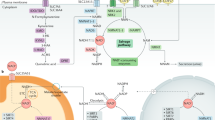
Abstract
The conceptual evolution of nicotinamide adenine dinucleotide (NAD+) from being seen as a simple metabolic cofactor to a pivotal cosubstrate for proteins regulating metabolism and longevity, including the sirtuin family of protein deacylases, has led to a new wave of scientific interest in NAD+. NAD+ levels decline during ageing, and alterations in NAD+ homeostasis can be found in virtually all age-related diseases, including neurodegeneration, diabetes and cancer. In preclinical settings, various strategies to increase NAD+ levels have shown beneficial effects, thus starting a competitive race to discover marketable NAD+ boosters to improve healthspan and lifespan. Here, we review the basics of NAD+ biochemistry and metabolism, and its roles in health and disease, and we discuss current challenges and the future translational potential of NAD+ research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Harden, A. & Young, W. The alcoholic ferment of yeast‐juice. Proc. R. Soc. Lond. B 77, 405–420 (1906).
von Euler, H. & Myrbäck, K. Co‐zymase. XVII. Hoppe-Seyler’s Z. Physiol. Chem. 190, 93–100 (1930).
Warburg, O. & Christian, W. Pyridine, the hydrogen transfusing component of fermentative enzymes. Helv. Chim. Acta 19, 79–88 (1936).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Haigis, M. C. & Sinclair, D. A. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 (2010).
Houtkooper, R. H., Pirinen, E. & Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012).
Kim, W. et al. Polyunsaturated fatty acid desaturation is a mechanism for glycolytic NAD+ recycling. Cell Metab. 29, 856–870.e7 (2019).
Lerner, F., Niere, M., Ludwig, A. & Ziegler, M. Structural and functional characterization of human NAD kinase. Biochem. Biophys. Res. Commun. 288, 69–74 (2001).
Arthur, K. Enzymatic synthesis of triphosphopyridine nucleotide. J. Biol. Chem. 182, 805–813 (1950).
Liu, L. et al. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab. 27, 1067–1080.e5 (2018).
Hanukoglu, I. & Rapoport, R. Routes and regulation of NADPH production in steroidogenic mitochondria. Endocr. Res. 21, 231–241 (1995).
Grabowska, D. & Chelstowska, A. The ALD6 gene product is indispensable for providing NADPH in yeast cells lacking glucose-6-phosphate dehydrogenase activity. J. Biol. Chem. 278, 13984–13988 (2003).
Ogawa, K., Suzuki, K., Okutsu, M., Yamazaki, K. & Shinkai, S. The association of elevated reactive oxygen species levels from neutrophils with low-grade inflammation in the elderly. Immun. Ageing 5, 13 (2008).
Clapper, D. L., Walseth, T. F., Dargie, P. J. & Lee, H. C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 262, 9561–9568 (1987).
Huang, T.-T. et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum. Mol. Genet. 15, 1187–1194 (2006).
Freeman, H. C., Hugill, A., Dear, N. T., Ashcroft, F. M. & Cox, R. D. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55, 2153–2156 (2006).
Ronchi, J. A. et al. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 63, 446–456 (2013).
Pollak, N., Dölle, C. & Ziegler, M. The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem. J. 402, 205–218 (2007).
Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 10, 179–206 (2008).
Klar, A. J. & Fogel, S. Activation of mating type genes by transposition in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 76, 4539–4543 (1979).
Rine, J., Strathern, J. N., Hicks, J. B. & Herskowitz, I. A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics 93, 877–901 (1979).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Landry, J. et al. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc. Natl Acad. Sci. USA 97, 5807–5811 (2000).
Frye, R. A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 273, 793–798 (2000).
Houtkooper, R. H., Cantó, C., Wanders, R. J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010).
Ghanta, S., Grossmann, R. E. & Brenner, C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: chemical and metabolic logic of acetyl-lysine modifications. Crit. Rev. Biochem. Mol. Biol. 48, 561–574 (2013).
Bai, P. & Cantó, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 16, 290–295 (2012).
Mouchiroud, L. et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013).
Braidy, N. et al. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One 6, e19194 (2011).
Berger, N. A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat. Res. 101, 4–15 (1985).
Feijs, K. L., Forst, A. H., Verheugd, P. & Lüscher, B. Macrodomain-containing proteins: regulating new intracellular functions of mono(ADP-ribosyl)ation. Nat. Rev. Mol. Cell Biol. 14, 443–451 (2013).
Guse, A. H. Regulation of calcium signaling by the second messenger cyclic adenosine diphosphoribose (cADPR). Curr. Mol. Med. 4, 239–248 (2004).
Quarona, V. et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytom. B Clin. Cytom. 84, 207–217 (2013).
Hussain, A. M., Lee, H. C. & Chang, C. F. Functional expression of secreted mouse BST-1 in yeast. Protein Expr. Purif. 12, 133–137 (1998).
Bhan, A. K., Reinherz, E. L., Poppema, S., McCluskey, R. T. & Schlossman, S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J. Exp. Med. 152, 771–782 (1980).
Aksoy, P., White, T. A., Thompson, M. & Chini, E. N. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 345, 1386–1392 (2006).
Zhao, Y. J., Lam, C. M. C. & Lee, H. C. The membrane-bound enzyme CD38 exists in two opposing orientations. Sci. Signal. 5, ra67 (2012).
Sauve, A. A., Munshi, C., Lee, H. C. & Schramm, V. L. The reaction mechanism for CD38: a single intermediate is responsible for cyclization, hydrolysis, and base-exchange chemistries. Biochemistry 37, 13239–13249 (1998).
Cakir-Kiefer, C., Muller-Steffner, H., Oppenheimer, N. & Schuber, F. Kinetic competence of the cADP-ribose-CD38 complex as an intermediate in the CD38/NAD+ glycohydrolase-catalysed reactions: implication for CD38 signalling. Biochem. J. 358, 399–406 (2001).
Summers, D. W., Gibson, D. A., DiAntonio, A. & Milbrandt, J. SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation. Proc. Natl Acad. Sci. USA 113, E6271–E6280 (2016).
Essuman, K. et al. The SARM1 Toll/interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343.e5 (2017).
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 (2015).
Kowtoniuk, W. E., Shen, Y., Heemstra, J. M., Agarwal, I. & Liu, D. R. A chemical screen for biological small molecule-RNA conjugates reveals CoA-linked RNA. Proc. Natl Acad. Sci. USA 106, 7768–7773 (2009).
Chen, Y. G., Kowtoniuk, W. E., Agarwal, I., Shen, Y. & Liu, D. R. LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nat. Chem. Biol. 5, 879–881 (2009).
