Abstract
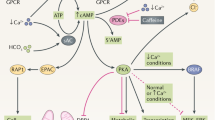
Metabolic reprogramming is emerging as a key pathological contributor to the progression of autosomal dominant polycystic kidney disease (ADPKD), but the molecular mechanisms underlying dysregulated cellular metabolism in cystic cells remain elusive. Super-enhancers (SEs) are large clusters of transcriptional enhancers that drive robust expression of cell identity and disease genes. Here, we show that SEs undergo extensive remodelling during cystogenesis and that SE-associated transcripts are most enriched for metabolic processes in cystic cells. Inhibition of cyclin-dependent kinase 7 (CDK7), a transcriptional kinase required for assembly and maintenance of SEs, or AMP deaminase 3 (AMPD3), one of the SE-driven and CDK7-controlled metabolic target genes, delays cyst growth in ADPKD mouse models. In a cohort of people with ADPKD, CDK7 expression was frequently elevated, and its expression was correlated with AMPD3 expression and disease severity. Together, our findings elucidate a mechanism by which SE controls transcription of metabolic genes during cystogenesis, and identify SE-driven metabolic reprogramming as a promising therapeutic target for ADPKD treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The RNA-seq and ChIP–seq data can be found at the Gene Expression Omnibus database under accession number GSE141281. Source data are provided with this paper.
References
Bergmann, C. et al. Polycystic kidney disease. Nat. Rev. Dis. Primers 4, 50 (2018).
Cornec-Le Gall, E., Alam, A. & Perrone, R. D. Autosomal dominant polycystic kidney disease. Lancet 393, 919–935 (2019).
Padovano, V., Podrini, C., Boletta, A. & Caplan, M. J. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat. Rev. Nephrol. 14, 678–687 (2018).
Menezes, L. F. & Germino, G. G. The pathobiology of polycystic kidney disease from a metabolic viewpoint. Nat. Rev. Nephrol. 15, 735–749 (2019).
Rowe, I. & Boletta, A. Defective metabolism in polycystic kidney disease: potential for therapy and open questions. Nephrol. Dial. Transplant. 29, 1480–1486 (2014).
Rowe, I. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 (2013).
Chiaravalli, M. et al. 2-Deoxy-d-glucose ameliorates PKD progression. J. Am. Soc. Nephrol. 27, 1958–1969 (2016).
Menezes, L. F., Lin, C. C., Zhou, F. & Germino, G. G. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 5, 183–192 (2016).
Lakhia, R. et al. PPARα agonist fenofibrate enhances fatty acid beta-oxidation and attenuates polycystic kidney and liver disease in mice. Am. J. Physiol. Renal Physiol. 314, F122–F131 (2018).
Hwang, V. J. et al. The cpk model of recessive PKD shows glutamine dependence associated with the production of the oncometabolite 2-hydroxyglutarate. Am. J. Physiol. Renal Physiol. 309, F492–F498 (2015).
Soomro, I. et al. Glutamine metabolism via glutaminase 1 in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 33, 1343–1353 (2018).
Podrini, C. et al. Dissection of metabolic reprogramming in polycystic kidney disease reveals coordinated rewiring of bioenergetic pathways. Commun. Biol. 1, 194 (2018).
Lin, C. C. et al. A cleavage product of polycystin-1 is a mitochondrial matrix protein that affects mitochondria morphology and function when heterologously expressed. Sci. Rep. 8, 2743 (2018).
Kuo, I. Y. et al. Polycystin 2 regulates mitochondrial Ca2+ signaling, bioenergetics, and dynamics through mitofusin 2. Sci. Signal 12, eaat7397 (2019).
Ishimoto, Y. et al. Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease. Mol. Cell. Biol. 37, e00337-17 (2017).
Harris, P. C. & Torres, V. E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Invest. 124, 2315–2324 (2014).
Padovano, V. et al. The polycystins are modulated by cellular oxygen-sensing pathways and regulate mitochondrial function. Mol. Biol. Cell 28, 261–269 (2017).
