Abstract
Obesity is mainly due to excessive food intake. IRX3 and IRX5 have been suggested as determinants of obesity in connection with the intronic variants of FTO, but how these genes contribute to obesity via changes in food intake remains unclear. Here, we show that mice doubly heterozygous for Irx3 and Irx5 mutations exhibit lower food intake with enhanced hypothalamic leptin response. By lineage tracing and single-cell RNA sequencing using the Ins2-Cre system, we identify a previously unreported radial glia-like neural stem cell population with high Irx3 and Irx5 expression in early postnatal hypothalamus and demonstrate that reduced dosage of Irx3 and Irx5 promotes neurogenesis in postnatal hypothalamus leading to elevated numbers of leptin-sensing arcuate neurons. Furthermore, we find that mice with deletion of Irx3 in these cells also exhibit a similar food intake and hypothalamic phenotype. Our results illustrate that Irx3 and Irx5 play a regulatory role in hypothalamic postnatal neurogenesis and leptin response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
References
Dietrich, M. O. & Horvath, T. L. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 36, 65–73 (2013).
Timper, K. & Bruning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model Mech. 10, 679–689 (2017).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754–764 (2019).
Lee, D. A. et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 15, 700–702 (2012).
Haan, N. et al. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 33, 6170–6180 (2013).
Goodman, T. & Hajihosseini, M. K. Hypothalamic tanycytes—masters and servants of metabolic, neuroendocrine and neurogenic functions. Front. Neurosci. 9, 387 (2015).
Rizzoti, K. & Lovell-Badge, R. Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol. Cell. Endocrinol. 445, 7–13 (2017).
Yoo, S. & Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 170, 53–66 (2018).
Smemo, S. et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507, 371–375 (2014).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373, 895–907 (2015).
Bjune, J. I. et al. IRX5 regulates adipocyte amyloid precursor protein and mitochondrial respiration in obesity. Int. J. Obes. https://doi.org/10.1038/s41366-018-0275-y (2018).
Cecil, J. E., Tavendale, R., Watt, P., Hetherington, M. M. & Palmer, C. N. An obesity-associated FTO gene variant and increased energy intake in children. N. Engl. J. Med. 359, 2558–2566 (2008).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Dina, C. et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726 (2007).
Tanofsky-Kraff, M. et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am. J. Clin. Nutr. 90, 1483–1488 (2009).
Speakman, J. R., Rance, K. A. & Johnstone, A. M. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity 16, 1961–1965 (2008).
Herman, M. A. & Rosen, E. D. Making biological sense of GWAS data: lessons from the FTO Locus. Cell Metab. 22, 538–539 (2015).
Tung, Y. C., Yeo, G. S., O’Rahilly, S. & Coll, A. P. Obesity and FTO: changing focus at a complex locus. Cell Metab. 20, 710–718 (2014).
de Araujo, T. M. et al. The partial inhibition of hypothalamic IRX3 exacerbates obesity. EBioMedicine 39, 448–460 (2019).
Kim, K. H., Rosen, A., Bruneau, B. G., Hui, C. C. & Backx, P. H. Iroquois homeodomain transcription factors in heart development and function. Circ. Res. 110, 1513–1524 (2012).
Zhang, S. S. et al. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His–Purkinje network. Proc. Natl Acad. Sci. USA 108, 13576–13581 (2011).
Kim, K. H. et al. Irx3 is required for postnatal maturation of the mouse ventricular conduction system. Sci. Rep. 6, 19197 (2016).
Tan, Z. et al. IRX3 and IRX5 inhibit adipogenic differentiation of hypertrophic chondrocytes and promote osteogenesis. J. Bone Miner. Res. https://doi.org/10.1002/jbmr.4132 (2020).
Cain, C. J. et al. Loss of Iroquois homeobox transcription factors 3 and 5 in osteoblasts disrupts cranial mineralization. Bone Rep. 5, 86–95 (2016).
