Abstract
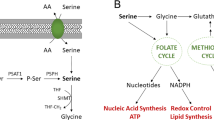
Cancer cells reprogramme their metabolism to support unrestrained proliferation and survival in nutrient-poor conditions. Whereas non-transformed cells often have lower demands for serine and glycine, several cancer subtypes hyperactivate intracellular serine and glycine synthesis and become addicted to de novo production. Copy-number amplifications of serine- and glycine-synthesis genes and genetic alterations in common oncogenes and tumour-suppressor genes enhance serine and glycine synthesis, resulting in high production and secretion of these oncogenesis-supportive metabolites. In this Review, we discuss the contribution of serine and glycine synthesis to cancer progression. By relying on de novo synthesis pathways, cancer cells are able to enhance macromolecule synthesis, neutralize high levels of oxidative stress and regulate methylation and tRNA formylation. Furthermore, we discuss the immunosuppressive potential of serine and glycine, and the essentiality of both amino acids to promoting survival of non-transformed neighbouring cells. Finally, we point to the emerging data proposing moonlighting functions of serine- and glycine-synthesis enzymes and examine promising small molecules targeting serine and glycine synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). This seminal paper summarizes the key changes involved in cancerous cell transformation, including metabolic rewiring.
Deberardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Phan, L. M., Yeung, S. C. J. & Lee, M. H. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 11, 1–19 (2014).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Deberardinis, R. J. & Chandel, N. S. We need to talk about the Warburg effect. Nat. Metab. 2, 127–129 (2020).
Sullivan, M. R. & Vander Heiden, M. G. When cancer needs what’s non-essential. Nat. Cell Biol. 19, 418–420 (2017).
de Koning, T. J. et al. l-Serine in disease and development. Biochem. J. 371, 653–661 (2003).
Locasale, J. W. et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 (2011).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011). This is a milestone paper describing the role of serine and glycine synthesis addiction in cancer.
Locasale, J. W. & Cantley, L. C. Genetic selection for enhanced serine metabolism in cancer development. Cell Cycle 10, 3812–3813 (2011).
Amelio, I., Cutruzzolá, F., Antonov, A., Agostini, M. & Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 39, 191–198 (2014).
Chaneton, B. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458–462 (2012).
Hitosugi, T. et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell 22, 585–600 (2012).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Wubben, T. J. et al. Small molecule activation of metabolic enzyme pyruvate kinase muscle isozyme 2, PKM2, circumvents photoreceptor apoptosis. Sci. Rep. 10, 2990 (2020).
Kung, C. et al. Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem. Biol. 19, 1187–1198 (2012).
Mullarky, E., Mattaini, K. R., Vander Heiden, M. G., Cantley, L. C. & Locasale, J. W. PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 24, 1112–1115 (2011).
Kim, S. K., Jung, W. H. & Koo, J. S. Differential expression of enzymes associated with serine/glycine metabolism in different breast cancer subtypes. PLoS ONE 9, e101004 (2014).
Liao, L. et al. Upregulation of phosphoserine phosphatase contributes to tumor progression and predicts poor prognosis in non-small cell lung cancer patients. Thorac. Cancer 10, 1203–1212 (2019).
Kim, D. et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520, 363–367 (2015).
Paone, A. et al. SHMT1 knockdown induces apoptosis in lung cancer cells by causing uracil misincorporation. Cell Death Dis. 5, e1525 (2014).
Tan, E. H. et al. A multicentre phase II gene expression profiling study of putative relationships between tumour biomarkers and clinical response with erlotinib in non-small-cell lung cancer. Ann. Oncol. 21, 217–222 (2010).
Reis-Filho, J. S. et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J. Pathol. 209, 445–453 (2006).
DeBerardinis, R. J. Serine metabolism: some tumors take the road less traveled. Cell Metab. 14, 285–286 (2011).
Pacold, M. E. et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 12, 452–458 (2016).
Mullarky, E. et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl Acad. Sci. USA 113, 1778–1783 (2016).
Zhu, J. et al. High expression of PHGDH predicts poor prognosis in non-small cell lung cancer. Transl. Oncol. 9, 592–599 (2016).