Cahová, H., Winz, M. L., Höfer, K., Nübel, G. & Jäschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 519, 374–377 (2015).
Bird, J. G. et al. The mechanism of RNA 5′ capping with NAD+, NADH and desphospho-CoA. Nature 535, 444–447 (2016).
Walters, R. W. et al. Identification of NAD+ capped mRNAs in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 114, 480–485 (2017).
Jiao, X. et al. 5′ end nicotinamide adenine dinucleotide cap in human cells promotes RNA decay through DXO-mediated deNADding. Cell 168, 1015–1027.e10 (2017).
Malygin, A. G. & Shemyakin, M. F. Adenosine, NAD and FAD can initiate template-dependent RNA synthesis catalyzed by Escherichia coli RNA polymerase. FEBS Lett. 102, 51–54 (1979).
Julius, C. & Yuzenkova, Y. Bacterial RNA polymerase caps RNA with various cofactors and cell wall precursors. Nucleic Acids Res. 45, 8282–8290 (2017).
Julius, C., Riaz-Bradley, A. & Yuzenkova, Y. RNA capping by mitochondrial and multi-subunit RNA polymerases. Transcription 9, 292–297 (2018).
Bird, J. G. et al. Highly efficient 5′ capping of mitochondrial RNA with NAD+ and NADH by yeast and human mitochondrial RNA polymerase. eLife 7, e42179 (2018).
Bieganowski, P. & Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502 (2004).
Cantó, C., Menzies, K. J. & Auwerx, J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 (2015).
Rongvaux, A., Andris, F., Van Gool, F. & Leo, O. Reconstructing eukaryotic NAD metabolism. BioEssays 25, 683–690 (2003).
Belenky, P., Bogan, K. L. & Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 (2007).
Preiss, J. & Handler, P. Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J. Biol. Chem. 233, 488–492 (1958).
Katsyuba, E. et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Palzer, L. et al. Alpha-amino-beta-carboxy-muconate-semialdehyde decarboxylase controls dietary niacin requirements for NAD+ synthesis. Cell Rep. 25, 1359–1370.e4 (2018).
Menzies, K. J., Zhang, H., Katsyuba, E. & Auwerx, J. Protein acetylation in metabolism—metabolites and cofactors. Nat. Rev. Endocrinol. 12, 43–60 (2016).
Cambronne, X. A. et al. Biosensor reveals multiple sources for mitochondrial NAD+. Science 352, 1474–1477 (2016).
Di Lisa, F., Menabò, R., Canton, M., Barile, M. & Bernardi, P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J. Biol. Chem. 276, 2571–2575 (2001).
Yang, H. et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 (2007).
Pittelli, M. et al. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 285, 34106–34114 (2010).
Barile, M., Passarella, S., Danese, G. & Quagliariello, E. Rat liver mitochondria can synthesize nicotinamide adenine dinucleotide from nicotinamide mononucleotide and ATP via a putative matrix nicotinamide mononucleotide adenylyltransferase. Biochem. Mol. Biol. Int. 38, 297–306 (1996).
Nakagawa, I., Takahashi, T., Suzuki, T. & Masana, Y. Effect in man of the addition of tryptophan oniacin to the diet on the excretion of their metabolites. J. Nutr. 99, 325–330 (1969).
van Roermund, C. W., Elgersma, Y., Singh, N., Wanders, R. J. & Tabak, H. F. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 14, 3480–3486 (1995).
Di Lisa, F. & Ziegler, M. Pathophysiological relevance of mitochondria in NAD+ metabolism. FEBS Lett. 492, 4–8 (2001).
Brown, K. et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 3, 319–327 (2013).
Cantó, C. et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
Nikiforov, A., Dölle, C., Niere, M. & Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 286, 21767–21778 (2011).
Davila, A. et al. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. eLife 7, e33246 (2018).
Pittelli, M. et al. Pharmacological effects of exogenous NAD on mitochondrial bioenergetics, DNA repair, and apoptosis. Mol. Pharmacol. 80, 1136–1146 (2011).
Todisco, S., Agrimi, G., Castegna, A. & Palmieri, F. Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J. Biol. Chem. 281, 1524–1531 (2006).
Agrimi, G. et al. Deletion or overexpression of mitochondrial NAD+ carriers in Saccharomyces cerevisiae alters cellular NAD and ATP contents and affects mitochondrial metabolism and the rate of glycolysis. Appl. Environ. Microbiol. 77, 2239–2246 (2011).
VanLinden, M. R. et al. Subcellular distribution of NAD+ between cytosol and mitochondria determines the metabolic profile of human cells. J. Biol. Chem. 290, 27644–27659 (2015).
Hara, N. et al. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J. Biol. Chem. 282, 24574–24582 (2007).
Hara, N. et al. Molecular identification of human glutamine- and ammonia-dependent NAD synthetases: carbon-nitrogen hydrolase domain confers glutamine dependency. J. Biol. Chem. 278, 10914–10921 (2003).
Emanuelli, M. et al. Molecular cloning, chromosomal localization, tissue mRNA levels, bacterial expression, and enzymatic properties of human NMN adenylyltransferase. J. Biol. Chem. 276, 406–412 (2001).
Yalowitz, J. A. et al. Characterization of human brain nicotinamide 5′-mononucleotide adenylyltransferase-2 and expression in human pancreas. Biochem. J. 377, 317–326 (2004).
Berger, F., Lau, C., Dahlmann, M. & Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 280, 36334–36341 (2005).
Zhang, X. et al. Structural characterization of a human cytosolic NMN/NaMN adenylyltransferase and implication in human NAD biosynthesis. J. Biol. Chem. 278, 13503–13511 (2003).
Ryu, K. W. et al. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science 360, eaan5780 (2018).
Kitani, T., Okuno, S. & Fujisawa, H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 544, 74–78 (2003).
Rongvaux, A. et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 32, 3225–3234 (2002).
Felici, R., Lapucci, A., Ramazzotti, M. & Chiarugi, A. Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria. PLoS One 8, e76938 (2013).
Hikosaka, K. et al. Deficiency of nicotinamide mononucleotide adenylyltransferase 3 (nmnat3) causes hemolytic anemia by altering the glycolytic flow in mature erythrocytes. J. Biol. Chem. 289, 14796–14811 (2014).
Yamamoto, M. et al. Nmnat3 is dispensable in mitochondrial NAD level maintenance in vivo. PLoS One 11, e0147037 (2016).
Nakagawa, T., Lomb, D. J., Haigis, M. C. & Guarente, L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 (2009).
