Abstract
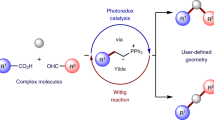
The development of a conjugation method initiated by irradiation of long-wavelength light (>500 nm) to prepare densely functionalized molecules while avoiding undesired photodegradation has attracted considerable attention. Here we show an amide bond formation method based on the photoreaction of 3-acylindolizines in the presence of amines triggered via red-light irradiation. Photooxidation of 3-acylindolizines using a catalytic amount of a photosensitizer and red light-emitting diodes (660 nm) affords the corresponding conjugated amides in nearly quantitative yields within <5 min. This transformation can be performed in aqueous organic solvents and is applicable to diverse aliphatic amines with various functional groups, including the moieties responsive to short-wavelength light.
Similar content being viewed by others
Introduction
Photoinduced conjugation has been used for synthesizing functional materials in a broad range of fields such as bioconjugation chemistry1,2 and polymer science3 because properties of light enable the spatiotemporal control of the chemical reactions. In fact, various reactions involving a photoinitiation process have been developed4,5,6,7,8,9,10. However, these methods require irradiation with short-wavelength light (<500 nm), which has high energy and can induce undesired photodegradation of the target molecules having absorption at these wavelengths. Therefore, to apply this technology for the modification of densely functionalized molecules, development of photoreactions triggered via long-wavelength light irradiation is urgently required.
Triggering chemical reactions via irradiation with long-wavelength light is a challenging task because the energy associated with long-wavelength light is generally insufficient to directly activate covalent bonds. In this context, using a photosensitizer, which can employ long-wavelength light for generating highly reactive singlet oxygen via energy transfer to triplet oxygen, has emerged as an attractive strategy. For instance, Fox et al. developed a red light-induced inverse-electron-demand Diels–Alder reaction involving photooxidation to provide tetrazines that smoothly react with trans-cyclooctenes (Fig. 1a)11. Truong and Forsythe employed red light-induced photooxidation of dihydrogen tetrazines for activation of inverse-electron-demand Diels–Alder conjugation of tetrazines and norbornenes12. Truong and Barner-Kowollik reported a photoinitiated oxime ligation reaction involving the generation of aldehydes by the photooxidation of furans (Fig. 1b)13. These works successfully applied long-wavelength light for the in situ preparation of agents used in well-established bioorthogonal reactions.
We recently reported a 3-acylindolizine-based photouncaging system that liberates carboxylic acids from indolizines upon red-light irradiation in the presence of a sensitizer such as methylene blue (Fig. 1c)14,15. Although the indolizine core is chemically stable, it is responsive to singlet oxygen to produce β-pyridylacrylic acids or the corresponding esters by capturing a solvent molecule such as water or alcohols, respectively, with the release of carboxylic acids16. The formation of these byproducts indicates that a nucleophilic agent such as an amine could bond with the pyridine moiety upon red-light irradiation (Fig. 1c). We anticipated that this approach would provide a practical conjugation method because the uncaging occurred within a few minutes. Herein, we report a red light-induced amidation reaction for the chemical conjugation of 3-acylindolizines with amines. Owing to the use of ubiquitous amino groups, this method is advantageous in terms of practical application for modifying bio/functional molecules.
Results and discussion
To verify our hypothesis, we commenced our investigation with the photoreaction of various 3-acylindolizines 2 with benzylamine (1a) as the model substrate under red-light (660 nm) irradiation in the presence of 1 mol% methylene blue (PS1, Table 1). As expected, the reaction using 3-acetylindolizine 2a afforded amide 3a with an excellent yield within only 3 min of irradiation. The screening of diverse 3-acyl groups such as benzoyl (2b), methoxycarbonyl (2c), and phenoxycarbonyl (2d) revealed that the use of 2a afforded amide 3a in the highest yield. The installation of a substituent at the 1- or 5-position of indolizine was tolerated, affording the products 2e and 2f, respectively, whereas a 4-methyl derivative resulted in a poor yield presumably due to steric hindrance (Supplementary Table S1). The reaction with the substrates having methyl (2g) or phenyl (2h) groups at the 2-position instead of the methoxy group also occurred, although prolonged photoirradiation time was required. In the course of our investigation, we did not observe the formation of any byproduct derived from benzylamine (1a), such as oxygenized17 or oxidatively dimerized compounds18, probably because of the short irradiation time. The photoreaction of 2a in the presence of water-d2 (17 v/v%) also afforded 3a in a comparable yield, suggesting that the amidation preferentially proceeded than the carboxylic acid formation via the reaction with water due to the high nucleophilicity of the amine.