Xian, Y. et al. Phosphoglycerate dehydrogenase is a novel predictor for poor prognosis in gastric cancer. Onco. Targets Ther. 9, 5553–5560 (2016).
Yin, K. Positive correlation between expression level of mitochondrial serine hydroxymethyltransferase and breast cancer grade. Onco. Targets Ther. 8, 1069–1074 (2015).
Sun, L. et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 25, 429–444 (2015). This paper identified cMyc as an upstream regulator of serine and glycine synthesis.
Zhang, B. et al. PHGDH defines a metabolic subtype in lung adenocarcinomas with poor prognosis. Cell Rep. 19, 2289–2303 (2017).
Kampen, K. R. et al. Translatome analysis reveals altered serine and glycine metabolism in T-cell acute lymphoblastic leukemia cells. Nat. Commun. 10, 2542 (2019). This paper highlights translational regulation of de novo serine and glycine synthesis.
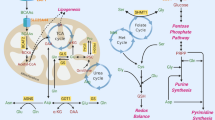
Fan, T. W. M. et al. De novo synthesis of serine and glycine fuels purine nucleotide biosynthesis in human lung cancer tissues. J. Biol. Chem. 294, 13464–13477 (2019).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Newman, A. C. & Maddocks, O. D. K. One-carbon metabolism in cancer. Br. J. Cancer 116, 1499–1504 (2017).
Tibbetts, A. S. & Appling, D. R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 30, 57–81 (2010).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Finkelstein, J. D. Methionine metabolism in mammals. J. Nutr. Biochem. 1, 228–237 (1990).
Kinney, A. J. & Moore, T. S. Phosphatidylcholine synthesis in castor bean endosperm. Plant Physiol. 84, 78–81 (1987).
Hwang, S. et al. Serine-dependent sphingolipid synthesis is a metabolic liability of aneuploid cells. Cell Rep. 21, 3807–3818 (2017).
Gao, X. et al. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 22, 3507–3520 (2018).
Schwartz, N. U. et al. Decreased ceramide underlies mitochondrial dysfunction in Charcot–Marie–Tooth 2F. FASEB J. 32, 1716–1728 (2018).
Muthusamy, T. et al. Serine restriction alters sphingolipid diversity to constrain tumour growth. Nature 586, 790–795 (2020).
Bartke, N. & Hannun, Y. A. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 50, S96 (2009).
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 4, 1406–1417 (2014).
Da Veiga Moreira, J. et al. The redox status of cancer cells supports mechanisms behind the Warburg effect. Metabolites 6, 33 (2016).
Davis, S. R. et al. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am. J. Physiol. Metab. 286, E272–E279 (2004).
Maddocks, O. D. K., Labuschagne, C. F., Adams, P. D. & Vousden, K. H. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol. Cell 61, 210–221 (2016).
Yu, W. et al. One-carbon metabolism supports S-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol. Cell 75, 1147–1160 (2019).
Tran, T. Q., Lowman, X. H. & Kong, M. Molecular pathways: metabolic control of histone methylation and gene expression in cancer. Clin. Cancer Res. 23, 4004–4009 (2017).
Carey, B. W., Finley, L. W. S., Cross, J. R., Allis, C. D. & Thompson, C. B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015).
Minton, D. R. et al. Serine catabolism by SHMT2 is required for proper mitochondrial translation initiation and maintenance of formylmethionyl-tRNAs. Mol. Cell 69, 610–621 (2018).
Morscher, R. J. et al. Mitochondrial translation requires folate-dependent tRNA methylation. Nature 554, 128–132 (2018).
Adams, C. M. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J. Biol. Chem. 282, 16744–16753 (2007).
DeNicola, G. M. et al. NRF2 regulates serine biosynthesis in non–small cell lung cancer. Nat. Genet. 47, 1475–1481 (2015). This paper identified NRF2 as an upstream regulator of serine and glycine synthesis.
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Ye, J. et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl Acad. Sci. USA 109, 6904–6909 (2012).
Mossmann, D., Park, S. & Hall, M. N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 18, 744–757 (2018).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Park, Y., Reyna-Neyra, A., Philippe, L. & Thoreen, C. C. mTORC1 balances cellular amino acid supply with demand for protein synthesis through post-transcriptional control of ATF4. Cell Rep. 19, 1083–1090 (2017).
