Abstract
Macrophages generate mitochondrial reactive oxygen species and mitochondrial reactive electrophilic species as antimicrobials during Toll-like receptor (TLR)-dependent inflammatory responses. Whether mitochondrial stress caused by these molecules impacts macrophage function is unknown. Here, we demonstrate that both pharmacologically driven and lipopolysaccharide (LPS)-driven mitochondrial stress in macrophages triggers a stress response called mitohormesis. LPS-driven mitohormetic stress adaptations occur as macrophages transition from an LPS-responsive to LPS-tolerant state wherein stimulus-induced pro-inflammatory gene transcription is impaired, suggesting tolerance is a product of mitohormesis. Indeed, like LPS, hydroxyoestrogen-triggered mitohormesis suppresses mitochondrial oxidative metabolism and acetyl-CoA production needed for histone acetylation and pro-inflammatory gene transcription, and is sufficient to enforce an LPS-tolerant state. Thus, mitochondrial reactive oxygen species and mitochondrial reactive electrophilic species are TLR-dependent signalling molecules that trigger mitohormesis as a negative feedback mechanism to restrain inflammation via tolerance. Moreover, bypassing TLR signalling and pharmacologically triggering mitohormesis represents a new anti-inflammatory strategy that co-opts this stress response to impair epigenetic support of pro-inflammatory gene transcription by mitochondria.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All datasets generated and analysed in this study are available from G.A.T. upon reasonable request. Raw mass spectrometry data are provided in Supplementary Tables 4–6. Processed isoTOP-ABPP and metabolomics data are provided as source data. RNA-seq and ChIP–seq data are available at the GEO under accession GSE169731. Source data are provided with this paper.
Code availability
Code used in isoTOP-ABPP (https://github.com/NomuraRG/) and metabolomics (https://github.com/graeberlab-ucla/MetabR/) data processing and analysis is available at GitHub.
References
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Hotchkiss, R. S., Monneret, G. & Payen, D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874 (2013).
Sun, L. et al. Activating a collaborative innate-adaptive immune response to control breast and ovarian cancer metastasis. Preprint at bioRxiv https://doi.org/10.1101/2020.07.13.200477 (2020).
Baik, S. H. et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell. Metab. 30, 493–507 (2019).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell. Metab. 24, 158–166 (2016).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Yu, W. et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol. Cell 75, 1147–1160 (2019).
Regdon, Z. et al. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic. Biol. Med. 131, 184–196 (2019).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015).
Shpilka, T. & Haynes, C. M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19, 109–120 (2018).
Ristow, M. & Schmeisser, K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 12, 288–341 (2014).
Calippe, B. et al. 17β-estradiol promotes TLR4-triggered pro-inflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 185, 1169–1176 (2010).
Ghisletti, S., Meda, C., Maggi, A. & Vegeto, E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-κB intracellular localization. Mol. Cell. Biol. 25, 2957–2968 (2005).
Torres, M. J. et al. 17β-estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell. Metab. 27, 167–179 (2018).
Saijo, K., Collier, J. G., Li, A. C., Katzenellenbogen, J. A. & Glass, C. K. An ADIOL–ERβ–CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145, 584–595 (2011).
Eliassen, A. H. et al. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women. Cancer Res. 72, 696–706 (2012).
Ezeriņa, D., Takano, Y., Hanaoka, K., Urano, Y. & Dick, T. P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell. Chem. Biol. 25, 447–459 (2018).
Kobayashi, E. H. et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624 (2016).
Holmstrom, K. M., Kostov, R. V. & Dinkova-Kostova, A. T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 1, 80–91 (2016).
Mendillo, M. L. et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 150, 549–562 (2012).
Boos, F. et al. Mitochondrial protein-induced stress triggers a global adaptive transcriptional programme. Nat. Cell Biol. 21, 442–451 (2019).
Quiros, P. M. et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 216, 2027–2045 (2017).
