Abstract
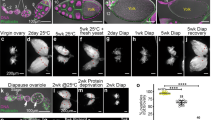
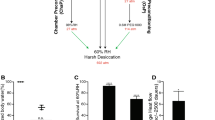
A moderate reduction in body temperature can induce a remarkable lifespan extension. Here we examine the link between cold temperature, germline fitness and organismal longevity. We show that low temperature reduces age-associated exhaustion of germline stem cells (GSCs) in Caenorhabditis elegans, a process modulated by thermosensory neurons. Notably, robust self-renewal of adult GSCs delays reproductive ageing and is required for extended lifespan at cold temperatures (10 °C, 15 °C). These cells release prostaglandin E2 (PGE2) to induce cbs-1 expression in the intestine, increasing the somatic production of hydrogen sulfide, a gaseous signalling molecule that prolongs lifespan. Loss of adult GSCs reduces intestinal cbs-1 expression and cold-induced longevity, whereas application of exogenous PGE2 rescues these phenotypes. Importantly, tissue-specific intestinal overexpression of cbs-1 mimics cold-temperature conditions and extends longevity even at warm temperatures (25 °C). Thus, our results indicate that GSCs communicate with somatic tissues to coordinate extended reproductive capacity with longevity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its supplementary files. Transcriptome data have been deposited in Gene Expression Omnibus (GEO) under the accession code GSE123054. All the other data are also available from the corresponding author upon request.
References
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span–from yeast to humans. Science 328, 321–326 (2010).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Robinson, J. D. & Powell, J. R. Long-term recovery from acute cold shock in Caenorhabditis elegans. BMC Cell Biol. 17, 2 (2016).
Conti, B. Considerations on temperature, longevity and aging. Cell Mol. Life Sci. 65, 1626–1630 (2008).
Hosono, R., Mitsui, Y., Sato, Y., Aizawa, S. & Miwa, J. Life span of the wild and mutant nematode Caenorhabditis elegans. Effects of sex, sterilization, and temperature. Exp. Gerontol. 17, 163–172 (1982).
Klass, M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 6, 413–429 (1977).
Wu, D., Rea, S. L., Cypser, J. R. & Johnson, T. E. Mortality shifts in Caenorhabditis elegans: remembrance of conditions past. Aging Cell 8, 666–675 (2009).
Lamb, M. J. Temperature and lifespan in Drosophila. Nature 220, 808–809 (1968).
Liu, R. K. & Walford, R. L. Increased growth and life-span with lowered ambient temperature in annual fish Cynolebias adloffi. Nature 212, 1277 (1966). &.
Valenzano, D. R., Terzibasi, E., Cattaneo, A., Domenici, L. & Cellerino, A. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell 5, 275–278 (2006).
Lee, S. J. & Kenyon, C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 19, 715–722 (2009).
Xiao, R. et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152, 806–817 (2013).
Conti, B. et al. Transgenic mice with a reduced core body temperature have an increased life span. Science 314, 825–828 (2006).
Loeb, J. & Northrop, J. H. Is there a temperature coefficient for the duration of life? Proc. Natl Acad. Sci. USA 2, 456–457 (1916).
Zhang, B. et al. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep. 11, 1414–1424 (2015).
Zhang, B. et al. Brain-gut communications via distinct neuroendocrine signals bidirectionally regulate longevity in C. elegans. Genes Dev. 32, 258–270 (2018).
Horikawa, M., Sural, S., Hsu, A. L. & Antebi, A. Co-chaperone p23 regulates C. elegans lifespan in response to temperature. PLoS Genet. 11, e1005023 (2015).
Partridge, L., Gems, D. & Withers, D. J. Sex and death: what is the connection? Cell 120, 461–472 (2005).
Kirkwood, T. B. Evolution of ageing. Nature 270, 301–304 (1977).
Arantes-Oliveira, N., Apfeld, J., Dillin, A. & Kenyon, C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 295, 502–505 (2002).
Hsin, H. & Kenyon, C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399, 362–366 (1999).
Vilchez, D. et al. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 489, 263–268 (2012).
Berman, J. R. & Kenyon, C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124, 1055–1068 (2006).
Lapierre, L. R., Gelino, S., Melendez, A. & Hansen, M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr. Biol. 21, 1507–1514 (2011).
