Abstract
Leigh syndrome is a fatal neurometabolic disorder caused by defects in mitochondrial function. Mechanistic target of rapamycin (mTOR) inhibition with rapamycin attenuates disease progression in a mouse model of Leigh syndrome (Ndufs4 knock-out (KO) mouse); however, the mechanism of rescue is unknown. Here we identify protein kinase C (PKC) downregulation as a key event mediating the beneficial effects of rapamycin treatment of Ndufs4 KO mice. Assessing the impact of rapamycin on the brain proteome and phosphoproteome of Ndufs4 KO mice, we find that rapamycin restores mitochondrial protein levels, inhibits signalling through both mTOR complexes and reduces the abundance and activity of multiple PKC isoforms. Administration of PKC inhibitors increases survival, delays neurological deficits, prevents hair loss and decreases inflammation in Ndufs4 KO mice. Thus, PKC may be a viable therapeutic target for treating severe mitochondrial disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All mass spectrometry raw files and searches have been deposited in the MassIVE repository with dataset identifier PXD012158. Protein and phosphorylation quantification results are provided as Supplementary Tables 1–6. Source data are provided with this paper.
References
Rich, P. Chemiosmotic coupling: the cost of living. Nature 421, 583 (2003).
Darin, N., Oldfors, A., Moslemi, A. R., Holme, E. & Tulinius, M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA abnormalities. Ann. Neurol. 49, 377–383 (2001).
Quintana, A., Kruse, S. E., Kapur, R. P., Sanz, E. & Palmiter, R. D. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc. Natl Acad. Sci. USA 107, 10996–11001 (2010).
Johnson, S. C. et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342, 1524–1528 (2013).
Ising, C. et al. Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Mol. Med. 7, 275–287 (2015).
Khan, N. A. et al. mTORC1 regulates mitochondrial integrated stress response and mitochondrial myopathy progression. Cell Metab. 26, 419–428.e5 (2017).
Loewith, R. et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 (2002).
Jacinto, E. et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 (2004).
Sarbassov, D. D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Sarbassov, D. D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Guertin, D. A. et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCɑ, but not S6K1. Dev. Cell. 11, 859–871 (2006).
Gould, C. M. & Newton, A. C. The life and death of protein kinase C. Curr. Drug Targets 9, 614–625 (2008).
Wang, Y. et al. Sequential posttranslational modifications regulate PKC degradation. Mol. Biol. Cell 27, 410–420 (2016).
Ikenoue, T. et al. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signaling. EMBO J. 27, 1919–1931 (2008).
Moon, J.-S. et al. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep. 12, 102–115 (2015).
Savaskan, N. E., Bräuer, A. U. & Nitsch, R. Molecular cloning and expression regulation of PRG-3, a new member of the plasticity-related gene family. Eur. J. Neurosci. 19, 212–220 (2004).
Shibuya, N. et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 11, 703–714 (2009).
Wesseling, H., Elgersma, Y. & Bahn, S. A brain proteomic investigation of rapamycin effects in the Tsc1+/− mouse model. Mol. Autism 8, 41 (2017).
Peretti, D. et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature 518, 236–239 (2015).
Zhu, X., Bührer, C. & Wellmann, S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell. Mol. Life Sci. 73, 3839–3859 (2016).
Wu, R. et al. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat. Methods 8, 677–683 (2011).
Hebert-Chatelain, E. et al. A cannabinoid link between mitochondria and memory. Nature 539, 555–559 (2016).
Niemi, N. M. et al. Pptc7 is an essential phosphatase for promoting mammalian mitochondrial metabolism and biogenesis. Nat. Commun. 10, 3197 (2019).
Hijazi, M. et al. Reconstructing kinase network topologies from phosphoproteomics data reveals cancer-associated rewiring. Nat. Biotechnol. 38, 493–502 (2020).
Ochoa, D. et al. An atlas of human kinase regulation. Mol. Syst. Biol. 12, 888 (2016).