Nargund, A. M., Fiorese, C. J., Pellegrino, M. W., Deng, P. & Haynes, C. M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPRmt. Mol. Cell 58, 123–133 (2015).
Jazwinski, S. M. & Kriete, A. The yeast retrograde response as a model of intracellular signaling of mitochondrial dysfunction. Front. Physiol. 3, 139 (2012).
Lauterbach, M. A. et al. Toll-like receptor signaling rewires macrophage metabolism and promotes histone acetylation via ATP-citrate lyase. Immunity 51, 997–1011 (2019).
Langston, P. K. et al. Glycerol phosphate shuttle enzyme GPD2 regulates macrophage inflammatory responses. Nat. Immunol. 20, 1186–1195 (2019).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337 (2016).
Divakaruni, A. S. et al. Etomoxir inhibits macrophage polarization by disrupting CoA homeostasis. Cell. Metab. 28, 490–503 (2018).
Srinivasan, B. et al. Extracellular 4′-phosphopantetheine is a source for intracellular coenzyme A synthesis. Nat. Chem. Biol. 11, 784–792 (2015).
Jung, H. W., Chung, Y. S., Kim, Y. S. & Park, Y. K. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-κB in LPS-stimulated BV-2 microglial cells. Exp. Mol. Med. 39, 715–721 (2007).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470 (2016).
Drobnica, L. & Sturdik, E. The reaction of carbonyl cyanide phenylhydrazones with thiols. Biochim. Biophys. Acta 585, 462–476 (1979).
Bie, A. S. et al. A cell model to study different degrees of Hsp60 deficiency in HEK293 cells. Cell Stress Chaperones 16, 633–640 (2011).
Katiyar, A. et al. HSF1 is required for induction of mitochondrial chaperones during the mitochondrial unfolded protein response. FEBS Open Bio 10, 1135–1148 (2020).
Cox, C. S. et al. Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell. Metab. 28, 776–786 (2018).
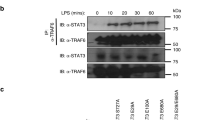
Herb, M. et al. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal. https://doi.org/10.1126/scisignal.aar5926 (2019).
Ahmed, D. et al. Differential remodeling of the electron transport chain is required to support TLR3 and TLR4 signaling and cytokine production in macrophages. Sci. Rep. 9, 18801–18804 (2019).
Seeley, J. J. & Ghosh, S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 101, 107–119 (2017).
Bailey, J. D. et al. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation. Cell. Rep. 28, 218–230 (2019).
Li, Y. et al. Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J. Biol. Chem. 288, 16225–16234 (2013).
Lavie, J. et al. Ubiquitin-dependent degradation of mitochondrial proteins regulates energy metabolism. Cell. Rep. 23, 2852–2863 (2018).
Bergenfelz, C. et al. Systemic monocytic MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS ONE 10, e0127028 (2015).
Washburn, M. L. et al. T cell- and monocyte-specific RNA-sequencing analysis in septic and nonseptic critically ill patients and in patients with cancer. J. Immunol. 203, 1897–1908 (2019).
Baumann, T. et al. Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal. Nat. Immunol. 21, 555–566 (2020).
Yang, W. et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 10, 1076–1078 (2019).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383(2020).
Gottwald, E. M. et al. The targeted antioxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue. Physiol. Rep. 6, e13667 (2018).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295 (2007).
Yoo, H. S. & Napoli, J. L. Quantification of dehydroepiandrosterone, 17β-estradiol, testosterone, and their sulfates in mouse tissues by LC–MS/MS. Anal. Chem. 91, 14624–14630 (2019).
Roberts, A. M. et al. Chemoproteomic screening of covalent ligands reveals UBA5 as a novel pancreatic cancer target. ACS Chem. Biol. 12, 899–904 (2017).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, 1251–1257 (2016).
Buescher, J. M. et al. A roadmap for interpreting 13C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 34, 189–201 (2015).
Costantini, L. M. et al. A palette of fluorescent proteins optimized for diverse cellular environments. Nat. Commun. 6, 7670 (2015).