Cohen, D. R., Cheng, C. W., Cheng, S. H. & Hui, C. C. Expression of two novel mouse Iroquois homeobox genes during neurogenesis. Mech. Dev. 91, 317–321 (2000).
Gaborit, N. et al. Cooperative and antagonistic roles for Irx3 and Irx5 in cardiac morphogenesis and postnatal physiology. Development 139, 4007–4019 (2012).
Li, D. et al. Formation of proximal and anterior limb skeleton requires early function of Irx3 and Irx5 and is negatively regulated by Shh signaling. Dev. Cell 29, 233–240 (2014).
Fu, A. et al. Dynamic expression patterns of Irx3 and Irx5 during germline nest breakdown and primordial follicle formation promote follicle survival in mouse ovaries. PLoS Genet. 14, e1007488 (2018).
Cui, X. et al. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol. Rep. https://doi.org/10.14814/phy2.12799 (2016).
Allison, M. B. & Myers, M. G. Jr. 20 years of leptin: connecting leptin signaling to biological function. J. Endocrinol. 223, T25–T35 (2014).
Campbell, J. N. et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 20, 484–496 (2017).
Henry, F. E., Sugino, K., Tozer, A., Branco, T. & Sternson, S. M. Cell-type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife https://doi.org/10.7554/eLife.09800 (2015).
Robins, S. C. et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 4, 2049 (2013).
Padilla, S. L., Carmody, J. S. & Zeltser, L. M. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med. 16, 403–405 (2010).
Ishii, Y. & Bouret, S. G. Embryonic birthdate of hypothalamic leptin-activated neurons in mice. Endocrinology 153, 3657–3667 (2012).
McNay, D. E., Briancon, N., Kokoeva, M. V., Maratos-Flier, E. & Flier, J. S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Invest. 122, 142–152 (2012).
Wojtowicz, J. M. & Kee, N. BrdU assay for neurogenesis in rodents. Nat. Protoc. 1, 1399–1405 (2006).
Song, J., Xu, Y., Hu, X., Choi, B. & Tong, Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis 48, 628–634 (2010).
Kong, D. et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151, 645–657 (2012).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Zywitza, V., Misios, A., Bunatyan, L., Willnow, T. E. & Rajewsky, N. Single-cell transcriptomics characterizes cell types in the subventricular zone and uncovers molecular defects impairing adult neurogenesis. Cell Rep. 25, 2457–2469 (2018).
Shah, P. T. et al. Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 173, 1045–1057 (2018).
Romanov, R. A. et al. Molecular design of hypothalamus development. Nature 582, 246–252 (2020).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Tabula Muris, C. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Han, X. et al. Mapping the Mouse Cell Atlas by Microwell-seq. Cell 172, 1091–1107 (2018).
Pierce, A. A. & Xu, A. W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci. 30, 723–730 (2010).
Kokoeva, M. V., Yin, H. & Flier, J. S. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310, 679–683 (2005).
Choudhury, A. I. et al. The role of insulin receptor substrate 2 in hypothalamic and beta-cell function. J. Clin. Invest. 115, 940–950 (2005).
Harrison, L. et al. Fluorescent blood–brain barrier tracing shows intact leptin transport in obese mice. Int. J. Obes. 43, 1305–1318 (2019).
Balland, E. et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 19, 293–301 (2014).
Hui, C. C. & Joyner, A. L. A mouse model of greig cephalo-polysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 3, 241–246 (1993).
Jandura, A., Hu, J., Wilk, R. & Krause, H. M. High-resolution fluorescent in situ hybridization in Drosophila embryos and tssues using tyramide signal amplification. J. Vis. Exp. https://doi.org/10.3791/56281 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies and species. Nat. Biotechnol. 36, 411–420 (2018).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Acknowledgements
We thank T. Paton, B. Thiruv, J. Zhang, B. Innes and G. Bader for assisting with the computational analysis of scRNA-seq data. The research was supported by Canadian Institutes of Health Research (MOP-136821) and the Canada Research Chairs program to C.-C.H. J.E.S. was supported by a postdoctoral fellowship award from Diabetes Canada.