Kottakis, F. et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature 539, 390–395 (2016).
Reina-Campos, M. et al. Increased serine and one-carbon pathway metabolism by PKCλ/ι deficiency promotes neuroendocrine prostate cancer. Cancer Cell 35, 385–400 (2019).
Ma, L. et al. Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell 152, 599–611 (2013).
Kim, Y. R. et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J. Pathol. 220, 446–451 (2010).
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Tao, S. et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res 74, 7430–7441 (2014).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372 (2017). This work showed the difference between cancer cells that depend on dietary serine and glycine versus de novo serine and glycine synthesis.
Sun, W. Y., Kim, H. M., Jung, W.-H. & Koo, J. S. Expression of serine/glycine metabolism-related proteins is different according to the thyroid cancer subtype. J. Transl. Med. 14, 168 (2016).
Xia, Y. et al. Metabolic reprogramming by MYCN confers dependence on the serine-glycine-one-carbon biosynthetic pathway. Cancer Res 79, 3837–3850 (2019).
Nilsson, L. M. et al. Mouse genetics suggests cell-context dependency for myc-regulated metabolic enzymes during tumorigenesis. PLOS Genet. 8, e1002573 (2012).
Yang, X. et al. SHMT2 desuccinylation by SIRT5 drives cancer cell proliferation. Cancer Res. 78, 372–386 (2018).
Lu, W., Zuo, Y., Feng, Y. & Zhang, M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumor Biol. 35, 10699–10705 (2014).
Wang, Y.-Q. et al. Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation-dependent manner. Nat. Commun. 9, 545 (2018).
Wei, Z. et al. Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat. Commun. 9, 4468 (2018).
Guo, H. et al. NRF2 SUMOylation promotes de novo serine synthesis and maintains HCC tumorigenesis. Cancer Lett. 466, 39–48 (2019).
Ding, J. et al. The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab. 18, 896–907 (2013).
Svoboda, L. K. et al. Menin regulates the serine biosynthetic pathway in Ewing sarcoma. J. Pathol. 245, 324–336 (2018).
Yang, L. et al. Novel impact of the DNMT3A R882H mutation on GSH metabolism in a K562 cell model established by TALENs. Oncotarget 8, 30395–30409 (2017).
Zhao, E. et al. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep. 14, 506–519 (2016).
Dalton, W. B. et al. Hotspot SF3B1 mutations induce metabolic reprogramming and vulnerability to serine deprivation. J. Clin. Invest 129, 4708–4723 (2019).
Maddocks, O. D. K. et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 493, 542–546 (2013).
Ou, Y., Wang, S.-J., Jiang, L., Zheng, B. & Gu, W. p53 protein-mediated regulation of phosphoglycerate dehydrogenase (PHGDH) is crucial for the apoptotic response upon serine starvation. J. Biol. Chem. 290, 457–466 (2015).
Liu, J. et al. Phosphoglycerate dehydrogenase induces glioma cells proliferation and invasion by stabilizing forkhead box M1. J. Neurooncol. 111, 245–255 (2013).
Liao, Y. et al. The cardiomyocyte RNA-binding proteome: links to intermediary metabolism and heart disease. Cell Rep. 16, 1456–1469 (2016).
Castello, A. et al. Comprehensive identification of rna-binding domains in human cells. Mol. Cell 63, 696–710 (2016).
Ma, X., Li, B., Liu, J., Fu, Y. & Luo, Y. Phosphoglycerate dehydrogenase promotes pancreatic cancer development by interacting with eIF4A1 and eIF4E. J. Exp. Clin. Cancer Res. 38, 66 (2019).
Metcalf, S. et al. Selective loss of phosphoserine aminotransferase 1 (PSAT1) suppresses migration, invasion, and experimental metastasis in triple negative breast cancer. Clin. Exp. Metastasis 37, 187–197 (2020).
Liu, B. et al. Overexpression of phosphoserine aminotransferase 1 (PSAT1) predicts poor prognosis and associates with tumor progression in human esophageal squamous cell carcinoma. Cell. Physiol. Biochem. 39, 395–406 (2016).