Acknowledgements
We thank the following people for their essential contributions to this paper: H. Nolla and A. Valeros for assistance with cell sorting and flow cytometry; the Dillin Laboratory for JC-9 reagent; C. F. Chang, P. Zushin and G. Dempsey for reagents, assistance with Seahorse respirometry and helpful discussion; H. S. Yoo and J. Napoli for assistance with steroid extraction; M. Gaidt for helpful advice and reagents; members of the UC Berkeley Functional Genomics Laboratory and Vincent J. Coates Genomic Sequencing Laboratory for assistance with next-generation sequencing; A. Killilea and the UC Berkeley Cell Culture facility for cell lines; C. Rosso, K. Shohat, J. Uyeki and D. Tan of the UCLA Metabolomics Center for LC–MS data processing and analysis; members of the Welch Lab for assistance with SpeedVac; members of the Saijo Lab and UC Berkeley immunology community for helpful feedback; A. Dillin and S. Lewis for critical reading of the paper; L. Lau for emotional support/baked goods. This work was supported by: UC Berkeley/Aduro Immunotherapeutics and Vaccine Research Initiative (IVRI) award and Pew Scholar Award to K.S.; American Diabetes Association Postdoctoral Fellowship no. 1–19-PDF-058 to G.A.T.; 1F32CA236156-01A1 (NIH F32 Postdoctoral Award), 5T32CA108462-15 (NIH T32) and Sandler Program for Breakthrough Biomedical Research (postdoctoral independence award) to K.M.T. This work used: the Vincent J. Coates Genomics Sequencing Laboratory at UC Berkeley, supported by NIH grant S10 OD018174 Instrumentation Grant; the QB3/Chemistry Mass Spectrometry Facility, supported by NIH grant 1S10OD020062-01; the UCLA Metabolomics Center, supported by NIH Instrumentation Grant S10 OD016387. Images and schematics (Figs. 1a,h, 2a,c,f, 3a, 5b,g,h, 6a–c,e, 7e,f and 8a,g, and Extended Data Figs. 1f, 4e and 6l) were created using BioRender.com (licence to G.A.T.). ChemDraw Professional (v.16.0) was also used to draw chemical structures used in Figs. 3a, 5g and 8g.
Author information
Authors and Affiliations
Contributions
G.A.T. and K.S. conceptualized the project. G.A.T., K.M.T., A.T.I., J.t.H., D.K.N., A.S. and K.S. procured funding and resources. G.A.T., K.M.T., B.F., A.T.I. and J.t.H. designed experimental methodology. G.A.T., K.M.T., B.F., J.M.W., J.W., S.Z., R.I.K., S.K.L., A.T.I. and J.t.H. performed experiments and analysed data. G.A.T. curated data, wrote the original manuscript, edited the manuscript with input from all authors and prepared the revised manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.A.T. and K.S. are co-inventors on provisional patent application no. 63/091,217 filed by UC Berkeley describing methods of use of hydroxyoestrogen for treatment of inflammatory and metabolic disease. G.A.T. is co-founder of a start-up focused on therapeutic applications of hydroxyoestrogen derivatives. D.K.N. is a shareholder and adviser for Frontier Medicines. The other authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Eicke Latz, Michael Ristow and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: George Caputa.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Hydroxyestrogens are anti-inflammatory in macrophages in vitro.