Yang, T., Chan, N. Y. & Sauve, A. A. Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. J. Med. Chem. 50, 6458–6461 (2007).
Tischler, M. E., Friedrichs, D., Coll, K. & Williamson, J. R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch. Biochem. Biophys. 184, 222–236 (1977).
Bender, D. A., Magboul, B. I. & Wynick, D. Probable mechanisms of regulation of the utilization of dietary tryptophan, nicotinamide and nicotinic acid as precursors of nicotinamide nucleotides in the rat. Br. J. Nutr. 48, 119–127 (1982).
Lin, L. F. & Henderson, L. M. Pyridinium precursors of pyridine nucleotides in perfused rat kidney and in the testis. J. Biol. Chem. 247, 8023–8030 (1972).
Jackson, T. M., Rawling, J. M., Roebuck, B. D. & Kirkland, J. B. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 125, 1455–1461 (1995).
Hagino, Y., Lan, S. J., Ng, C. Y. & Henderson, L. M. Metabolism of pyridinium precursors of pyridine nucleotides in perfused rat liver. J. Biol. Chem. 243, 4980–4986 (1968).
Ijichi, H., Ichiyama, A. & Hayaishi, O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. 3. Comparative in vivo studies on nicotinic acid, nicotinamide, and quinolinic acid as precursors of nicotinamide adenine dinucleotide. J. Biol. Chem. 241, 3701–3707 (1966).
Williams, G. T., Lau, K. M., Coote, J. M. & Johnstone, A. P. NAD metabolism and mitogen stimulation of human lymphocytes. Exp. Cell Res. 160, 419–426 (1985).
Mori, V. et al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PLoS One 9, e113939 (2014).
Collins, P. B. & Chaykin, S. Comparative metabolism of nicotinamide and nicotinic acid in mice. Biochem. J. 125, 117P (1971).
Yang, S. J. et al. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J. Nutr. Biochem. 25, 66–72 (2014).
Jacobson, E. L., Dame, A. J., Pyrek, J. S. & Jacobson, M. K. Evaluating the role of niacin in human carcinogenesis. Biochimie 77, 394–398 (1995).
Revollo, J. R. et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 6, 363–375 (2007).
Shibata, K., Hayakawa, T. & Iwai, K. Tissue distribution of the enzymes concerned with the biosynthesis of NAD in rats. Agr. Biol. Chem. 50, 3037–3041 (1986).
Bender, D. A. & Olufunwa, R. Utilization of tryptophan, nicotinamide and nicotinic acid as precursors for nicotinamide nucleotide synthesis in isolated rat liver cells. Br. J. Nutr. 59, 279–287 (1988).
Trammell, S. A. et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 7, 12948 (2016).
Yoshino, J., Mills, K. F., Yoon, M. J. & Imai, S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Ratajczak, J. et al. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat. Commun. 7, 13103 (2016).
Fletcher, R. S. et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 6, 819–832 (2017).
Li, J., Mayne, R. & Wu, C. A novel muscle-specific beta 1 integrin binding protein (MIBP) that modulates myogenic differentiation. J. Cell Biol. 147, 1391–1398 (1999).
Sasaki, Y., Araki, T. & Milbrandt, J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J. Neurosci. 26, 8484–8491 (2006).
Grozio, A. et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 1, 47–57 (2019).
Schmidt, M. S. & Brenner, C. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat. Metab. 1, 660–661 (2019).
Diguet, N. et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256–2273 (2018).
Frederick, D. W. et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24, 269–282 (2016).
Spector, R. & Johanson, C. E. Vitamin transport and homeostasis in mammalian brain: focus on vitamins B and E. J. Neurochem. 103, 425–438 (2007).
Cantó, C. et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219 (2010).
Chen, D. et al. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 22, 1753–1757 (2008).
Qin, W. et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 281, 21745–21754 (2006).
Sato, S. et al. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell 170, 664–677.e11 (2017).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Fulco, M. et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 14, 661–673 (2008).
Cantó, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Costford, S. R. et al. Skeletal muscle NAMPT is induced by exercise in humans. Am. J. Physiol. Endocrinol. Metab. 298, E117–E126 (2010).
Koltai, E. et al. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 131, 21–28 (2010).
Gariani, K. et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 63, 1190–1204 (2016).
Gariani, K. et al. Inhibiting poly ADP-ribosylation increases fatty acid oxidation and protects against fatty liver disease. J. Hepatol. 66, 132–141 (2017).
Trammell, S. A. et al. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 6, 26933 (2016).
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M. & Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009).
Ramsey, K. M. et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009).
Mauvoisin, D. et al. Circadian and feeding rhythms orchestrate the diurnal liver acetylome. Cell Rep. 20, 1729–1743 (2017).
Peek, C. B. et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 1243417 (2013).
Asher, G. et al. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142, 943–953 (2010).
Asher, G. et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 (2008).
Chang, H. C. & Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460 (2013).
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 (2008).
Bellet, M. M. et al. Histone deacetylase SIRT1 controls proliferation, circadian rhythm, and lipid metabolism during liver regeneration in mice. J. Biol. Chem. 291, 23318–23329 (2016).
Masri, S. et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158, 659–672 (2014).
Masri, S. & Sassone-Corsi, P. Sirtuins and the circadian clock: bridging chromatin and metabolism. Sci. Signal. 7, re6 (2014).
Rutter, J., Reick, M., Wu, L. C. & McKnight, S. L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293, 510–514 (2001).
Sahar, S., Nin, V., Barbosa, M. T., Chini, E. N. & Sassone-Corsi, P. Altered behavioral and metabolic circadian rhythms in mice with disrupted NAD+ oscillation. Aging (Albany N. Y.) 3, 794–802 (2011).
Yoshino, J., Baur, J. A. & Imai, S. I. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 27, 513–528 (2018).
Imai, S. & Guarente, L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2, 16017 (2016).
Yang, Y., Mohammed, F. S., Zhang, N. & Sauve, A. A. Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J. Biol. Chem. 294, 9295–9307 (2019).
Hsu, C. P., Oka, S., Shao, D., Hariharan, N. & Sadoshima, J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ. Res. 105, 481–491 (2009).
Yoshida, M. et al. Extracellular vesicle-contained enampt delays aging and extends lifespan in mice. Cell Metab. 30, 329–342.e5 (2019).
Wang, G. et al. P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158, 1324–1334 (2014).
Gardell, S. J. et al. Boosting NAD+ with a small molecule that activates NAMPT. Nat. Commun. 10, 3241 (2019).
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004).
Williams, P. A. et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760 (2017).