We conducted several experiments to gain insight into the mechanism of the photooxidative amide bond formation. The absorption spectra of 2a or its mixture with 1a showed no absorbance in the red-light region, which suggests that the red-light irradiation does not excite the substrates but PS1 (Supplementary Fig. S1). Control experiments under dark (Table 2, entry 2), under argon (entry 3), or in the absence of PS1 (entry 4) resulted in no reaction, indicating that the process involves photooxidation mediated by PS1. Singlet oxygen, generated by photoirradiation to PS1 under air, is likely to be the reactive species as the addition of sodium azide (NaN3, 10 equiv), a known scavenger for singlet oxygen, shut down the process (entry 5). A similar trend was observed when a chlorin e6 derivative (PS2) was used as the photosensitizer instead of PS1 (entries 6 and 7). We also conducted the reaction using 18O-labeled compounds in the absence of an amine nucleophile to validate the reaction with a nucleophile with 3-acylindolizines (Fig. 2a, b, and Supplementary Fig. S3). The reaction of 2b in a mixture of acetonitrile and 18O-labeled water (18O[H2O]) afforded 18O-labeled β-pyridylacrylic acid 5 (Fig. 2a), whereas the presence of 18O-labeled oxygen (18O[O2]) provided a mixture of 5 and 18O-labeled benzoic acid (Fig. 2b). These results are consistent with the mechanism involving nucleophilic incorporation of water to provide a carboxylic acid. Overall, we consider that the amidation occurs via singlet oxygen-mediated oxidative ring-opening of an indolizine ring followed by nucleophilic addition of an amine (Fig. 2c). Interestingly, when 2a was irradiated with 370 nm LED in the absence of PS1, product 3a was obtained in 63% yield (Table 2, entry 8). This result indicates that direct activation of 2a by 370 nm light can produce singlet oxygen.
We then investigated the substrate scope of the photoreaction (Fig. 3). The reactions proceeded smoothly with primary amines having linear or branched chains to afford the corresponding amides (3f and 3g). tert-Butylamine and 1-aminoadamantane afforded the products 3h and 3i, respectively. We established the structure of the product 3i as an E-isomer by single-crystal X-ray diffractometry. We did not observe the formation of the Z-isomer using 1H NMR spectroscopy measurements of the reaction mixtures. The reaction was applicable to cyclic secondary amines to provide morpholine and piperidine derivatives 3j and 3k, respectively, whereas acyclic secondary amines such as diethylamine, dibenzylamine, and N-methylbenzylamine did not furnish the expected products (Supplementary Table S1). The reaction with the less nucleophilic aniline was also unsuccessful (Supplementary Table S1). When using amino alcohols, amide bond formation occurred preferentially over ester formation to afford the products 3k–3m. Tyrosine was conjugated with the pyridine moiety without the loss of enantiopurity. Further, amines having various functional groups, such as the acetal, allyl, propargyl, and aromatic azido groups, participated in the reaction to furnish the corresponding amides 3n–3r. Moreover, the reaction proceeded with amines containing heteroaryl rings such as the pyridinyl, thienyl, and indolyl rings (3s–3u). Amine hydrochlorides could also be used in the reaction by adding a stoichiometric amount of sodium carbonate in aqueous acetonitrile solutions, affording the products 3v and 3w. Overall, these results demonstrate the excellent functional group tolerance of this conjugation method involving the photooxidation of indolizine rings.
Notably, this method tolerates the substrates that are sensitive to short-wavelength light because the reaction occurs under irradiation with red light, which has long wavelength. Thus, we achieved the conjugation of the coumarin-linked indolizine 2i, whose (coumarin-4-yl)methyl ester is cleavable upon 350 nm-light19. 2-Thiophenemethylamine smoothly underwent the red light-induced amide formation to furnish the functionalized amide 3x in high yield (Fig. 4a). Moreover, an amine with a diazirinyl group, which generates carbene species under 365-nm ultraviolet-light irradiation20,21, was successfully conjugated to provide 3w (Fig. 3). Emission spectrum of the red LED light source and the absorption spectrum of the substrates showed no overlap, exemplifying the wavelength selectivity (Supplementary Fig. S2). Motivated by this result, we performed a continuous photoreaction using diazirinyl amine hydrochloride. As shown in Fig. 4b, red light-induced amide formation in methanol-d4/water-d2 (13/1) followed by irradiation with 365-nm ultraviolet light for 8 min afforded the methanol adduct 6 in high yield without purification.