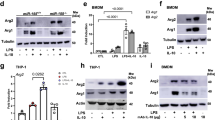
a. BMDMs pretreated for 1h with vehicle or 1 μM hydroxyestrogens before 6h LPS stimulation and qPCR to validate targets identified in RNA-seq data. b. RAW macrophages pretreated for 1h with vehicle or 5 μM estrogens before 6h LPS stimulation and Il1b qPCR. c. THP-1s and U937s pretreated for 1h with vehicle control or 10 μM estrogens before 6h LPS stimulation and qPCR for indicated transcripts. d. RAW macrophages pretreated with ethanol or 5 μM 4-OHE1 for 1h, followed by 6h LPS stimulation before pro-IL-1β measurement by western blot (left, n = 2 biological replicates), or by intracellular staining and flow cytometry (right, representative data from 1 independent biological replicate for each condition shown). e. RAW macrophages pretreated with EtOH or 2.5 μM estrogens for 1h, followed by 3h stimulation with LPS (TLR4), Pam3CSK4 (PAM, TLR2), polyinosinic-polycytidylic acid (pIC, TLR3), or CpG oligodeoxynucleotides (CpG, TLR9), and Il1b qPCR. Percentages indicate induction relative to max (100%) in the ‘EtOH +TLR ligand’ control BMDMs for each ligand. f. BMDMs pretreated with EtOH or 1 μM hydroxyestrogens in the absence (left) or presence (right) of 10 μM ICI 182780 for 1h, followed by 6h LPS stimulation and Il1b qPCR. Each data point is an independent biological replicate. For n = 2, data represented as mean; for n = 3, mean ± SEM. P values from unpaired, two-sided Student’s T Test versus ‘EtOH +LPS’ sample (planned comparison). qPCR and flow cytometry representative of 2 independent experiments. Western blot was performed once.
Extended Data Fig. 2 Hydroxyestrogens are anti-inflammatory in vivo.
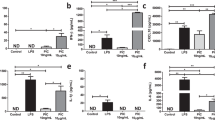
a. Representative gating strategy for identifying forward scatter/side scatter (FS/SS) live gate+, DAPI-, CD45+, F4/80+CD11b+ visceral white adipose tissue (vWAT) macrophages for sorting and flow cytometry analysis. b. vWAT mass in mice after 30 days HFD feeding and EtOH control or estrogen injections. n = 5 mice per group. c. vWAT macrophage cellularity in mice after 30 days HFD feeding and EtOH control or estrogen injections. n = 5 mice per group. d. Relative expression* of select pro-inflammatory genes in vWAT macrophages from HFD-fed mice injected with EtOH, 4-OHE1, or E2 (*log2-transformed RPKM centered on the mean of each gene). n = 5 mice per group. e. Blood glucose levels at 30min and 1h post-glucose injection in mice from Fig. 2f. n = 10, 10, and 11 mice per condition. f. qPCR for Nos2 expression in splenocytes isolated from mice in Fig. 2h. n = 3, 5, 5, and 5 mice per condition. For bar graphs, each data point is an independent biological replicate, and data is represented as mean ± SEM. All P values from unpaired, two-sided Student’s T Test (planned comparisons). HFD chronic inflammation model was performed once for transcriptional profiling (a-d), and a second time for metabolic studies (e,f).
Extended Data Fig. 3 Hydroxyestrogens activate NRF2, but NRF2 is dispensable for their anti-inflammatory effects.
a. GO analysis of 341 genes significantly upregulated in hydroxyestrogen-pretreated, LPS-stimulated BMDMs relative to control pretreatments. Red highlights GO categories involved in oxidative stress resistance (OSR) and detoxification of reactive oxygen species (ROS). b. WT and Nrf2 KO BMDMs were pretreated 1h with EtOH or indicated concentrations of 4-OHE1 before 6h LPS stimulation and qPCR for the NRF2 target gene Hmox1. Data represented as mean ± SEM. n = 3 independent biological replicates per condition. P values from unpaired, two-sided Student’s T Test against corresponding WT sample (planned comparisons). Data representative of 2 independent experiments.