Liu, D. et al. Identification of key genes and candidated pathways in human autosomal dominant polycystic kidney disease by bioinformatics analysis. Kidney Blood Press. Res. 44, 533–552 (2019).
de Almeida, R. M. et al. Transcriptome analysis reveals manifold mechanisms of cyst development in ADPKD. Hum. Genomics 10, 37 (2016).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Sengupta, S. & George, R. E. Super-enhancer-driven transcriptional dependencies in cancer. Trends Cancer 3, 269–281 (2017).
Compe, E. & Egly, J. M. Nucleotide excision repair and transcriptional regulation: TFIIH and Beyond. Annu. Rev. Biochem. 85, 265–290 (2016).
Shin, H. Y. Targeting super-enhancers for disease treatment and diagnosis. Mol. Cells 41, 506–514 (2018).
Niederriter, A. R., Varshney, A., Parker, S. C. & Martin, D. M. Super enhancers in cancers, complex disease, and developmental disorders. Genes (Basel) 6, 1183–1200 (2015).
Chapin, H. C. & Caplan, M. J. The cell biology of polycystic kidney disease. J. Cell Biol. 191, 701–710 (2010).
Podrini, C., Cassina, L. & Boletta, A. Metabolic reprogramming and the role of mitochondria in polycystic kidney disease. Cell. Signal. 67, 109495 (2019).
Bustamante, E., Morris, H. P. & Pedersen, P. L. Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J. Biol. Chem. 256, 8699–8704 (1981).
Cho, H. P., Nakamura, M. T. & Clarke, S. D. Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase. J. Biol. Chem. 274, 471–477 (1999).
Kaur, H., Kumar, C., Junot, C., Toledano, M. B. & Bachhawat, A. K. Dug1p Is a Cys-Gly peptidase of the gamma-glutamyl cycle of Saccharomyces cerevisiae and represents a novel family of Cys-Gly peptidases. J. Biol. Chem. 284, 14493–14502 (2009).
Kim, J. T., Li, V. L., Terrell, S. M., Fischer, C. R. & Long, J. Z. Family-wide annotation of enzymatic pathways by parallel in vivo metabolomics. Cell Chem. Biol. 26, 1623–1629 (2019). e1623.
Ahmad, S. et al. Catalytic characterization of human microsomal glutathione S-transferase 2: identification of rate-limiting steps. Biochemistry 52, 1755–1764 (2013).
Morisaki, T., Sabina, R. L. & Holmes, E. W. Adenylate deaminase. A multigene family in humans and rats. J. Biol. Chem. 265, 11482–11486 (1990).
Patterson, S. L., Sluka, K. A. & Arnold, M. A. A novel transverse push-pull microprobe: in vitro characterization and in vivo demonstration of the enzymatic production of adenosine in the spinal cord dorsal horn. J. Neurochem. 76, 234–246 (2001).
Mahnke-Zizelman, D. K. & Sabina, R.L. Cloning of human AMP deaminase isoform E cDNAs. Evidence for a third AMPD gene exhibiting alternatively spliced 5′-exons. J. Biol. Chem. 267, 20866–20877 (1992).
Song, X. et al. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 18, 2328–2343 (2009).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Chen, L. et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J. Clin. Invest. 125, 2399–2412 (2015).
Calvet, J. P. Polycystic kidney disease: primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int. 43, 101–108 (1993).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Saint-Andre, V. et al. Models of human core transcriptional regulatory circuitries. Genome Res. 26, 385–396 (2016).
Wang, X., Cairns, M. J. & Yan, J. Super-enhancers in transcriptional regulation and genome organization. Nucleic Acids Res. 47, 11481–11496 (2019).
Sun, Y. et al. Activation of P-TEFb by cAMP–PKA signaling in autosomal dominant polycystic kidney disease. Sci. Adv. 5, eaaw3593 (2019).
Zippo, A. et al. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell 138, 1122–1136 (2009).
Kanno, T. et al. BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat. Struct. Mol. Biol. 21, 1047–1057 (2014).