Photoconjugation with substrates bearing photoreactive (coumarin-4-yl)methyl ester (a) and diazirinyl (b) moieties. aRed light-emitting diode (LED) (660 nm), methylene blue (1 mol %), MeCN, under air, room temperature, 3 min. bIsolated yield. cRed LED (660 nm), methylene blue (1 mol %), Na2CO3 (1 equiv), PhCF3 (internal standard, 1 equiv), methanol-d4/water-d2 (13/1), under air, room temperature, 3 min. dYield determined by 19F NMR measurement.
Red-light irradiation of a mixture of indometacin–indolizine conjugate 2j and C-protected tyrosine afforded free indometacin and tyrosine-derived amide 3m in high yield (Fig. 5), indicating that the photooxidation of 3-acylindolizines enables the simultaneous amide conjugation and release of functional carboxylic acids.
The compatibility of the method in an aqueous solution (Table 1) prompted us to perform the amidation in a water medium without organic co-solvent. The reaction in water using water-soluble 3-acylindolizine 2k and propargylamine afforded desired amide 3p quantitatively, while increased amount of the amine (5 equiv) and PS1 (5 mol%) and extended reaction time (5 min) was required (Table 3, entry 1). This is probably due to the reduced singlet oxygen lifetime in water (~3 μs) compared to that in acetonitrile (~80 μs)22. Using a nearly neutral buffered solution (pH 7.4) lowered the yield, which could be attributed to the decrease in nucleophilicity of the amine due to the protonation (pKb = 9) (entry 2). Indeed, increasing the pH of the buffered solution to 8.5 provided amide 3p in high yield (entry 3). These results indicated a potential applicability of this method to biofunctional molecules that are only soluble in water, although careful control of the pH is necessary.
Conclusion
In summary, we developed a red light-induced conjugation method of amines with chemically stable 3-acylindolizines. The reaction, which involved photooxidation and amide bond formation, proceeded rapidly with a broad substrate scope. This method is expected to be applied to the modification of biological and functional materials, although consideration to the potential damage caused by singlet oxygen should be paid. Further studies including the application of this method for the synthesis of functional molecules are currently ongoing in our laboratory.
Methods
General information
See Supplementary Methods, general information (page S6).
Chemicals
See Supplementary Methods, chemicals (page S7).
Synthesis of substrates
See Supplementary Methods, synthesis of substrates (pages S8–S14).
Procedures for photoreactions
See Supplementary Methods, procedures for photoreactions (pages S15–S26).
NMR charts
See Supplementary Data 1 for NMR charts of all synthesized compounds.
Chiral HPLC charts
See Supplementary Methods, HPLC charts (page S27) for 3m (Fig. 3).
ORTEP diagram and crystallographic data
See Supplementary Methods, ORTEP diagram and crystallographic data (page S28–S29) for 3i (Fig. 3).
Cif file
See Supplementary Data 2 for a cif file of 3i (Fig. 3).
Data availability
Supplementary Information includes Supplementary Methods, absorption spectra of 2a in the absence and presence of benzylamine (Fig. S1), absorption spectra of substrates and emission spectra of red LEDs (Fig. S2), SI mass spectra for 18O-labeling experiments (Fig. S3), unapplicable substrates (Table S1). Supplementary Data 1 includes NMR charts of all synthesized compounds. Supplementary Data 2 includes a cif file of 3i (Fig. 3). The X-ray crystallographic data of 3i (Fig. 3) has been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition number CCDC 2149818. The data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. Extra data are available from the corresponding authors upon request.
References
Lee, M., Rizzo, R., Surman, F. & Wong, M. Guiding lights: tissue bioprinting using photoactivated materials. Chem. Rev. 120, 10950–11027 (2020).
Holland, J. P. et al. Photochemical reactions in the synthesis of protein–drug conjugates. Chem. Eur. J. 26, 33–48 (2020).
Corrigan, N. et al. Seeing the light: advancing materials chemistry through photopolymerization. Angew. Chem. Int. Ed. 58, 5170–5189 (2019).
Fairbanks, B. D. et al. Photoclick chemistry: a bright idea. Chem. Rev. 121, 6915–6990 (2021).
Poloukhtine, A. A. et al. Selective labeling of living cells by a photo-triggered click reaction. J. Am. Chem. Soc. 131, 15769–15776 (2009).