Extended Data Fig. 4 Hydroxyestrogens cause mitochondrial stress.
a. RAW macrophages treated with 5 μM E2, or 5 μM 4-OHE1 (with or without 30min MitoQ pretreatment, 1 μM) for 1h before Hmox1 qPCR. n = 3 independent biological replicates per condition. Data represented as mean ± SEM. P values from unpaired, two-sided Student’s T Test (planned comparisons). Data representative of 2 independent experiments. b-d. GO analysis of 341 genes significantly upregulated in hydroxyestrogen-pretreated, LPS-stimulated BMDMs relative to control pretreatments. Red highlights GO categories indicative of HSF1 and ATF4 activity, and upregulation of glycolysis/pentose phosphate pathway (PPP) genes. e. Experimental setup for steroid extraction and liquid chromatography/mass spectrometry (LC-MS) to measure 4-OHE1 extracted from cell culture media (top, control), or from whole cell and mitochondrial fractions prepared from RAW macrophages treated with 5 μM 4-OHE1 for 1h (n = 2 independent biological replicates per condition). f. Uncropped western blot confirming enrichment of mitochondrial marker VDAC, and depletion of cytoplasmic marker vinculin, in mitochondrial fractions versus whole cell lysates prepared from RAW macrophages. Blot is representative of two independent fractionations for LC-MS. g. top – Extracted ion chromatograms for 4-OHE1 (m/z = 287.1642). Peaks at retention time (RT, minutes) = 13.14 and RT=13.23 for the 4-OHE1 standard and media +4-OHE1 sample (denoted by red arrows) confirm our ability to extract and detect 4-OHE1. Lack of a defined, quantifiable chromatographic peak at RT = 13.1–13.2 for whole cell or mitochondrial extracts prepared from RAW macrophages indicates lack detectable, free 4-OHE1 in these samples. Data is representative of two independent fractionations and LC-MS runs with n = 2 independent biological replicates per condition. bottom – Mass spectrum (positive ion mode) measured for 1mg/mL 4-OHE1 standard (injection volume = 2 μL) showing detail for the [M+H]+ ion of 4-OHE1 at m/z = 287.1642. Mass range displayed: m/z = 287.1624–287.1660. h. Chi-square test comparing the observed frequency of mitochondrial targets (18) in our isoTOP-ABPP target list (118 total targets) versus the expected frequency of mitochondrial targets from MitoCarta 2.0.
Extended Data Fig. 5 Hydroxyestrogens impair mitochondrial acetyl-CoA production and histone acetylation required for LPS-induced proinflammatory gene transcription.
a. BMDMs pretreated 1h with EtOH or 4-OHE1 before 6h LPS stimulation and qPCR. The average percent induction at each 4-OHE1 concentration relative to control for each gene was calculated (n = 3 independent biological replicates) and plotted. b. Abundance (area under the curve, AUC) of TCA cycle metabolites in EtOH versus 4-OHE1-treated BMDMs. c. Fractional contribution (FC) of 13C6-glucose-derived carbons to TCA cycle metabolites and amino acids in EtOH versus 4-OHE1-treated BMDMs (significant labeling in bold). d. UCSC genome browser H3K27ac ChIP-seq tracks displaying regions of decreased read density (arrows) in the Il1b, Cd69, Ifitm3, and Nfkbid loci. Read density was locally scaled, and black bar added to provide common reference point across tracks. e. RAW macrophages cultured in CoA (500 μM, top) or acetyl-CoA (Ac-CoA, 500 μM, bottom) (red bars) for 3h prior to 1h EtOH or 5 μM 4-OHE1 pretreatment, 6h LPS stimulation, and Il1b qPCR. f. RAW macrophages cultured in sodium acetate (5mM, purple bar) or CoA (500 μM, red bar) for 15 minutes prior to 1h EtOH or 5 μM 4-OHE1 pretreatment, 1h LPS stimulation, and Il1b qPCR. g. Data from reference24. Acetyl-CoA levels in HeLa cells treated with vehicle or 10 μM FCCP for 24h. n = 7 independent biological replicates per condition. h. RAW macrophages pretreated with vehicle or itaconate (7.5mM) for 1h before 3h LPS stimulation and Il1b qPCR. i. RAW macrophages cultured in acetyl-CoA (Ac-CoA, 200 μM, bottom) for 2h prior to pretreatment with vehicle or itaconate for 1h before 3h LPS stimulation and Il1b qPCR. Each data point is an independent biological replicate. For n = 2, data represented as mean; for n = 3 or more, data represented as mean ± SEM. All P values from unpaired, two-sided Student’s T Test (planned comparisons). qPCR data representative of at least 2 independent experiments. Metabolomics and ChIP-seq studies were performed once.