Yang, Y. et al. PSAT1 regulates cyclin D1 degradation and sustains proliferation of non-small cell lung cancer cells. Int. J. Cancer 136, E39–E50 (2015).
Park, S.-M. et al. Phosphoserine phosphatase promotes lung cancer progression through the dephosphorylation of IRS-1 and a noncanonical l-serine-independent pathway. Mol. Cells 42, 604–616 (2019).
Collet, J.-F., Stroobant, V. & Van Schaftingen, E. Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J. Biol. Chem. 274, 33985–33990 (1999).
Liu, X., Reig, B., Nasrallah, I. M. & Stover, P. J. Human cytoplasmic serine hydroxymethyltransferase is an mRNA binding protein. Biochemistry 39, 11523–11531 (2000).
Castello, A. et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406 (2012).
Guiducci, G. et al. The moonlighting RNA-binding activity of cytosolic serine hydroxymethyltransferase contributes to control compartmentalization of serine metabolism. Nucleic Acids Res. 47, 4240–4254 (2019).
Hentze, M. W. & Preiss, T. The REM phase of gene regulation. Trends Biochem. Sci. 35, 423–426 (2010).
Zheng, H. et al. A BRISC–SHMT complex deubiquitinates IFNAR1 and regulates interferon responses. Cell Rep. 5, 180–193 (2013).
Walden, M. et al. Metabolic control of BRISC–SHMT2 assembly regulates immune signalling. Nature 570, 194–199 (2019).
Cao, J. et al. HDAC11 regulates type I interferon signaling through defatty-acylation of SHMT2. Proc. Natl Acad. Sci. USA 116, 5487–5492 (2019).
Goveia, J. et al. Meta-analysis of clinical metabolic profiling studies in cancer: challenges and opportunities. EMBO Mol. Med. 8, 1134–1142 (2016).
He, F. et al. l-Serine lowers the inflammatory responses during pasteurella multocida infection. Infect. Immun. 87, e00677–19 (2019).
Rodriguez, A. E. et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 29, 1003–1011 (2019).
Su, S. et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell 175, 442–457 (2018).
Wilson, J. L. et al. Inverse data-driven modeling and multiomics analysis reveals phgdh as a metabolic checkpoint of macrophage polarization and proliferation. Cell Rep. 30, 1542–1552(2020).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017).
Ron-Harel, N. et al. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 24, 104–117 (2016).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). This work emphasizes the importance of metabolic competition in the tumor microenvironment.
Vandekeere, S. et al. Serine synthesis via PHGDH is essential for heme production in endothelial cells. Cell Metab. 28, 573–587 (2018).
Graham, K. & Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 13, 6049–6058 (2018).
Samanta, D. et al. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 76, 4430–4442 (2016).
Engel, A. L. et al. Serine-dependent redox homeostasis regulates glioblastoma cell survival. Br. J. Cancer 122, 1391–1398 (2020).
Zhang, X. & Bai, W. Repression of phosphoglycerate dehydrogenase sensitizes triple‑negative breast cancer to doxorubicin. Cancer Chemother. Pharmacol. 78, 655–659 (2016).
Ross, K. C., Andrews, A. J., Marion, C. D., Yen, T. J. & Bhattacharjee, V. Identification of the serine biosynthesis pathway as a critical component of BRAF inhibitor resistance of melanoma, pancreatic, and non-small cell lung cancer cells. Mol. Cancer Ther. 16, 1596–1609 (2017).
Yoshino, H. et al. PHGDH as a key enzyme for serine biosynthesis in HIF2α-targeting therapy for renal cell carcinoma. Cancer Res. 77, 6321–6329 (2017).
Labuschagne, C. F., van den Broek, N. J. F., Mackay, G. M., Vousden, K. H. & Maddocks, O. D. K. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 7, 1248–1258 (2014).
Gravel, S. P. et al. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 74, 7521–7533 (2014).
Humpton, T. J., Hock, A. K., Maddocks, O. D. K. & Vousden, K. H. p53-mediated adaptation to serine starvation is retained by a common tumour-derived mutant. Cancer Metab. 6, 18 (2018).
Wang, Q. et al. Rational design of selective allosteric inhibitors of PHGDH and serine synthesis with anti-tumor activity. Cell Chem. Biol. 24, 55–65 (2017).