Adzima, B. J., Tao, Y. H., Kloxin, C. J. & DeForest, C. J. Spatial and temporal control of the alkyne-azide cycloaddition by photoinitiated Cu(II) reduction. Nat. Chem. 3, 256–259 (2011).
Oehlenschlaeger, K. K. et al. Light-induced modular ligation of conventional raft polymers. Angew. Chem. Int. Ed. 52, 762–766 (2013).
Feist, F. et al. Light-induced ligation of o-quinodimethanes with gated fluorescence self-reporting. J. Am. Chem. Soc. 142, 7744–7748 (2020).
Hoyle, C. E. & Bowman, C. N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 49, 1540–1573 (2010).
Fairbanks, B. D., Sims, E. A., Anseth, K. S. & Bowman, C. N. Reaction rates and mechanisms for radical, photoinitated addition of thiols to alkynes, and implications for thiol-yne photo-polymerizations and click reactions. Macromolecules 43, 4113–4119 (2010).
Zhang, H. et al. Rapid bioorthogonal chemistry turn-on through enzymatic or long wavelength photocatalytic activation of tetrazine ligation. J. Am. Chem. Soc. 138, 5978–5983 (2016).
Truong, V. X., Tsang, K. M., Ercole, F. & Forsythe, J. S. Red light activation of tetrazine-norbornene conjugation for bioorthogonal polymer cross-linking across tissue. Chem. Mater. 29, 3678–3685 (2017).
Truong, V. X. & Barner-Kowollik, C. Red-Light driven photocatalytic oxime ligation for bioorthogonal hydrogel design. ACS Macro Lett. 10, 78–83 (2021).
Watanabe, K. et al. Indolizines enabling rapid uncaging of alcohols and carboxylic acids by red light-induced photooxidation. Org. Lett. 22, 5434–5438 (2020).
Watanabe, K., Terao, N., Niwa, T. & Hosoya, T. Direct 3-acylation of indolizines by carboxylic acids for the practical synthesis of red light-releasable caged carboxylic acids. J. Org. Chem. 86, 11822–11834 (2021).
Li, Y. et al. Reaction modes and mechanism in indolizine photooxygenation reactions. J. Org. Chem. 69, 2332–2339 (2004).
Weil, L. Photochemical oxidation of nicotine in the presence of methylene blue. Science 107, 426–427 (1948).
Huang, L. et al. BODIPY derivatives as organic triplet photosensitizers for aerobic photoorganocatalytic oxidative coupling of amines and photooxidation of dihydroxylnaphthalenes. J. Org. Chem. 78, 5627–5637 (2013).
Suzuki, A. Z. et al. Coumarin-4-ylmethoxycarbonyls as phototriggers for alcohols and phenols. Org. Lett. 5, 4867–4870 (2003).
Hosoya, T. et al. Novel bifunctional probe for radioisotope-free photoaffinity labeling: compact structure comprised of photospecific ligand ligation and detectable tag anchoring units. Org. Biomol. Chem. 2, 637–641 (2004).
Watanabe, K. et al. Divergent synthesis of photoaffinity probe candidates by click reactions of azido-substituted aryltrifluoromethyldiazirines. Heterocycles 99, 1366–1387 (2019).
Bregnhøj, M., Westberg, M., Jensen, F. & Ogilby, P. R. Solvent-dependent singlet oxygen lifetimes: temperature effects implicate tunneling and charge-transfer interactions. Phys. Chem. Chem. Phys. 18, 22946–22961 (2016).
Acknowledgements
This research was supported by JSPS KAKENHI Grant No. JP22K05325 (K.W.); Japan Agency for Medical Research and Development (AMED) under Grant Nos. JP21am0401021 (Science and Technology Platform Program for Advanced Biological Medicine) and JP21am0101098 (Platform Project for Supporting Drug Discovery and Life Science Research, BINDS); the Cooperative Research Project of Research Center for Biomedical Engineering; and the Pioneering Project “Chemical Probe” and the Incentive Projects from RIKEN.
Author information
Authors and Affiliations
Contributions
K.W., conceptualization, investigation, methodology, writing draft, funding acquisition; A.K., investigation, methodology; D.H., X-ray data collection, structure refinement; T.N., writing, editing, revision, supervision, funding acquisition; T.H., writing, editing, revision, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Vinh Truong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, K., Kuratsu, A., Hashizume, D. et al. Red light-induced conjugation of amines through amide bond formation triggered via photooxidation of 3-acylindolizines. Commun Chem 5, 91 (2022). https://doi.org/10.1038/s42004-022-00712-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-022-00712-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.