Extended Data Fig. 6 Hydroxyestrogen-driven mitochondrial stress triggers mitohormesis.
a. Oxygen consumption in RAW macrophages after treatment with vehicle or 4-OHE1. 5 repeated measurements per condition were taken over 45min and averaged (n = 4 independent biological replicates). b. Mitochondrial membrane potential in RAW macrophages treated 20min with vehicle, oligomycin, FCCP, or 4-OHE1 (5 μM). c. RAW matrix-oxGFP macrophages treated 8h with vehicle or 4-OHE1 and fluorescence quantified by flow cytometry. d. qPCR for matrix-oxGFP transcript in parental macrophages and RAW matrix-oxGFP macrophages treated 8h with vehicle or estrogens (5 μM). e. Mitochondrial DNA content in RAW macrophages treated with vehicle or 5 μM 4-OHE1. f. RAW matrix-oxGFP macrophages pretreated 1h with NAC (5mM) or MitoQ (1 μM) prior to 8h vehicle or 5 μM 4-OHE1 treatment. g. RAW matrix-oxGFP macrophages pretreated 1h with KRIBB11 (10, 7.5, 5 μM) before 8h treatment with 5 μM 4-OHE1. h. i. RAW macrophages and BMDMs treated 8h with vehicle or estrogens (5 μM) before MitoTracker Green staining and flow cytometry. j. THP-1 and U937 matrix-oxGFP cells treated 24h with vehicle or estrogens (5 μM). k. BMDMs pretreated 1h with vehicle or estrogens (5 μM) before 6h LPS stimulation, MitoPY1 staining, and flow cytometry. l. left – roGFP experimental strategy. right – RAW matrix-roGFP 405nm/488nM excitation ratio after H2O2 and DTT treatment. m. RAW macrophages pretreated 1h with NAC (5mM) or MitoQ (1 μM) prior to vehicle or 5 μM 4-OHE1 treatment (18h). Cells were then treated 2.5h with vehicle or 50 μM menadione before viability assessment by flow cytometry. n. BMDMs treated overnight with vehicle or 5 μM 4-OHE1 before vehicle (4h) or 50 μM menadione (2,4h) treatment and viability assessment. o. THP-1 and U937s treated 7h with vehicle or estrogens (5 μM) before H2O2 challenge, CM-H2DCFDA staining, and flow cytometry. Each data point is an independent biological replicate. For n = 2, data represented as mean; for n = 3 or more, data represented as mean ± SEM. All P values from unpaired, two-sided Student’s T Test (planned comparisons). All data representative of at least 2 independent experiments.
Extended Data Fig. 7 LPS-driven mitochondrial stress triggers mitohormesis.
a. GO analysis of 1622 genes upregulated by both 4-OHE1 and LPS at 6h in BMDMs. b. Heatmap showing relative expression* of select genes upregulated by both 4-OHE1 and LPS at 6h in BMDMs. (*DESeq2 counts centered on the mean of each gene). c. RAW matrix-oxGFP macrophages pretreated with vehicle or 10 μM KRIBB11 for 1h before treatment with 4-OHE1 (5 μM, left) or LPS (right) for 8h. Matrix-oxGFP fluorescence was quantified by flow cytometry. d. MitoTracker Green signal in RAW macrophages and BMDMs measured by flow cytometry after 24h LPS simulation. e. Mitochondrial DNA/genomic DNA (mtDNA/gDNA) ratio in RAW macrophages treated with PBS vehicle control or LPS for indicated times. f. THP-1 and U937 matrix-oxGFP reporter cells treated 24h with LPS before matrix-oxGFP fluorescence was quantified by flow cytometry. g. WT (left) and Nrf2 KO (right) BMDMs were treated with EtOH vehicle control, or 4-OHE1/LPS (5 μM/100ng/mL) for 7h before Prdx6 qPCR. h. THP-1 cells were left untreated, or stimulated with LPS for 7h, before H2O2 treatment (500 μM, 5min) and intracellular oxidative stress measurement via CM-H2DCFDA staining and flow cytometry. Each data point is an independent biological replicate. For n = 2, data represented as mean; for n = 3, mean ± SEM. P values from unpaired, two-sided Student’s T Test (planned comparisons). RNA-seq study was performed once. Flow cytometry and qPCR data representative of 2 independent experiments.