Gaikwad, A., Long, D. J. II, Stringer, J. L. & Jaiswal, A. K. In vivo role of NAD(P)H:quinone oxidoreductase 1 (NQO1) in the regulation of intracellular redox state and accumulation of abdominal adipose tissue. J. Biol. Chem. 276, 22559–22564 (2001).
Khadka, D. et al. Augmentation of NAD+ levels by enzymatic action of NAD(P)H quinone oxidoreductase 1 attenuates adriamycin-induced cardiac dysfunction in mice. J. Mol. Cell. Cardiol. 124, 45–57 (2018).
Kim, H.-J. et al. Augmentation of NAD+ by NQO1 attenuates cisplatin-mediated hearing impairment. Cell Death Dis. 5, e1292 (2014).
Nazari Soltan Ahmad, S. et al. Dunnione protects against experimental cisplatin-induced nephrotoxicity by modulating NQO1 and NAD+ levels. Free Radic. Res. 52, 808–817 (2018).
Oh, G.-S. et al. Pharmacological activation of NQO1 increases NAD+ levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int. 85, 547–560 (2014).
Pandit, A. et al. Dunnione ameliorates cisplatin-induced small intestinal damage by modulating NAD+ metabolism. Biochem. Biophys. Res. Commun. 467, 697–703 (2015).
Shen, A. et al. NAD+ augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Sci. Rep. 7, 3006 (2017).
Oh, G.-S. et al. Increased cellular NAD+ level through NQO1 enzymatic action has protective effects on bleomycin-induced lung fibrosis in mice. Tuberc. Respir. Dis. (Seoul.) 79, 257–266 (2016).
Diaz-Ruiz, A. et al. Overexpression of CYB5R3 and NQO1, two NAD+-producing enzymes, mimics aspects of caloric restriction. Aging Cell 17, e12767 (2018).
Pellicciari, R. et al. α-Amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD) inhibitors as novel modulators of de novo nicotinamide adenine dinucleotide (NAD+) biosynthesis. J. Med. Chem. 61, 745–759 (2018).
Kraus, D. et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508, 258–262 (2014).
Neelakantan, H. et al. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochem. Pharmacol. 147, 141–152 (2018).
Neelakantan, H. et al. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem. Pharmacol. 163, 481–492 (2019).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
Pirinen, E. et al. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 19, 1034–1041 (2014).
Waldman, M. et al. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1α axis. Exp. Cell Res. 373, 112–118 (2018).
Wang, H. et al. PARP-1 inhibition attenuates cardiac fibrosis induced by myocardial infarction through regulating autophagy. Biochem. Biophys. Res. Commun. 503, 1625–1632 (2018).
Xia, Q. et al. PARP-1 inhibition rescues short lifespan in hyperglycemic C. Elegans and improves GLP-1 secretion in human cells. Aging Dis. 9, 17–30 (2018).
Bian, C. et al. NADP+ is an endogenous PARP inhibitor in DNA damage response and tumor suppression. Nat. Commun. 10, 693 (2019).
Aksoy, P. et al. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem. Biophys. Res. Commun. 349, 353–359 (2006).
Barbosa, M. T. et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 21, 3629–3639 (2007).
Boslett, J., Hemann, C., Zhao, Y. J., Lee, H. C. & Zweier, J. L. Luteolinidin protects the postischemic heart through CD38 inhibition with preservation of NAD(P)(H). J. Pharmacol. Exp. Ther. 361, 99–108 (2017).
Boslett, J., Helal, M., Chini, E. & Zweier, J. L. Genetic deletion of CD38 confers post-ischemic myocardial protection through preserved pyridine nucleotides. J. Mol. Cell. Cardiol. 118, 81–94 (2018).
Boslett, J., Reddy, N., Alzarie, Y. A. & Zweier, J. L. Inhibition of CD38 with the thiazoloquin(az)olin(on)e 78c protects the heart against postischemic injury. J. Pharmacol. Exp. Ther. 369, 55–64 (2019).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016).
Tarragó, M. G. et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab. 27, 1081–1095.e10 (2018).
Geisler, S. et al. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J. Exp. Med. 216, 294–303 (2019).
Chiarugi, A., Dölle, C., Felici, R. & Ziegler, M. The NAD metabolome: a key determinant of cancer cell biology. Nat. Rev. Cancer 12, 741–752 (2012).
Rajman, L., Chwalek, K. & Sinclair, D. A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 27, 529–547 (2018).
Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213 (2015).
Katsyuba, E. & Auwerx, J. Modulating NAD+ metabolism, from bench to bedside. EMBO J. 36, 2670–2683 (2017).
Fang, E. F. et al. NAD+ in aging: molecular mechanisms and translational implications. Trends Mol. Med. 23, 899–916 (2017).
Imai, S. & Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 (2014).
Johnson, V. E., Stewart, W. & Smith, D. H. Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 (2013).
Lingor, P., Koch, J. C., Tönges, L. & Bähr, M. Axonal degeneration as a therapeutic target in the CNS. Cell Tissue Res. 349, 289–311 (2012).
Lunn, E. R., Perry, V. H., Brown, M. C., Rosen, H. & Gordon, S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur. J. Neurosci. 1, 27–33 (1989).
Wang, J. et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 170, 349–355 (2005).
Mack, T. G. et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 4, 1199–1206 (2001).
Di Stefano, M. et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 22, 731–742 (2015).
Zhao, Z. Y. et al. A cell-permeant mimetic of NMN activates SARM1 to produce cyclic ADP-ribose and induce non-apoptotic cell death. iScience 15, 452–466 (2019).
Sasaki, Y., Nakagawa, T., Mao, X., DiAntonio, A. & Milbrandt, J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. eLife 5, e19749 (2016).
Pieper, A. A. et al. Discovery of a proneurogenic, neuroprotective chemical. Cell 142, 39–51 (2010).
De Jesús-Cortés, H. et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc. Natl Acad. Sci. USA 109, 17010–17015 (2012).
Tesla, R. et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 109, 17016–17021 (2012).
Yin, T. C. et al. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 8, 1731–1740 (2014).
Choi, S. H. et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821 (2018).
Voorhees, J. R. et al. (–)-P7C3-S243 protects a rat model of Alzheimer’s disease from neuropsychiatric deficits and neurodegeneration without altering amyloid deposition or reactive glia. Biol. Psychiatry 84, 488–498 (2018).
Liu, D. et al. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol. Aging 34, 1564–1580 (2013).
Zhou, M. et al. Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain 138, 992–1008 (2015).
Schöndorf, D. C. et al. The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 23, 2976–2988 (2018).
Blacher, E. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480 (2019).