Zhou, X., Yang, W. & Li, J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-κB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-ɑ production in lipopolysaccharide-stimulated rat peritoneal macrophages. J. Biol. Chem. 281, 31337–31347 (2006).
Lieu, Q. et al. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca2+-dependent PKC/p38MAPK/NF-κB pathway. J. Neuroinflammation 16, 10 (2019).
Leitges, M. et al. Immunodeficiency in protein kinase Cβ-deficient mice. Science 273, 788–791 (1996).
Jope, R. S., Yuskaitis, C. J. & Beurel, E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 32, 577–595 (2007).
Mochly-Rose, D., Das, K. & Grimes, K. V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 11, 937–957 (2012).
Aiello, L. P. et al. Oral protein kinase C β inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the protein kinase C β inhibitor-diabetic retinopathy study and the protein kinase C β inhibitor-diabetic retinopathy study 2. Retina 31, 2084–2094 (2011).
Jin, Z., Wei, W., Yang, M., Du, Y. & Wan, Y. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 20, 483–498 (2014).
Lamming, D. W. et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell 13, 911–917 (2014).
Fang, Y. et al. Effects of rapamycin on growth hormone receptor knockout mice. Proc. Natl Acad. Sci. USA 115, E1495–E1503 (2018).
Sage-Schwaede, A. et al. Exploring mTOR inhibition as treatment for mitochondrial disease. Ann. Clin. Transl. Neurol. 6, 1877–1881 (2019).
Johnson, S. C. et al. mTOR inhibitors may benefit kidney transplant recipients with mitochondrial diseases. Kidney Int. 95, 455–466 (2019).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Cox, J. & Mann, M. 1D and 2D annotation enrichment: a statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinform. 13, S12 (2012).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, D1251–D1257 (2016).
Eden, E. et al. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 10, 48 (2009).
Hornbeck, P. V. et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520 (2015).
Schwartz, D. & Gygi, S. P. An iterative statistical approach to the identification of protein phosphorylation motifs from large-scale data sets. Nat. Biotechnol. 23, 1391–1398 (2005).
Acknowledgements
We thank V. V. Pineda, N. J. LeTexier, J. Phillips, J. Tan, Y. Lee, T. Nguyen, S. Khessib, N. Lim, C. Lu, S. Mekvanich, C. Bodart, V. T. Ha, S. A. Huff, D. Kim, S. Narayan and A. O. Zimmermann for assisting with animal experiments. We thank J. Snyder and the Histology Imaging Core at the University of Washington for assistance with histology and helpful discussions. We thank J. An for assistance with anaesthesia. We thank S.C. Johnson at the Seattle Children’s Research Institute for helpful discussions during revisions. This work was supported by NIH grants no. R01 NS098329 and no. P30 AG013280 (to M.K. and J.V.), and grant no. R35 GM119536 (to J.V.). T.K.I. was supported by a JSPS Postdoctoral Fellowship and a Uehara Memorial Foundation Postdoctoral Research Fellowship. A.S.G. was supported by NIH Ruth L. Kirschstein NRSA Fellowship grant no. F32 NS110109. S.W.E. was supported by NIH no. T32 HG00035 Interdisciplinary training grant in Genome Sciences and a Samuel and Althea Stroum Endowed Graduate Fellowship. A.S.V. was supported by NIH no. T32 LM012419 Big Data in Genomics and Neurosciences training grant.