Extended Data Fig. 8 Mitohormesis in macrophages involves metabolic reprogramming that enforces an LPS-tolerant state.
a. Basal and maximal OCR from RAW macrophage mitochondrial stress test Fig. 8c. Data is average of 3 repeated measurements prior to oligomycin injection, or following FCCP injection, respectively. b. Mitochondrial stress test in BMDMs treated 20h with EtOH, 4-OHE1 (5 μM), LPS, or both. n = 5 independent biological replicates per condition. c. Il1b qPCR in BMDMs treated overnight (18–24h) with EtOH, 4-OHE1 (5 μM), LPS, or both, before treatment wash out, recovery (1–2h), and 6h secondary LPS stimulation. Data is plotted as secondary LPS fold-induction versus cells with the same primary treatment but no secondary LPS. d. RAW macrophages treated overnight with EtOH, 4-OHE1 (5 μM), LPS, or both, before wash out, recovery, and 6h secondary LPS stimulation for MitoPY1 staining and flow cytometry. Data plotted as percent change in MitoPY1 fluorescence versus no secondary LPS control for each primary treatment. P values are from comparison of treatment vs. naïve control. e. BMDMs treated overnight with EtOH or estrogens (5 μM) before wash out, recovery, and 6h secondary LPS stimulation (100ng/mL, 2.5 μM nigericin added the last hour) for IL-1β ELISA. f. IL1B qPCR in THP-1s treated overnight with EtOH, LPS, or 4-OHE1 (5 μM) before wash out, recovery, and 6h secondary LPS stimulation. Data plotted as LPS fold-induction for cells with the same primary treatment but no secondary LPS. g. Mitochondrial membrane potential (mtMP) in RAW macrophages treated with LPS and stained with JC-9 for flow cytometry. Except for b, each data point is an independent biological replicate. For n = 2, data represented as mean; for n = 3 or greater, mean ± SEM. P values are from unpaired, two-sided Student’s T Test (planned comparisons). BMDM Seahorse and ELISA were performed once. All other data representative of 2 independent experiments.
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blot from Fig. 1e.
Source Data Fig. 3
Unprocessed western blot from Fig. 3f.
Source Data Fig. 4
Source data for Fig. 4e,f.
Source Data Fig. 5
Statistical source data for Fig. 5c–e.
Source Data Extended Data Fig. 1
Unprocessed western blot from Extended Data Fig. 1d.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5c.
Rights and permissions
About this article
Cite this article
Timblin, G.A., Tharp, K.M., Ford, B. et al. Mitohormesis reprogrammes macrophage metabolism to enforce tolerance. Nat Metab 3, 618–635 (2021). https://doi.org/10.1038/s42255-021-00392-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00392-w
This article is cited by
-
Mitochondrial stress activates YAP/TAZ through RhoA oxidation to promote liver injury
Cell Death & Disease (2024)
-
The immunomodulatory effects of metformin in LPS-induced macrophages: an in vitro study
Inflammation Research (2024)
-
Transcriptional characteristics and functional validation of three monocyte subsets during aging
Immunity & Ageing (2023)
-
Pnpt1 mediates NLRP3 inflammasome activation by MAVS and metabolic reprogramming in macrophages
Cellular & Molecular Immunology (2023)
-
Tamoxifen for the treatment of myeloproliferative neoplasms: A Phase II clinical trial and exploratory analysis
Nature Communications (2023)