Ward, J. M. et al. Metabolic and organelle morphology defects in mice and human patients define spinocerebellar ataxia type 7 as a mitochondrial disease. Cell Rep. 26, 1189–1202.e6 (2019).
Stein, L. R. & Imai, S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 33, 1321–1340 (2014).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016).
Fukuwatari, T., Morikawa, Y., Sugimoto, E. & Shibata, K. Effects of fatty liver induced by niacin-free diet with orotic acid on the metabolism of tryptophan to niacin in rats. Biosci. Biotechnol. Biochem. 66, 1196–1204 (2002).
Mukhopadhyay, P. et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 66, 589–600 (2017).
Amano, H. et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab. 29, 1274–1290.e9 (2019).
Mukhopadhyay, P. et al. Poly (ADP-ribose) polymerase-1 is a key mediator of liver inflammation and fibrosis. Hepatology 59, 1998–2009 (2014).
Tummala, K. S. et al. Inhibition of de novo NAD+ synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 26, 826–839 (2014).
Ear, P. H. et al. Maternal nicotinamide riboside enhances postpartum weight loss, juvenile offspring development, and neurogenesis of adult offspring. Cell Rep. 26, 969–983.e4 (2019).
Li, Z. et al. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int. 93, 881–892 (2018).
Morigi, M. et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Invest. 125, 715–726 (2015).
Chang, J. W. et al. Up-regulation of SIRT1 reduces endoplasmic reticulum stress and renal fibrosis. Nephron 133, 116–128 (2016).
He, W. et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 120, 1056–1068 (2010).
Koyama, T. et al. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic. Biol. Med. 51, 1258–1267 (2011).
Hasegawa, K. et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat. Med. 19, 1496–1504 (2013).
Hong, Q. et al. Increased podocyte Sirtuin-1 function attenuates diabetic kidney injury. Kidney Int. 93, 1330–1343 (2018).
Ogura, Y. et al. Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep. 23, 153–159 (2018).
Papadimitriou, A. et al. Theobromine increases NAD+/Sirt-1 activity and protects the kidney under diabetic conditions. Am. J. Physiol. Ren. Physiol. 308, F209–F225 (2015).
Poyan Mehr, A. et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 24, 1351–1359 (2018).
Wang, Y.-M., Han, R.-L., Song, S.-G., Yuan, X.-P. & Ren, X.-S. Inhibition of PARP overactivation protects acute kidney injury of septic shock. Eur. Rev. Med. Pharmacol. Sci. 22, 6049–6056 (2018).
Guan, Y. et al. Nicotinamide mononucleotide, an NAD+ precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352 (2017).
Tran, M. T. et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 531, 528–532 (2016).
Ugur, S. et al. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren. Fail. 37, 332–336 (2015).
Chen, Y. et al. Endogenous Nampt upregulation is associated with diabetic nephropathy inflammatory-fibrosis through the NF-κB p65 and Sirt1 pathway: NMN alleviates diabetic nephropathy inflammatory-fibrosis by inhibiting endogenous Nampt. Exp. Ther. Med. 14, 4181–4193 (2017).
Zakaria, E. M., El-Maraghy, N. N., Ahmed, A. F., Ali, A. A. & El-Bassossy, H. M. PARP inhibition ameliorates nephropathy in an animal model of type 2 diabetes: focus on oxidative stress, inflammation, and fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 390, 621–631 (2017).
Zhuo, L. et al. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell. Physiol. Biochem. 27, 681–90 (2011).
Cho, K. H., Kim, H. J., Rodriguez-Iturbe, B. & Vaziri, N. D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Ren. Physiol. 297, F106–F113 (2009).
Zheng, M. et al. Nicotinamide reduces renal interstitial fibrosis by suppressing tubular injury and inflammation. J. Cell. Mol. Med. 23, 3995–4004 (2019).
Gerner, R. R. et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut 67, 1813–1823 (2018).
Lo Sasso, G. et al. Loss of Sirt1 function improves intestinal anti-bacterial defense and protects from colitis-induced colorectal cancer. PLoS One 9, e102495 (2014).
Wellman, A. S. et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology 153, 772–786 (2017).
Igarashi, M. & Guarente, L. mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166, 436–450 (2016).
Mihaylova, M. M. et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22, 769–778.e4 (2018).
Igarashi, M. et al. NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell 18, e12935 (2019).
Toropova, Y. G. et al. Nicotinamide riboside has protective effects in a rat model of mesenteric ischaemia-reperfusion. Int. J. Exp. Pathol. 99, 304–311 (2018).
Seita, J. & Weissman, I. L. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 640–653 (2010).
Vannini, N. et al. Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat. Commun. 7, 13125 (2016).
Vannini, N. et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24, 405–418.e7 (2019).
Giammona, L. M. et al. Mechanistic studies on the effects of nicotinamide on megakaryocytic polyploidization and the roles of NAD+ levels and SIRT inhibition. Exp. Hematol. 37, 1340–1352.e3 (2009).
Peled, T. et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp. Hematol. 40, 342–55.e1 (2012).
Konieczna, I. M. et al. Administration of nicotinamide does not increase platelet levels in mice. Blood Cells Mol. Dis. 50, 171–176 (2013).
Delabie, W. et al. The senotherapeutic nicotinamide riboside raises platelet nicotinamide adenine dinucleotide levels but cannot prevent storage lesion. Transfusion https://doi.org/10.1111/trf.15556 (2019).
Livingston, D. H. et al. Bone marrow failure following severe injury in humans. Ann. Surg. 238, 748–753 (2003).
Robinson, Y., Hostmann, A., Matenov, A., Ertel, W. & Oberholzer, A. Erythropoiesis in multiply injured patients. J. Trauma 61, 1285–1291 (2006).
Sims, C. A. et al. Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI Insight 3, 120182 (2018).
Meng, Z. H., Dyer, K., Billiar, T. R. & Tweardy, D. J. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am. J. Physiol. Cell Physiol. 280, C343–C351 (2001).
Stensballe, J. et al. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol. Scand. 53, 515–521 (2009).
Minhas, P. S. et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Lee, C.-U., Song, E.-K., Yoo, C.-H., Kwak, Y.-K. & Han, M.-K. Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Mol. Cells 34, 573–576 (2012).
Cerutti, R. et al. NAD+-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 19, 1042–1049 (2014).
Khan, N. A. et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol. Med. 6, 721–731 (2014).
Wallace, G. Q. & McNally, E. M. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu. Rev. Physiol. 71, 37–57 (2009).
Ryu, D. et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci. Transl. Med. 8, 361ra139 (2016).