Noormohammadi, A. et al. Somatic increase of CCT8 mimics proteostasis of human pluripotent stem cells and extends C. elegans lifespan. Nat. Commun. 7, 13649 (2016).
Shemesh, N., Shai, N. & Ben-ZviA. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell 12, 814–822 (2013).
Hine, C. et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 (2015).
Miller, D. L. & Roth, M. B. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl Acad. Sci. U S A 104, 20618–20622 (2007).
Priess, J. R., Schnabel, H. & Schnabel, R. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell 51, 601–611 (1987).
Curran, S. P., Wu, X., Riedel, C. G. & Ruvkun, G. A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature 459, 1079–1084 (2009).
Syntichaki, P., Troulinaki, K. & Tavernarakis, N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature 445, 922–926 (2007).
Andux, S. & Ellis, R. E. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 4, e1000295 (2008).
Barton, M. K. & Kimble, J. fog-1, a regulatory gene required for specification of spermatogenesis in the germ line of Caenorhabditis elegans. Genetics 125, 29–39 (1990).
L’Hernault, S. W. Spermatogenesis. WormBook 2006, 1–14 (2006).
Schedl, T. & Kimble, J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119, 43–61 (1988).
Varkey, J. P., Muhlrad, P. J., Minniti, A. N., Do, B. & Ward, S. The Caenorhabditis elegans spe-26 gene is necessary to form spermatids and encodes a protein similar to the actin-associated proteins kelch and scruin. Genes Dev. 9, 1074–1086 (1995).
Hubbard, E. J., & Greenstein, D. Introduction to the germ line.WormBook 2005, 1–4 (2005).
Crittenden, S. L., Leonhard, K. A., Byrd, D. T. & Kimble, J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol. Biol. Cell 17, 3051–3061 (2006).
Kimble, J. & Seidel, H. C. elegans germline stem cells and their niche. StemBook https://dx.doi.org/10.3824/stembook.1.95.1 (Harvard Stem Cell Institute, 2008).
Morita, Y. & Tilly, J. L. Oocyte apoptosis: like sand through an hourglass. Dev. Biol. 213, 1–17 (1999).
Hodgkin, J. & Barnes, T. M. More is not better: brood size and population growth in a self-fertilizing nematode. Proc. Biol. Sci. 246, 19–24 (1991).
Garrity, P. A., Goodman, M. B., Samuel, A. D. & Sengupta, P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and drosophila. Genes Dev. 24, 2365–2382 (2010).
Glauser, D. A. et al. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics 188, 91–U150 (2011).
Kindt, K. S. et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat. Neurosci. 10, 568–577 (2007).
Perkins, L. A., Hedgecock, E. M., Thomson, J. N. & Culotti, J. G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487 (1986).
Satterlee, J. S. et al. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron 31, 943–956 (2001).
Joshi, P. M., Riddle, M. R., Djabrayan, N. J. & Rothman, J. H. Caenorhabditis elegans as a model for stem cell biology. Dev. Dyn. 239, 1539–1554 (2010).
Graham, P. L., Schedl, T. & Kimble, J. More mog genes that influence the switch from spermatogenesis to oogenesis in the hermaphrodite germ line of Caenorhabditis elegans. Dev. Genet. 14, 471–484 (1993).
Puoti, A. & Kimble, J. The Caenorhabditis elegans sex determination gene mog-1 encodes a member of the DEAH-Box protein family. Mol. Cell Biol. 19, 2189–2197 (1999).
Puoti, A. & Kimble, J. The hermaphrodite sperm/oocyte switch requires the Caenorhabditis elegans homologs of PRP2 and PRP22. Proc. Natl Acad. Sci. USA 97, 3276–3281 (2000).
Kawasaki, I. et al. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics 167, 645–661 (2004).
Kawasaki, I. et al. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94, 635–645 (1998).
Kuznicki, K. A. et al. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development 127, 2907–2916 (2000).
Hanazawa, M. et al. The Caenorhabditis elegans eukaryotic initiation factor 5A homologue, IFF-1, is required for germ cell proliferation, gametogenesis and localization of the P-granule component PGL-1. Mech. Dev. 121, 213–224 (2004).