Author information
Authors and Affiliations
Contributions
J.E.S., X.L., X.H. and C.-C.H. conceived and designed the study. J.E.S., Z.D., K.-H.K., V.S.B.C. and X.C. performed the mouse experiments. J.E.S., Z.D., R.M. and X.Z. carried out the RNA and protein analyses. J.E.S., Z.D., S.W., and T.K. performed the scRNA-seq analysis. J.E.S. and C.-C.H. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Metabolism thanks Seth Blackshaw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Isabella Samuelson.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 General phenotypes of Irx3/5dHet mice.
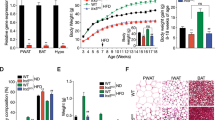
a-b, RNA expression analysis of (a) Irx3 and (b) Irx5 in hypothalamus, PWAT and BAT (male mice; n = 6 WT, n = 6 Irx3/5dHet). c, Representative photograph of WT vs. Irx3KO, WT vs. Irx5KO, and WT vs. Irx3/5dHet male mice at weaning (21 days of age). d, Body length (nose-to-anus distance) of WT and Irx3/5dHet mice at 14 weeks of age (male mice; n = 8/11). e, Body weight changes of WT and Irx3/5dHet female mice. The inset bar-graph shows body weight of 11 weeks old mice (n = 9/9). Data are represented as means ± SEM values. *P < 0.05; **P < 0.01 by two-tailed unpaired t test. ns: not significant.
Extended Data Fig. 2 Metabolic phenotypes of Irx3/5dHet mice.
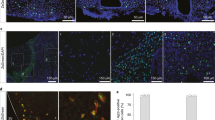
a, Frequency distribution and b, average of adipocyte diameter in PWAT of WT and Irx3/5dHet mice (male mice; n = 6/10). c, Linear regression analysis of energy expenditure as a function of lean mass. d-e, qPCR analysis of Ucp1 mRNA expression in (d) IWAT and (e) BAT (male mice; n = 7 WT, n = 6 Irx3/5dHet). f, Representative images and quantification of the number of p-STAT3+ cells in the VMH and DMH following leptin injection (male mice; n = 3 WT-Leptin, n = 4 Irx3/5dHet-Leptin). Scale bar, 100 μm. Data are represented as means ± SEM values. *P < 0.05; **P < 0.01 by two-tailed unpaired t test. ns: not significant. g, Visualization of fluorescence-conjugated leptin (Leptin650) in the ventricular hypothalamus of wild-type and Irx3/5dHet mice. Leptin650 was found only in the blood vessels at 2 minutes post-leptin injection. Leptin650 diffused into the parenchyma of the ventricular hypothalamus at 10 minutes post-leptin injection (Top). Leptin650 uptake by tanycytes. Dashed lines indicate the ventricular tanycytic layer (Bottom). Scale bar, 100 μm. h, Representative Images of ZO-1 immunostaining in the ventricular tanycytic layer of WT and Irx3/5dHet mice. Scale bar, 50 μm.
Extended Data Fig. 3 Cellular characterization of ARC-ME from wild-type and Irx3/5dHet mice using single cell RNA sequencing (scRNA-Seq) analysis.
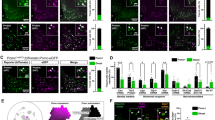
a, Schematic of the micro-dissected ARC-ME preparation for scRNA-Seq analysis. b, Heatmap of top marker genes for each cell cluster and annotation. c, UMAP plot of sequenced ARC-ME cells (2208 WT cells, 1407 Irx3/5dHet cells) and annotated according to known cell type markers. d, Percentage of orexigenic (Agrp+/Npy+) and anorexigenic (Pomc+/Cartpt+) neurons out of 2208 WT cells or 1407 Irx3/5dHet cells as revealed by scRNA-Seq. The number of Agrp+/Npy+ neurons is 124 for WT and 186 for Irx3/5dHet. For Pomc+/Cartpt+ neurons, the numbers are 397 and 400 for WT and Irx3/5dHet, respectively. Data represented as data-points ± 95% confidence intervals. *P < 0.05; **P < 0.01 determined using the chi-square test of independence with Bonferroni-adjusted P values. e, Gene expression analysis in micro-dissected ARC-ME (male mice; n = 14 WT, n = 16 Irx3/5dHet). Data are represented as means ± SEM values. The asterisks indicate a significant difference (*P < 0.05; **P < 0.01). P value was determined by two-tailed unpaired t test.