Zhou, X. et al. Therapeutic targeting of BET bromodomain protein, Brd4, delays cyst growth in ADPKD. Hum. Mol. Genet. 24, 3982–3993 (2015).
Seeger-Nukpezah, T., Geynisman, D. M., Nikonova, A. S., Benzing, T. & Golemis, E. A. The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 11, 515–534 (2015).
Lian, X. et al. The combination of metformin and 2-deoxyglucose significantly inhibits cyst formation in miniature pigs with polycystic kidney disease. Br. J. Pharmacol. 176, 711–724 (2019).
Hajarnis, S. et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 8, 14395 (2017).
Lee, E. C. et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat. Commun. 10, 4148 (2019).
Takiar, V. et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl Acad. Sci. USA 108, 2462–2467 (2011).
Leonhard, W. N. et al. Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. EBioMedicine 47, 436–445 (2019).
Seliger, S. L. et al. A randomized clinical trial of metformin to treat autosomal dominant polycystic kidney disease. Am. J. Nephrol. 47, 352–360 (2018).
Pisani, A., Riccio, E., Bruzzese, D. & Sabbatini, M. Metformin in autosomal dominant polycystic kidney disease: experimental hypothesis or clinical fact? BMC Nephrol. 19, 282 (2018).
Sarvaria, A., Topp, Z. & Saven, A. Current therapy and new directions in the treatment of hairy cell leukemia: a review. JAMA Oncol. 2, 123–129 (2016).
Thike, A. A., Chng, M. J., Fook-Chong, S. & Tan, P. H. Immunohistochemical expression of hormone receptors in invasive breast carcinoma: correlation of results of H-score with pathological parameters. Pathology 33, 21–25 (2001).
Shibazaki, S. et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 17, 1505–1516 (2008).
Yang, Y. et al. Interactions between macrophages and cyst-lining epithelial cells promote kidney cyst growth in Pkd1-deficient mice. J. Am. Soc. Nephrol. 29, 2310–2325 (2018).
Cao, X. et al. Targeting super-enhancer-driven oncogenic transcription by CDK7 inhibition in anaplastic thyroid carcinoma. Thyroid 29, 809–823 (2019).
Acknowledgements
This work was supported by grants (31571337 and 81770658 to Y.C. and 31700144 to Z.M.) from the National Natural Science Foundation of China, a grant (2017YFA0504102 to Y.C.) from the National Key Research and Development Program of China, a grant (to Y.C.) from the Excellent Talent Project of Tianjin Medical University, grants (19JCJQJC63800 to Y.C. and 17JCQNJC10600 to Z.M.) from Tianjin Municipal Science and Technology Commission and a grant (2019ZD027 to Y.C.) from Tianjin Municipal Education Commission.
Author information
Authors and Affiliations
Contributions
Z.M. performed mouse and biochemistry studies. Y. Song performed mouse and biochemistry studies. X.C. performed bioinformatics analysis. Y.L., Y. Sun and C.H. provided expertise on mouse studies. Z.L. provided expertise on bioinformatics analysis. X.Z. performed bioinformatics analysis. M.G. performed mouse studies. B.L. provided expertise on biochemistry studies. H.X. provided human specimens. L.Z. designed the project and analysed data. Y.C. conceived, designed and supervised the project, analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Peer review information Primary Handling Editor: Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Enhancer characterization in PH and PN cells.
a, The heatmap of normalized H3K27ac ChIP-seq signal in PH and PN cells. The rows show 3 kb around the H3K27ac peak centre. b, Composite plots of normalized ChIP-seq signals for H3K27ac in PH and PN cells. The x-axis represents distance from the H3K27ac peaks and y-axis represents probability density. c, KEGG enrichment analysis of PN-lost SEs (n = 250 genes). Numbers beside bars indicate numbers of differently expressed genes in that KEGG category. P values obtained by hypergeometric test. d, Gene ontology enrichment analysis of the unchanged SEs (n = 347 genes). Numbers beside bars indicate numbers of differently expressed genes in that GO category. P values obtained by hypergeometric test.