Chalkiadaki, A., Igarashi, M., Nasamu, A. S., Knezevic, J. & Guarente, L. Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS Genet. 10, e1004490 (2014).
Choi, J. Y. et al. Age-associated repression of type 1 inositol 1, 4, 5-triphosphate receptor impairs muscle regeneration. Aging (Albany NY) 8, 2062–2080 (2016).
Cousin, W. et al. Regenerative capacity of old muscle stem cells declines without significant accumulation of DNA damage. PLoS One 8, e63528 (2013).
Gomes, A. P. et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 (2013).
Mohamed, J. S., Wilson, J. C., Myers, M. J., Sisson, K. J. & Alway, S. E. Dysregulation of SIRT-1 in aging mice increases skeletal muscle fatigue by a PARP-1-dependent mechanism. Aging (Albany NY) 6, 820–834 (2014).
Kourtzidis, I. A. et al. The NAD+ precursor nicotinamide riboside decreases exercise performance in rats. J. Int. Soc. Sports Nutr. 13, 32 (2016).
Karamanlidis, G. et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 18, 239–250 (2013).
Pillai, J. B., Isbatan, A., Imai, S. & Gupta, M. P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J. Biol. Chem. 280, 43121–43130 (2005).
Pillai, V. B. et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J. Biol. Chem. 285, 3133–3144 (2010).
Yamamoto, T. et al. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One 9, e98972 (2014).
Boslett, J., Hemann, C., Christofi, F. L. & Zweier, J. L. Characterization of CD38 in the major cell types of the heart: endothelial cells highly express CD38 with activation by hypoxia-reoxygenation triggering NAD(P)H depletion. Am. J. Physiol. Cell Physiol. 314, C297–C309 (2018).
Wang, L.-F. et al. CD38 deficiency protects heart from high fat diet-induced oxidative stress via activating Sirt3/FOXO3 pathway. Cell. Physiol. Biochem. 48, 2350–2363 (2018).
Xu, W. et al. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep. 13, 533–545 (2015).
Lee, C. F. et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134, 883–894 (2016).
Yano, M. et al. Monocyte-derived extracellular Nampt-dependent biosynthesis of NAD+ protects the heart against pressure overload. Sci. Rep. 5, 15857 (2015).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
Winnik, S., Auwerx, J., Sinclair, D. A. & Matter, C. M. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur. Heart J. 36, 3404–3412 (2015).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Dahl, T. et al. Nicotinamide phosphoribosyltransferase and lipid accumulation in macrophages. Eur. J. Clin. Invest. 41, 1098–1104 (2011).
Romani, M., Hofer, D. C., Katsyuba, E. & Auwerx, J. Niacin: an old lipid drug in a new NAD+ dress. J. Lipid Res. 60, 741–746 (2019).
Hughes-Large, J. M. et al. Niacin receptor activation improves human microvascular endothelial cell angiogenic function during lipotoxicity. Atherosclerosis 237, 696–704 (2014).
Borradaile, N. M. & Pickering, J. G. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell 8, 100–112 (2009).
Das, A. et al. Impairment of an endothelial NAD+-H2S signalling network is a reversible cause of vascular aging. Cell 173, 74–89.e20 (2018).
Tarantini, S. et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192 (2019).
Dollerup, O. L. et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am. J. Clin. Nutr. 108, 343–353 (2018).
Dollerup, O. L. et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J. Clin. Endocrinol. Metab. 104, 5703–5714 (2019).
Elhassan, Y. S. et al. Nicotinamide riboside augments the human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures in aged subjects: a placebo-controlled, randomized trial. Cell Rep. 28, 1717–1728.e6 (2019).
Trammell, S. A. & Brenner, C. Targeted, LCMS-based metabolomics for quantitative measurement of NAD+ metabolites. Comput. Struct. Biotechnol. J. 4, e201301012 (2013).
Sallin, O. et al. Semisynthetic biosensors for mapping cellular concentrations of nicotinamide adenine dinucleotides. eLife 7, e32638 (2018).
Yu, Q. et al. A biosensor for measuring NAD+ levels at the point of care. Nat. Metab. 1, 1219–1225 (2019).
Smith, B. C., Hallows, W. C. & Denu, J. M. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal. Biochem. 394, 101–109 (2009).
Pacholec, M. et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 285, 8340–8351 (2010).
Gerhart-Hines, Z. et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol. Cell 44, 851–863 (2011).
Jin, L. et al. Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3. Protein Sci. 18, 514–25 (2009).
Hirschey, M. D. et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 44, 177–190 (2011).
Laurent, G. et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 50, 686–698 (2013).
Madsen, A. S. et al. Investigating the sensitivity of NAD+-dependent sirtuin diacylation activities to NADH. J. Biol. Chem. 291, 7128–7141 (2016).
Roessler, C., Tüting, C., Meleshin, M., Steegborn, C. & Schutkowski, M. A novel continuous assay for the deacylase Sirtuin 5 and other deacetylases. J. Med. Chem. 58, 7217–7223 (2015).
Kugel, S. et al. Identification of and molecular basis for SIRT6 loss-of-function point mutations in cancer. Cell Rep. 13, 479–488 (2015).
Mendoza-Alvarez, H. & Alvarez-Gonzalez, R. Poly(ADP-ribose) polymerase is a catalytic dimer and the automodification reaction is intermolecular. J. Biol. Chem. 268, 22575–22580 (1993).
Amé, J. C. et al. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 274, 17860–17868 (1999).
Schweiger, M. et al. Characterization of recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 492, 95–100 (2001).
Raffaelli, N. et al. Identification of a novel human nicotinamide mononucleotide adenylyltransferase. Biochem. Biophys. Res. Commun. 297, 835–840 (2002).
The UniProt Consortium. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 (2019); https://www.uniprot.org/.
Pabarcus, M. K. & Casida, J. E. Kynurenine formamidase: determination of primary structure and modeling-based prediction of tertiary structure and catalytic triad. Biochim. Biophys. Acta 1596, 201–211 (2002).
Pabarcus, M. K. & Casida, J. E. Cloning, expression, and catalytic triad of recombinant arylformamidase. Protein Expr. Purif. 44, 39–44 (2005).
Uemura, T. & Hirai, K. Kynurenine 3-monooxygenase activity of rat brain mitochondria determined by high performance liquid chromatography with electrochemical detection. Adv. Exp. Med. Biol. 294, 531–534 (1991).
Alberati-Giani, D. et al. Cloning and functional expression of human kynurenine 3-monooxygenase. FEBS Lett. 410, 407–412 (1997).