Extended Data Fig. 4 BrdU labelling during adult period.
a, Representative images and b, Quantification of the number of BrdU-labelled cells in the ARC (n = 5 WT, n = 4 Irx3/5dHet). 8 weeks old male mice received BrdU from for 14 days through drinking water (1 mg/mL). Data are represented as means ± SEM values. ns: not significant. P value was determined by two-tailed unpaired t test. Scale bars, 100 μm.
Extended Data Fig. 5 Identification of Ins2-Cre+ cells.
a, Schematic of the generation of Ins2-Cre;Rosa26mT/mG mice. b, Representative mT/mG images of various tissues from Ins2-Cre;Rosa26mT/mG male mice at adult stage. c, Representative fluorescence RNA in situ hybridization images of tdTomato with Npy or Cartpt in the ARC from adult Ins2-Cre;Ai14 tdTomato male mice. Arrowheads denote co-labelled cells. Scale bar, 100 μm.
Extended Data Fig. 6 scRNA-Seq analysis of Ins2-Cre;Ai14 tdTomato+ ARC-ME cells at P10.
a, Heatmap of top marker genes for each cell cluster and annotation. b, Violin plots showing orexigenic (Agrp+/Npy+) neurons and anorexigenic (Pomc+/Cartpt+) neurons in the neuronal cluster. c, Feature plots of Htra1, Mfge8, Fgf10, and Gfap. The color key indicates expression levels (red, high; gray, low). d, Proportional distribution of cell types. e, Percentage of orexigenic (Agrp+/Npy+) and anorexigenic (Pomc+/Cartpt+) neurons out of 3479 control cells or 3939 Irx3/5dHet cells as revealed by scRNA-Seq. The number of Agrp+/Npy+ neurons is 1286 in control and 2081 in Irx3/5dHet. There are 1142 and 1494 Pomc+/Cartpt+ neurons in control and Irx3/5dHet, respectively. f, Proportional distribution of radial glia-like NSCs (427-control; 430-Irx3/5dHet) identified by reference-based integration with Sox2-eGFP SVZ dataset. Data represented as datapoints ± 95% confidence intervals. *P < 0.05; **P < 0.01 determined using the chi-square test of independence with Bonferroni-adjusted P values. ns: not significant.
Extended Data Fig. 7 Analysis of the developing whole hypothalamus scRNA-Seq dataset (GSE132730).
a, UMAP plots of radial glia/progenitors, tanycytes, and immature neurons from whole hypothalamus at different developmental timepoints. b, Differential gene expression analysis shows that embryonic and postnatal radial glia/progenitors from whole hypothalamus have distinct gene expression profiles. Radial glia/progenitors from P10 hypothalamus and Ins2-Cre;Ai14 tdTom+ RG-like NSCs display highly related gene expression profiles.
Extended Data Fig. 8 Pseudotime differential trajectory analysis of RG-like NSCs, tanycytes and neurons from the Ins2-Cre;Ai14 tdTomato+ scRNA-Seq dataset.