Author information
Authors and Affiliations
Contributions
M.M.-P. designed, conducted, analysed and interpreted all the proteomic experiments; prepared associated figures; wrote the initial draft; and revised and edited the manuscript. A.S.G. designed, conducted and interpreted phenotypic and lifespan experiments with the PKC-β inhibitor; conducted and interpreted all the western blot, cytokine and histological data; prepared associated figures; and wrote, revised and edited the manuscript. T.K.I. conceptualized the study; designed, conducted and interpreted phenotypic and lifespan experiments with rapamycin and broad-spectrum PKC inhibitors; and wrote, revised and edited the manuscript. A.S.V. conducted KSEA analysis and revised and edited the manuscript. J.H. conceptualized the study and obtained the brain tissue samples for proteomic analysis. S.W.E. assisted with the proteomic analysis and revised and edited the manuscript. H.Z.H. and D.K. assisted with mouse experiments. M.Y. assisted with statistical analysis and revised and edited the manuscript. M.K. conceived and coordinated the project; supervised the mouse work; provided animal resources and funding for T.K.I., A.S.G., J.H., H.Z.H. and D.K.; and revised and edited the manuscript. J.V. coordinated the project; supervised the proteomics work; provided instrumentation resources and funding for M.M.-P., A.S.V. and S.W.E.; and revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary handling editors: Elena Bellafante; Pooja Jha.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Proteome and phosphoproteome analysis statistics.
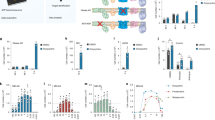
a, Number of total (all), mitochondrial (mitoc) and Complex I (C-I) proteins quantified (gray) and those with significant changes among WT, KO, and KR experimental groups (ANOVA test, FDR q-value < 0.05) (red). Mitochondrial proteins annotations were extracted from mouse MitoCarta 2.0 database. b, Correlations of log2 transformed LFQ-normalized protein abundance measurements between samples (N = 6-7 mice). c, Same as in (a) but for phosphorylation sites. d, Correlations of log2 transformed median-normalized phosphorylation site intensity values between samples and replicates. Two technical replicates of IMAC phosphopeptide enrichment were performed for each brain sample to increase phosphoproteome coverage (N = 12–14 samples; 6-7 mice and duplicated IMAC enrichment and LC-MS/MS analysis). e, Distribution of Pearson’s r correlation values among all samples and only for technical replicates. Box plots include the median line, the box denotes the interquartile range (IQR), whiskers denote ±1.5 × IQR. f, PCA analysis of log2 transformed median-normalized phosphorylation site intensities data (hollow/solid symbols indicates female/male samples respectively).
Extended Data Fig. 2 Global changes in respiratory chain related proteins.
a,b, Aggregated protein abundance changes in respiratory chain complexes (a) and respiratory chain assembly proteins (b). Box plots include the median line, the box denotes the interquartile range (IQR), whiskers denote ±1.5 × IQR. Sum of relative iBAQ intensities for all members of each complex or protein group were used (N = 6-7 mice). T-test significance p-values are indicated (* p < 0.05; ** p < 0.01; *** p < 0.001).
Extended Data Fig. 3 Western blot analysis of brain extracts from P30 and P50 mice treated with vehicle or rapamycin from P10 to P30 or P50.
a,b, Western blot analysis of mTORC1 and mTORC2 markers of brain lysates from P30 wild-type (WT) and Ndufs4 KO mice treated daily with vehicle (KO) or rapamycin (KR) from P10 to P30. c, Densitometry (relative to actin) of western blot data from (a) and (b) normalized to wild-type levels (N = 6 mice). d,e, Western blot analysis of PKC isoforms of brain lysates from P30 wild-type (WT) and Ndufs4 KO mice treated daily with vehicle (KO) or rapamycin (KR) from P10 to P30. f, Densitometry (relative to actin) of western blot data from (d) and (e) normalized to wild-type levels (N = 6 mice). g,h, Representative WB images and densitometry (relative to actin) normalized to wild-type (WT) levels showing relative phosphorylated and total levels of proteins involved in mTORC1 and mTORC2 (N = 4 mice). i,j, Representative WB images and densitometry (relative to actin) normalized to WT levels showing relative phosphorylated and total levels of PKC proteins (N = 4 mice). Each lane corresponds to a brain lysate from a single mouse. T-test significance p-values are indicated (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
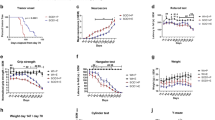
Extended Data Fig. 4 Rapamycin exerts similar effects in the brains of wild-type mice to Ndufs4 KO mice.