Erickson, J. B., Flanagan, E. M., Russo, S. & Reinhard, J. F.Jr. A radiometric assay for kynurenine 3-hydroxylase based on the release of 3H2O during hydroxylation of l-[3,5-3H]kynurenine. Anal. Biochem. 205, 257–262 (1992).
Alberati-Giani, D. et al. Isolation and expression of a cDNA clone encoding human kynureninase. Eur. J. Biochem. 239, 460–468 (1996).
Malherbe, P. et al. Molecular cloning and functional expression of human 3-hydroxyanthranilic-acid dioxygenase. J. Biol. Chem. 269, 13792–13797 (1994).
Allegri, G., Costa, C.V.L., Ragazzi, E., Steinhart, H. & Laresio, L. Developments in Tryptophan and Serotonin Metabolism (Springer, 2012).
Thul, P. J. et al. A subcellular map of the human proteome. Science 26, eaal3321 (2017); https://www.proteinatlas.org/
Seifert, R., Hoshino, J. & Kröger, H. Nicotinamide methylation: tissue distribution, developmental and neoplastic changes. Biochim Biophys. Acta 801, 259–264 (1984).
Riederer, M., Erwa, W., Zimmermann, R., Frank, S. & Zechner, R. Adipose tissue as a source of nicotinamide N-methyltransferase and homocysteine. Atherosclerosis 204, 412–417 (2009).
Pawlak, D., Tankiewicz, A., Matys, T. & Buczko, W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J. Physiol. Pharmacol. 54, 175–189 (2003).
Ito, H. et al. Ability of IDO to attenuate liver injury in alpha-galactosylceramide-induced hepatitis model. J. Immunol. 185, 4554–4560 (2010).
Dobrovolsky, V. N. et al. Effect of arylformamidase (kynurenine formamidase) gene inactivation in mice on enzymatic activity, kynurenine pathway metabolites and phenotype. Biochim. Biophys. Acta 1724, 163–172 (2005).
Kawai, J., Okuno, E. & Kido, R. Organ distribution of rat kynureninase and changes of its activity during development. Enzyme 39, 181–189 (1988).
Pucci, L., Perozzi, S., Cimadamore, F., Orsomando, G. & Raffaelli, N. Tissue expression and biochemical characterization of human 2-amino 3-carboxymuconate 6-semialdehyde decarboxylase, a key enzyme in tryptophan catabolism. FEBS J. 274, 827–840 (2007).
Sanada, H. & Miyazaki, M. Effect of high-protein diet on liver alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 30, 113–123 (1984).
Tanabe, A., Egashira, Y., Fukuoka, S., Shibata, K. & Sanada, H. Purification and molecular cloning of rat 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase. Biochem. J. 361, 567–575 (2002).
Sadanaga-Akiyoshi, F. et al. Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem. Res. 28, 1227–1234 (2003).
Kabra, D. G., Thiyagarajan, M., Kaul, C. L. & Sharma, S. S. Neuroprotective effect of 4-amino-1,8-napthalimide, a poly(ADP ribose) polymerase inhibitor in middle cerebral artery occlusion-induced focal cerebral ischemia in rat. Brain Res. Bull. 62, 425–433 (2004).
Kaundal, R. K., Shah, K. K. & Sharma, S. S. Neuroprotective effects of NU1025, a PARP inhibitor in cerebral ischemia are mediated through reduction in NAD depletion and DNA fragmentation. Life Sci. 79, 2293–2302 (2006).
Feng, Y., Paul, I. A. & LeBlanc, M. H. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res. Bull. 69, 117–122 (2006).
Zheng, C. et al. NAD+ administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci. Lett. 512, 67–71 (2012).
Xie, L., Wang, Z., Li, C., Yang, K. & Liang, Y. Protective effect of nicotinamide adenine dinucleotide (NAD+) against spinal cord ischemia-reperfusion injury via reducing oxidative stress-induced neuronal apoptosis. J. Clin. Neurosci. 36, 114–119 (2017).
Gong, B. et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 34, 1581–1588 (2013).
Turunc Bayrakdar, E., Uyanikgil, Y., Kanit, L., Koylu, E. & Yalcin, A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Aβ(1-42)-induced rat model of Alzheimer’s disease. Free Radic. Res. 48, 146–158 (2014).
Wang, X., Hu, X., Yang, Y., Takata, T. & Sakurai, T. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 1643, 1–9 (2016).
Sorrentino, V. et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552, 187–193 (2017).
Choi, S. H. et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821 (2018).
Hamity, M. V. et al. Nicotinamide riboside, a form of vitamin B3 and NAD+ precursor, relieves the nociceptive and aversive dimensions of paclitaxel-induced peripheral neuropathy in female rats. Pain 158, 962–972 (2017).
Brown, K. D. et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20, 1059–1068 (2014).
Stromsdorfer, K. L. et al. NAMPT-mediated NAD+ biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 16, 1851–1860 (2016).
Escande, C. et al. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62, 1084–1093 (2013).
Abdullah, K. M., Alam, M. M., Iqbal, Z. & Naseem, I. Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed. Pharmacother. 105, 1223–1231 (2018).
Demarin, V., Podobnik, S. S., Storga-Tomic, D. & Kay, G. Treatment of Alzheimer’s disease with stabilized oral nicotinamide adenine dinucleotide: a randomized, double-blind study. Drugs Exp. Clin. Res. 30, 27–33 (2004).
Wakade, C., Chong, R., Bradley, E. & Morgan, J. C. Low-dose niacin supplementation modulates GPR109A, niacin index and ameliorates Parkinson’s disease symptoms without side effects. Clin. Case Rep. 3, 635–637 (2015).
Alisky, J. M. Niacin improved rigidity and bradykinesia in a Parkinson’s disease patient but also caused unacceptable nightmares and skin rash—a case report. Nutr. Neurosci. 8, 327–329 (2005).
Phelan, M. J. & Phase, I. I. Clinical trial of nicotinamide for the treatment of mild to moderate Alzheimer’s disease. J. Geriatr. Med. Gerontol. 3, 021 (2017).
Vestergaard, E. T., Cichosz, S. L., Møller, N., Jørgensen, J. O. L. & Fleischer, J. Short-term acipimox treatment is associated with decreased cardiac parasympathetic modulation. Br. J. Clin. Pharmacol. 83, 2671–2677 (2017).
de la Rubia, J. E. et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 115–122 (2019).
Berge, K. G. & Canner, P. L. Coronary drug project: experience with niacin. Eur. J. Clin. Pharmacol. 40(Suppl. 1), S49–S51 (1991).