Unterlass, J. E. et al. Validating and enabling phosphoglycerate dehydrogenase (PHGDH) as a target for fragment-based drug discovery in PHGDH-amplified breast cancer. Oncotarget 9, 13139–13153 (2018).
Li, A. M. & Ye, J. The PHGDH enigma: do cancer cells only need serine or also a redox modulator? Cancer Lett. 476, 97–105 (2020).
Ravez, S. et al. α-Ketothioamide derivatives: a promising tool to interrogate phosphoglycerate dehydrogenase (PHGDH). J. Med. Chem. 60, 1591–1597 (2017).
Mullarky, E. et al. Inhibition of 3-phosphoglycerate dehydrogenase (PHGDH) by indole amides abrogates de novo serine synthesis in cancer cells. Bioorg. Med. Chem. Lett. 29, 2503–2510 (2019).
Weinstabl, H. et al. Intracellular trapping of the selective phosphoglycerate dehydrogenase (PHGDH) inhibitor BI-4924 disrupts serine biosynthesis. J. Med. Chem. 62, 7976–7997 (2019).
Daidone, F. et al. In silico and in vitro validation of serine hydroxymethyltransferase as a chemotherapeutic target of the antifolate drug pemetrexed. Eur. J. Med. Chem. 46, 1616–1621 (2011).
Paiardini, A. et al. Screening and in vitro testing of antifolate inhibitors of human cytosolic serine hydroxymethyltransferase. ChemMedChem 10, 490–497 (2015).
Ducker, G. S. et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 114, 11404–11409 (2017).
Geeraerts, S. et al. Repurposing the antidepressant sertraline as SHMT inhibitor to suppress serine/glycine synthesis addicted breast tumor growth. Mol. Cancer Ther. 20, 50–63 (2021). This paper describes the clinically used antidepressant sertraline as a dual SHMT1/2 targeting compound.
García-Cañaveras, J. C. et al. SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia. Leukemia https://doi.org/10.1038/s41375-020-0845-6 (2020).
Pikman, Y. et al. Targeting serine hydroxymethyltransferases 1 and 2 for T-cell acute lymphoblastic leukemia therapy. Preprint at bioRxiv https://doi.org/10.1101/2020.02.06.936286 (2020).
Zhang, W. C. et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 148, 259–272 (2012).
Acknowledgements
S.L.G. received a SB PhD fellowship from Fonds Wetenschappelijk Onderzoek (FWO) (1S14517N). E.H. is a FWO PhD fellow fundamental research (1106121N). K.D.K. was supported by a grant from Stichting Tegen Kanker (2016-112) and funding from the KU Leuven Research Council (C1 grant C14/18/104). K.R.K. was funded by the Emmanuel van der Schueren postdoctoral fellowship from Kom op tegen Kanker and a research grant from FWO (FWO KAN2018 1501419N).
Author information
Authors and Affiliations
Contributions
S.L.G., E.H., K.D.K. and K.R.K. wrote the review. S.L.G., E.H. and K.R.K. made the figures and tables. K.D.K. and K.R.K. supervised the entire study. All authors have participated in the manuscript review and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: George Caputa. Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Geeraerts, S.L., Heylen, E., De Keersmaecker, K. et al. The ins and outs of serine and glycine metabolism in cancer. Nat Metab 3, 131–141 (2021). https://doi.org/10.1038/s42255-020-00329-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00329-9
This article is cited by
-
Targeting serine/glycine metabolism improves radiotherapy response in non-small cell lung cancer
British Journal of Cancer (2024)
-
Unraveling the role of the mitochondrial one-carbon pathway in undifferentiated thyroid cancer by multi-omics analyses
Nature Communications (2024)
-
Causal associations between liver traits and Colorectal cancer: a Mendelian randomization study
BMC Medical Genomics (2023)
-
PHGDH arginine methylation by PRMT1 promotes serine synthesis and represents a therapeutic vulnerability in hepatocellular carcinoma
Nature Communications (2023)
-
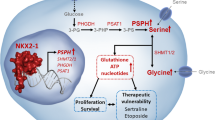
Transcription factor NKX2–1 drives serine and glycine synthesis addiction in cancer
British Journal of Cancer (2023)