Green, R. A. et al. A high-resolution C. elegans essential gene network based on phenotypic profiling of a complex tissue. Cell 145, 470–482 (2011).
Francis, R., Barton, M. K., Kimble, J. & Schedl, T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606 (1995).
Marin, V. A. & Evans, T. C. Translational repression of a C. elegans notch mRNA by the STAR/KH domain protein GLD-1. Development 130, 2623–2632 (2003).
Pinkston, J. M., Garigan, D., Hansen, M. & Kenyon, C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313, 971–975 (2006).
Van Voorhies, W. A. & Ward, S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc. Natl Acad. Sci. USA 96, 11399–11403 (1999).
Vozdek, R., Hnizda, A., Krijt, J., Kostrouchova, M. & Kozich, V. Novel structural arrangement of nematode cystathionine beta-synthases: characterization of Caenorhabditis elegans CBS-1. Biochem J 443, 535–547 (2012).
Yang, G. et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322, 587–590 (2008).
Paul, B. D. & Snyder, S. H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 13, 499–507 (2012).
Kudo, I. & Murakami, M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J. Biochem. Mol. Biol. 38, 633–638 (2005).
Tanioka, T. et al. Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem. Biophys. Res. Commun. 303, 1018–1023 (2003).
Lovgren, A. K., Kovarova, M. & Koller, B. H. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E-2 synthesis. Mol. Cell Biol. 27, 4416–4430 (2007).
Kochel, T. J. & Fulton, A. M. Multiple drug resistance-associated protein 4 (MRP4), prostaglandin transporter (PGT), and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) as determinants of PGE2 levels in cancer. Prostaglandins Other Lipid Mediat. 116-117, 99–103 (2015).
Taylor, R. C., Berendzen, K. M. & Dillin, A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat. Rev. Mol. Cell Biol. 15, 211–217 (2014).
Khodakarami, A., Mels, J., Saez, I. & Vilchez, D. Mediation of organismal aging and somatic proteostasis by the germline.Front. Mol. Biosci. 2, 3 (2015).
Prahlad, V., Cornelius, T. & Morimoto, R. I. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 320, 811–814 (2008).
Gandhi, S., Santelli, J., Mitchell, D. H., Stiles, J. W. & Raosanadi, D. A simple method for maintaining large, aging populations of Caenorhabditis elegans. Mech. Ageing Dev. 12, 137–150 (1980).
Mitchell, D. H., Stiles, J. W., Santelli, J. & Rao, S. D. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J. Gerontol. 34, 28–36 (1979).
Van Raamsdonk, J. M. & Hekimi, S. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mech. Ageing Dev. 132, 519–521 (2011).
Feldman, N., Kosolapov, L. & Ben-Zvi, A. Fluorodeoxyuridine improves Caenorhabditis elegans proteostasis independent of reproduction onset. PLoS One 9, e85964 (2014).
Scott, T. A. et al. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell 169, 442–456 e418 (2017).
Wei, Y. & Kenyon, C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, E2832–E2841 (2016).
O’Rourke, E. J., Kuballa, P., Xavier, R. & Ruvkun, G. Omega-6 polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 27, 429–440 (2013).
Gold, E. B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. North Am. 38, 425–440 (2011).
Ossewaarde, M. E. et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 16, 556–562 (2005).
Shadyab, A. H. et al. Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women’s health initiative. Menopause 24, 35–44 (2017).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Yigit, E. et al. Analysis of the C. elegans argonaute family reveals that distinct argonautes act sequentially during RNAi. Cell 127, 747–757 (2006).
Espelt, M. V., Estevez, A. Y., Yin, X. & Strange, K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J. Gen. Physiol. 126, 379–392 (2005).
Marre, J., Traver, E. C. & Jose, A. M. Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, 12496–12501 (2016).
Calixto, A., Chelur, D., Topalidou, I., Chen, X. & Chalfie, M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 7, 554–559 (2010).
Zou, L. et al. Construction of a germline-specific RNAi tool in C. elegans. Sci. Rep. 9, 2354 (2019).
Mello, C. C., Kramer, J. M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Yang, J. S. et al. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One 6, e23525 (2011).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell Proteomics 13, 2513–2526 (2014).
