-PCR (n = 5 per group; ***p = 0.0009). f, IL-22 production by shScram (scramble) or shCpt1a lentivirus infected LTi was assessed by intracellular cytokine staining. Proportion and IL-22 MFI of IL-22+ LTi were calculated (n = 5 per group; **p = 0.0027, *p = 0.0213). Data are representative of at least three independent experiments. Data are presented as the mean ± s.d., and statistical significance was determined by two-sided unpaired t-test (a, c-f). Ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 1
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Rights and permissions
About this article
Cite this article
Wu, D., Hu, L., Han, M. et al. PD-1 signaling facilitates activation of lymphoid tissue inducer cells by restraining fatty acid oxidation. Nat Metab 4, 867–882 (2022). https://doi.org/10.1038/s42255-022-00595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-022-00595-9