a, Integrated diffusion map of RG-like NSCs, tanycytes and neurons from the Ins2-Cre;Ai14 tdTomato dataset reveals a single linear differentiation trajectory (Left). A line with arrow indicates pseudotime trajectory inferred by the Slingshot method. Cells are color-coded for defined pseudotime score. Expression profiles of genes for stemness (Sox2), enriched in radial glia-like NSCs (Hes5) and neurons (Tubb3) in pseudo-trajectory (Right). Cells are color-coded according to expression level. Termination with higher Sox2/Hes5 expression was chosen as the root. b, Pseudotime trajectory analysis performed using minimum-spanning tree (MST) shows a similar trajectory to the Slingshot method. c, Integrated diffusion map of RG-like NSCs, tanycytes and orexigenic (Agrp+/Npy+)/anorexigenic (Pomc+/Cartpt+) neurons from postnatal Ins2-Cre;Ai14 tdTomato+ scRNA-seq dataset reveals a single linear differentiation trajectory (Left). A line with arrow indicates pseudotime trajectory defined by Slingshot. Cells are color-coded for defined pseudotime score. Expression profiles of genes for stemness (Sox2), enriched in radial glia-like NSCs (Hes5) and orexigenic/anorexigenic neurons (Agrp and Npy for orexigenic neurons; Pomc and Cartpt for anorexigenic neurons) in pseudo-trajectory (Right). Cells are color-coded according to expression level. Termination with higher Sox2 expression was chosen as the root.
Extended Data Fig. 9 General phenotypes of Ins2-Cre;Irx3KO mice.
a, Breeding scheme for the generation of Ins2-Cre;Irx3Fl/Fl (Ins2-Cre;Irx3KO) mice. b, qPCR analysis of Irx3 and Irx5 mRNA expression in micro-dissected ARC-ME (male mice; n = 12 Cont, n = 15 Ins2-Cre;Irx3KO). c, Additional images of the fluorescence RNA in situ hybridization analysis of Irx3 expression in the ventral hypothalamus of control (n = 3) and Ins2-Cre;Irx3KO (n = 3). Arrowheads denote positive signals along the walls of the third ventricle in the control, which are absent in Ins2-Cre;Irx3KO mice. d, Body length (nose-to-anus distance) of control and Ins2-Cre;Irx3KO mice at 14 weeks of age (male mice; n = 4 Cont, n = 7 Ins2-Cre;Irx3KO). e, Linear regression analysis of energy expenditure as a function of lean mass. f, Gene expression analysis in micro-dissected ARC-ME (male mice; n = 12/15). Data are represented as means ± SEM values. The asterisks indicate a significant difference (*P < 0.05; **P < 0.01, ns: not significant). P values were determined by two-tailed unpaired t test. g, Leptin650 injection study. Leptin650 was found only in the blood vessels at 2 minutes post-leptin injection. Leptin650 diffused into the parenchyma of the ventricular hypothalamus at 10 minutes post-leptin injection (Top). Leptin650 uptake by tanycytes (Bottom). Dashed lines indicate the ventricular tanycytic layer. Scale bar, 100 μm.
Extended Data Fig. 10 Pancreatic islet phenotypes of Ins2-Cre;Irx3KO mice.
a, Representative images of pancreatic islets. b, Quantification of the islet diameter, and c, Coverage of α-cells (Glu+) or β-cells (Ins+) per islet (male mice; n = 6 Cont, n = 8 Ins2-Cre;Irx3KO). d, Plasma insulin level at postprandial and fasting conditions (male mice; n = 11/13). Pancreatic phenotypes were assessed in 3 weeks old mice. Data are represented as means ± SEM values. The asterisks indicate a significant difference (ns: not significant). P values were determined by two-tailed unpaired t test. Scale bar, 100 μm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3
Supplementary Table 1
Sequences of oligonucleotides.
Rights and permissions
About this article
Cite this article
Son, J.E., Dou, Z., Kim, KH. et al. Irx3 and Irx5 in Ins2-Cre+ cells regulate hypothalamic postnatal neurogenesis and leptin response. Nat Metab 3, 701–713 (2021). https://doi.org/10.1038/s42255-021-00382-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-021-00382-y
This article is cited by
-
Application of Lineage Tracing in Central Nervous System Development and Regeneration
Molecular Biotechnology (2023)
-
Deficiency of Irx5 protects mice from obesity and associated metabolic abnormalities
International Journal of Obesity (2022)
-
Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis
Nature Metabolism (2022)