Extended Data Fig. 2 Comparison of differential expressed genes in ADPKD cells, mouse and human kidney tissues.
Comparison of upregulated (a) and downregulated (c) genes in cells, mouse and human kidney tissues. Gene ontology enrichment analysis of the overlap upregulated genes (b) and downregulated genes (d). Numbers beside bars indicate numbers of differently expressed genes in that GO category. P values obtained by hypergeometric test. e, Scatter plot showing expression changes of SE-driven genes in cells and mouse kidney tissues. x-axis: log2 (fold change) from PN versus PH cells; y-axis: log2 (fold change) from ADPKD mouse kidney tissues versus normal kidney tissues. f, Heatmap demonstrating expression values of 29 candidate genes in cells and mouse kidney tissues. Rows show Z scores calculated for each group.
Extended Data Fig. 3 Effects of THZ1 treatment on cell proliferation, apoptosis and body weight in ADPKD mouse model.
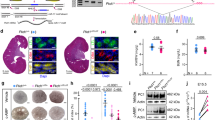
a, Immunohistochemistry analysis of PCNA expression in P29 Pkd1−/− mice injected with THZ1 or DMSO, n = 3 biologically independent mice per group; Data are represented as means ± s.e.m. Unpaired two-sided Student’s t-test was used for statistical analysis, **P = 0.0051. b, Immunofluorescence analysis of TUNEL level from P29 Pkd1−/− mice injected with THZ1 or DMSO, n = 3 biologically independent mice per group; Data are represented as means ± s.e.m. Unpaired two-sided Student’s t-test was used for statistical analysis, *P = 0.0161. c, Body weight of mice from late-onset ADPKD mouse model. Scale bars, 10 µm (a) and 50 µm (b).
Extended Data Fig. 4 Expression of THZ1-rescued genes in ADPKD mouse kidneys.
qRT-PCR analysis of THZ1 downregulated (a) and upregulated (b) genes mRNA level in THZ1 treated mouse kidney tissues (n = 3 biologically independent mice per group). Data are represented as means ± s.e.m., analysed by unpaired two-sided Student’s t-test, Sh3rf2 *P = 0.0127, Adamts5 **P = 0.0053, Arhgef2 ****P < 0.0001, Sh3bp2 **P = 0.0096, Mdh1 **P = 0.0071, Fdxr **P = 0.0038, Csrp2 ***P = 0.0006, Aldh9a1 **P = 0.0098.
Extended Data Fig. 5 The ratio of AMP to ATP decreased in PN cells.
a, HPLC analysis of total AMP and ATP concentrations from PH and PN cell perchlorate extracts. b, The ratio of AMP to ATP in PH and PN cells (n = 3 biologically independent experiments). Data are represented as means ± s.e.m., analysed by unpaired two-sided Student’s t-test, **P = 0.0073.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–9
Source data
Source Data Fig. 1
Statistical source Data
Source Data Fig. 2
Statistical source Data
Source Data Fig. 3
Statistical source Data
Source Data Fig. 3
Unprocessed Western Blots
Source Data Fig. 4
Statistical source Data
Source Data Fig. 4
Unprocessed Western Blots
Source Data Fig. 5
Statistical source Data
Source Data Fig. 6
Statistical source Data
Source Data Fig. 7
Statistical source Data
Source Data Fig. 7
Unprocessed Western Blots
Source Data Fig. 8
Statistical source Data
Source Data Extended Data Fig. 1
Statistical source Data
Source Data Extended Data Fig. 2
Statistical source Data
Source Data Extended Data Fig. 3
Statistical source Data
Source Data Extended Data Fig. 4
Statistical source Data
Source Data Extended Data Fig. 5
Statistical source Data
Rights and permissions
About this article
Cite this article
Mi, Z., Song, Y., Cao, X. et al. Super-enhancer-driven metabolic reprogramming promotes cystogenesis in autosomal dominant polycystic kidney disease. Nat Metab 2, 717–731 (2020). https://doi.org/10.1038/s42255-020-0227-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-0227-4