Westphal, S., Borucki, K., Taneva, E., Makarova, R. & Luley, C. Adipokines and treatment with niacin. Metabolism 55, 1283–1285 (2006).
Brown, G. et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N. Engl. J. Med. 323, 1289–1298 (1990).
Blankenhorn, D. H. et al. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. J. Am. Med. Assoc. 257, 3233–3240 (1987).
Blankenhorn, D. H. et al. The Cholesterol Lowering Atherosclerosis Study (CLAS): design, methods, and baseline results. Control. Clin. Trials 8, 356–387 (1987).
Brown, B. G. et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345, 1583–1592 (2001).
Taylor, A. J., Sullenberger, L. E., Lee, H. J., Lee, J. K. & Grace, K. A. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110, 3512–3517 (2004).
Guyton, J. R. et al. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J. Am. Coll. Cardiol. 51, 1564–1572 (2008).
Sang, Z. C. et al. Combined use of extended-release niacin and atorvastatin: safety and effects on lipid modification. Chin. Med. J. (Engl.) 122, 1615–1620 (2009).
Villines, T. C. et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J. Am. Coll. Cardiol. 55, 2721–2726 (2010).
AIM-HIGH Investigators et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
HPS2-THRIVE Collaborative Group. et al. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371, 203–212 (2014).
Takahashi, Y. et al. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 65, 1099–1104 (2004).
Airhart, S. E. et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One 12, e0186459 (2017).
Conze, D., Brenner, C. & Kruger, C. L. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci. Rep. 9, 9772 (2019).
Irie, J. et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr. J. https://doi.org/10.1507/endocrj.EJ19-0313 (2019).
Dellinger, R. W. et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech. Dis. 3, 17 (2017).
Sirtori, C. R. et al. Reduced triglyceridemia and increased high density lipoprotein cholesterol levels after treatment with acipimox, a new inhibitor of lipolysis. Atherosclerosis 38, 267–271 (1981).
Taskinen, M. R. & Nikkilä, E. A. Effects of acipimox on serum lipids, lipoproteins and lipolytic enzymes in hypertriglyceridemia. Atherosclerosis 69, 249–255 (1988).
Worm, D. et al. Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J. Clin. Endocrinol. Metab. 78, 717–721 (1994).
Bajaj, M. et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54, 3148–3153 (2005).
van de Weijer, T. et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 64, 1193–1201 (2015).
Salgin, B. et al. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am. J. Physiol. Endocrinol. Metab. 296, E454–E461 (2009).
Santomauro, A. T. et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48, 1836–1841 (1999).
Lehto, H.-R. et al. Effects of acute and one-week fatty acid lowering on cardiac function and insulin sensitivity in relation with myocardial and muscle fat and adiponectin levels. J. Clin. Endocrinol. Metab. 97, 3277–3284 (2012).
Vestergaard, E. T. et al. Acipimox acutely increases GLP-1 concentrations in overweight subjects and hypopituitary patients. J. Clin. Endocrinol. Metab. 78, 2581–2592 (2019).
Hansen, D. et al. Adipose tissue lipolytic inhibition enhances the glucoregulatory properties of exercise in type 2 diabetes patients. Eur. J. Sport Sci. 18, 1245–1254 (2018).
Siadat, A. H., Iraji, F., Khodadadi, M. & Jary, M. K. Topical nicotinamide in combination with calcipotriol for the treatment of mild to moderate psoriasis: A double-blind, randomized, comparative study. Adv. Biomed. Res. 2, 90 (2013).
Dollerup, O. L. et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin resistant men. J. Physiol. (Lond.) https://doi.org/10.1113/JP278752 (2019).
van Loon, L. J. C. et al. Inhibition of adipose tissue lipolysis increases intramuscular lipid use in type 2 diabetic patients. Diabetologia 48, 2097–2107 (2005).
Restrepo Valencia, C. A. & Cruz, J. [Safety and effectiveness of nicotinic acid in the management of patients with chronic renal disease and hyperlipidemia associated to hyperphosphatemia]. Nefrologia 28, 61–66 (2008).
Jin Kang, H. et al. Effects of low-dose niacin on dyslipidemia and serum phosphorus in patients with chronic kidney disease. Kidney Res. Clin. Pract. 32, 21–26 (2013).
Maccubbin, D., Tipping, D., Kuznetsova, O., Hanlon, W. A. & Bostom, A. G. Hypophosphatemic effect of niacin in patients without renal failure: a randomized trial. Clin. J. Am. Soc. Nephrol. 5, 582–589 (2010).
Shimoda, K. et al. [Niceritrol decreases serum phosphate levels in chronic hemodialysis patients]. Nihon Jinzo Gakkai Shi. 40, 1–7 (1998).
Flechtner-Mors, M., Jenkinson, C. P., Alt, A., Adler, G. & Ditschuneit, H. H. Effects of acipimox on the lipolysis rate in subcutaneous adipose tissue of obese subjects. Diabetes Metab. Res. Rev. 17, 387–390 (2001).
Acknowledgements
This work was supported by grants from the EPFL, the European Research Council (ERC-AdG-787702), the Swiss National Science Foundation (SNSF 310030B-160318 and SNF NAD 31003A_179435), the Fondation Suisse de Recherche sur les Maladies Musculaires (FSRMM), the AgingX program of the Swiss Initiative for Systems Biology (RTD 2013/153) and the NIH (R01AG043930).
Author information
Authors and Affiliations
Contributions
E.K., M.R., D.H. and J.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.A. is a consultant to Mitobridge-Astellas, MetroBiotech and TES pharma, companies that develop NAD+-boosting therapies. E.K., M.R. and D.H. declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Primary handling editor: Christoph Schmitt.
Rights and permissions
About this article
Cite this article
Katsyuba, E., Romani, M., Hofer, D. et al. NAD+ homeostasis in health and disease. Nat Metab 2, 9–31 (2020). https://doi.org/10.1038/s42255-019-0161-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0161-5
This article is cited by
-
Amifostine ameliorates bleomycin-induced murine pulmonary fibrosis via NAD+/SIRT1/AMPK pathway-mediated effects on mitochondrial function and cellular metabolism
European Journal of Medical Research (2024)
-
Absolute quantification of nicotinamide mononucleotide in biological samples by double isotope-mediated liquid chromatography-tandem mass spectrometry (dimeLC-MS/MS)
npj Aging (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Visualizing subcellular changes in the NAD(H) pool size versus redox state using fluorescence lifetime imaging microscopy of NADH
Communications Biology (2024)
-
Axonal energy metabolism, and the effects in aging and neurodegenerative diseases
Molecular Neurodegeneration (2023)