a, Experimental design to evaluate rapamycin-mediated effects in the brain of wild-type mice. b, Body weight gain in mice from the two experimental groups (mean ± s.d.; N = 4–6 mice). c, Total brain weight at the end of the experimental trial (N = 4–6 mice). T-test significance p-values are indicated (* p < 0.05). Box plots include the median line, the box denotes the interquartile range (IQR), whiskers denote ±1.5 × IQR. d, Comparison of rapamycin mediated changes between wild-type and knock-out mice in individual protein levels. Pearson’s correlation values are indicated. e, 2D-enrichment analysis of GO and KEGG terms comparing the effect of rapamycin between wild-type and knock-out mice in the proteome (Wilcoxon-Mann-Whitney test, FDR q-value < 0.05). f, Comparison of rapamycin mediated changes between wild-type and knock-out mice in phosphorylation sites. Pearson’s correlation values are indicated. g, 2D-enrichment analysis of GO and KEGG terms comparing the effect of rapamycin between wild-type and knock-out mice in the phosphoproteome (Wilcoxon-Mann-Whitney test, p-value < 0.01). In 2D enrichment analysis (panels (e) and (f)) most data points are close to the diagonal dashed line (that is identity function), indicating no differences in the effect of rapamycin on wild-type and Ndufs4 KO mice.
Extended Data Fig. 5 Correlation between rapamycin effects in Ndufs4 KO mice at the proteomic (x axis) and phosphoproteomic (y axis) levels.
Pearson’s r coefficient and goodness-of-fit test p-value of linear curve fitted line (dashed line) are indicated.
Extended Data Fig. 6 Phosphorylation changes in brain proteins of Ndufs4 KO mice upon rapamycin treatment.
a, Phosphorylation sites on proteins of the mTOR complexes and associated substrates that show significant changes among experimental groups (WT: wild-type; KO: Ndufs4 KO; KR: rapamycin-treated Ndufs4 KO). b, Average (mean ± s.e.m.) changes in phosphorylation of kinase substrates upon rapamycin treatment in Ndufs4 KO mice brain. Each dot represents an individual phosphorylation site substrate. Only kinases with more than 9 substrates found are shown. c, Significant changes in phosphorylation on activity regulatory sites of specific kinases (*activation loop sites, #inhibitory sites). d, Significant changes in activating phosphorylation sites of the main two calcium-release channels from the endoplasmic reticulum. All box plots include the median line, the box denotes the interquartile range (IQR), whiskers denote ±1.5 × IQR. T-test significance p-values are indicated (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; N = 12–14 samples; 6–7 mice and duplicated IMAC enrichment and LC-MS/MS analysis, if no missing values are found).
Extended Data Fig. 7 Treatment of Ndufs4 KO mice with PKC inhibitors largely prevents the alopecia phenotype at weaning (~P21).
a, Wild-type mice at weaning show no hair loss. b, Untreated Ndufs4 KO mice normally exhibit alopecia (that is hair loss) at 21-days old due to a TLR2/4 innate immune response. In contrast, minimal hair loss was observed in 21-day old Ndufs4 KO mice treated with c,d, GF109203X and ruboxistaurin from P10 to P21. e, Some hair loss was observed in 21-day old Ndufs4 KO mice treated with rapamycin from P10 to P21.