Wagle, P., Nikolic, M. & Frommolt, P. QuickNGS elevates next-generation sequencing data analysis to a new level of automation. BMC Genomics 16, 487 (2015).
Hoogewijs, D., Houthoofd, K., Matthijssens, F., Vandesompele, J. & Vanfleteren, J. R. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol. Biol. 9, 9 (2008).
Acknowledgements
This work was supported by the European Research Council (ERC Starting Grant-677427 StemProteostasis) and the Deutsche Forschungsgemeinschaft (DFG) (VI742/1-2 and CECAD). We are grateful to E. Llamas for the graphical model and the germline scheme. We thank U. Pham and N. Fritsma for their help in BrdU and lifespan experiments, respectively. We thank J. P. Derks for data analysis and preparation of heatmap figures with R stats package of proteomics experiments. We also thank J. Horák for generation of the tissue-specific cbs-1 expression plasmids. We thank T. Hoppe for critical comments on the manuscript. We are grateful to the CECAD Proteomics and Imaging Facilities for their advice and contribution to proteomics and imaging experiments, respectively. We also thank P. Wagle from the CECAD Bionformatics Facility for data analysis of RNA-sequencing experiments. This work was also supported by the grant of the German Research Council through Collaborative Research Centre 1218 (SFB1218 - TP B01) to A.T.
Author information
Authors and Affiliations
Contributions
H. J. L., A. N. and D. V. performed most of the experiments, data analysis and interpretation. S. K. assessed knockdown levels and contributed to lifespan assays and other experiments. G. C. performed some of the BrdU assays and helped with other experiments. M. S. S. generated the plasmids for tissue-specific overexpression that were used to clone cbs-1. M. H. performed oxygen consumption experiments. A. T. contributed expertise on metabolic rates and provided critical advice on the project. The manuscript was written by D. V. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Primary Handling Editor: Pooja Jha.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–33 and Supplementary Tables 1 and 2.
Supplementary Dataset 1
Differentially expressed proteins following iff-1 RNAi treatment or temperature increase. Log2-fold change of differentially expressed proteins in iff-1 RNAi-treated worms at 15 °C and EV RNAi-treated worms at 20 °C compared with EV RNAi-treated worms at 15 °C. The fer-15(b26);fem-1(hc17) control strain was raised at the restrictive temperature (25 °C) during development to obtain sterile worms with a proliferating germline, which were then shifted to the indicated temperatures and RNAi treatment until day 6 of adulthood. Statistical comparisons were made by two-tailed Student’s t-test (n=3, P value <0.05 was considered significant).
Supplementary Dataset 2
Transcriptome analysis of extruded germlines from worms following iff-1 RNAi treatment or temperature increase. Differentially expressed transcripts changed in the same direction in the germline of both iff-1 RNAi-treated worms at 15 °C and empty vector (EV) RNAi-treated worms at 20 °C compared with the germline of EV RNAi-treated worms at 15 °C. Statistical comparisons were made by two-tailed t-test, n=3 (each replicate contains 150 extruded germ lines from N2 wild-type worms), P <0.01 was considered significant. Germlines were extruded at day 6 of adulthood.
Supplementary Dataset 3
Statistical analysis and replicate data of lifespan experiments. All statistical comparisons were made by two-sided log-rank test, n=96 worms per condition.
Rights and permissions
About this article
Cite this article
Lee, H.J., Noormohammadi, A., Koyuncu, S. et al. Prostaglandin signals from adult germline stem cells delay somatic ageing of Caenorhabditis elegans. Nat Metab 1, 790–810 (2019). https://doi.org/10.1038/s42255-019-0097-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0097-9
This article is cited by
-
Screening for cold tolerance genes in C. elegans, whose expressions are affected by anticancer drugs camptothecin and leptomycin B
Scientific Reports (2024)
-
Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes
Nature Aging (2023)
-
Moderately cold temperatures prevent protein aggregation related to aging and disease
Nature Aging (2023)
-
FMO rewires metabolism to promote longevity through tryptophan and one carbon metabolism in C. elegans
Nature Communications (2023)
-
The metabolite alpha-ketobutyrate extends lifespan by promoting peroxisomal function in C. elegans
Nature Communications (2023)