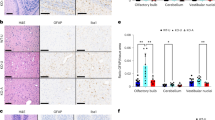
Extended Data Fig. 8 Histological analysis of skin pathology at P21 or P30.
a, Representative images (20X zoom) of haematoxylin and eosin staining of skin sections of P21 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P21. Each picture corresponds to an image from an individual mouse. b, Blinded skin inflammation pathology scores from haematoxylin and eosin staining of skin sections of P21 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P21. Increasing scores represent increasing severity of pathology. c, Blinded hair follicle pathology scores from haematoxylin and eosin staining of skin sections of P21 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P21. d, Representative images (20X zoom) of haematoxylin and eosin staining of skin sections of P30 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P30. Each picture corresponds to an image from an individual mouse. e, Blinded skin inflammation pathology scores from haematoxylin and eosin staining of skin sections of P30 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P30. Increasing scores represent increasing severity of pathology. f, Blinded hair follicle pathology scores from haematoxylin and eosin staining of skin sections of P30 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P30. Increasing scores represent increasing severity of pathology. Each point represents the score for an individual mouse. T-test significance p-values are indicated (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; N = 6 mice). g, Cytokine levels in mouse skin (N = 3–6 mice) of P30 WT and Ndufs4 KO mice treated with vehicle, rapamycin, or ruboxistaurin from P10 to P30. Cytokine levels were measured using a cytokine array and z-score normalized. Left, violin plot of combined z-score normalized values of median abundance of individual cytokines (each point represents an individual cytokine and the dashed line indicates median z-score value for each group; N = 14). Zero mean score is indicated by a dotted line. Right, individualized information in a heatmap. T-test significance p-values are indicated for the different treatments compared to their respective wild-type or knock-out mice values (^ p < 0.1; * p < 0.05; ** p < 0.01; N = 3–6 mice).
Extended Data Fig. 9 Untreated and vehicle-treated Ndufs4 KO mice exhibit similar symptoms of disease.
a, Vehicle treatment does not alter the lifespan of Ndufs4 KO mice compared to untreated controls. Untreated vs. vehicle p-value = 0.7898, log-rank. b, Vehicle treatment does not alter the onset of clasping of Ndufs4 KO mice compared to untreated controls. c, Vehicle treatment does not alter weight gain of Ndufs4 KO mice compared to untreated controls (mean ± s.d). N = 8 mice for vehicle and N = 11 mice for untreated controls in all plots.
Supplementary information
Supplementary Table 1
Proteins quantified (log2 LFQ intensity) by mass spectrometry in wild-type (WT), Ndufs4 knock-out (KO) and rapamycin-treated Ndufs4 knock-out (KR) mouse brains.
Supplementary Table 2
List of proteins belonging to clusters 1–4 from Fig. 2a.
Supplementary Table 3
Proteins quantified (log2 LFQ intensity) by mass spectrometry in wild-type (WT) and rapamycin-treated wild-type (WR) mouse brains.
Supplementary Table 4
Phosphosites quantified by mass spectrometry in wild-type (WT), Ndufs4 knock-out (KO) and rapamycin-treated Ndufs4 knock-out (KR) mouse brains.
Supplementary Table 5
Phosphosites quantified by mass spectrometry in wild-type (WT) and rapamycin-treated wild-type (WR) mouse brains.
Supplementary Table 6
Mitochondrial proteins (according to mouse MitoCarta 2.0 database) with significantly altered phosphorylation sites.
Source data
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Unprocessed western blots and/or gels.
Rights and permissions
About this article
Cite this article
Martin-Perez, M., Grillo, A.S., Ito, T.K. et al. PKC downregulation upon rapamycin treatment attenuates mitochondrial disease. Nat Metab 2, 1472–1481 (2020). https://doi.org/10.1038/s42255-020-00319-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-020-00319-x
This article is cited by
-
Targeting the biology of aging with mTOR inhibitors
Nature Aging (2023)
-
Cardiac Involvement in Mitochondrial Disorders
Current Heart Failure Reports (2023)
-
Acarbose suppresses symptoms of mitochondrial disease in a mouse model of Leigh syndrome
Nature Metabolism (2023)
-
The immune system as a driver of mitochondrial disease pathogenesis: a review of evidence
Orphanet Journal of Rare Diseases (2022)
-
Elamipretide effects on the skeletal muscle phosphoproteome in aged female mice
GeroScience (2022)