Abstract
The development of a unified and straightforward method for the synthesis of ɤ-alkynylated ketones, esters, and amides is an unmet challenge. Here we report a general and practical protocol to access ɤ-alkynylated esters, ketones, and amides with diverse substitution patterns enabled by dual-catalyzed spontaneous formation of Csp3–sp3 and Csp3–sp bond from alkenes at room temperature. This directing-group-free strategy is operationally simple, and allows for the straightforward introduction of an alkynyl group onto ɤ-position of carbonyl group along with the streamlined skeleton assembly, providing a unified protocol to synthesize various ɤ-alkynylated esters, acids, amides, ketones, and aldehydes, from readily available starting materials with excellent functional group compatibility.
Similar content being viewed by others
Introduction
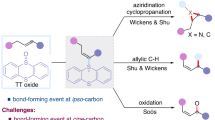
Alkynes and carbonyl derivatives are among the most important functional groups since they are ubiquitous in organic compounds as well as they serve as useful chemical handles transformed to other diverse functional groups1,2,3,4. The introduction of alkyne onto a specific remote position to carbonyl groups is of interest to organic chemists5,6,7,8,9,10,11,12,13,14. In particular, the alkynylation at ɤ-positon of carbonyl functional group is challenging. Yu and Chatani reported a coordination-assisted strategy for alkynylation of amides via Pd/Rh catalyzed C–H activation (Fig. 1a)15,16. This strategy gave only two examples for ɤ-alkynylation with poor results (<40% yield) and is only applicable to the terminal methyl group of amide with directing group. Recently, Zhu and Studer independently developed an elegant alkynyl migration of propargyl alcohol with a pendant olefin via a radical initiated chain reaction to give ɤ-alkynylated ketones (Fig. 1b)17,18,19. Despite significant progress in this field20, the existing methods suffer from several major limitations and drawbacks: (1) Requiring additional steps to synthesize the backbone of sophisticated substrates. (2) Restricted to specific carbonyl functional groups (amide or ketone). (3) Limited substitution patterns at ɤ-position (R=H, or CnFmCH2). Thus, a general, practical, and straightforward method to introduce ɤ-alkynylation for diverse carbonyl derivatives, including ketones, esters, and amides, from easily available and cost-effective starting materials is highly desirable. On the other hand, intermolecular carbo-difunctionalization of alkenes is unarguably an attractive alternative to build molecular complexity via simultaneous formation of two C–C bonds by backbone assembly 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35.
Herein, we establish a unified and general protocol for the direct synthesis of ɤ-alkynylated aryl or alkyl ketones, aldehydes, esters, acids, secondary and tertiary amides using α-bromomethyl carbonyl precursors with alkynes in the presence of alkenes at room temperature (Fig. 1c). Over the past years, reports disclosed that α-halomethyl carbonyl compounds could be converted into alkyl radicals (A)36,37,38,39,40,41,42,43,44,45,46 to initiate coupling with unsaturated systems, such as alkenes and alkynes, to produce C-centered radical (B) (Fig. 1c). We hypothesize that the direct utilization of the radical intermediate (B) with copper catalysis47,48,49,50,51 by the merger of photocatalysis52,53,54,55,56,57 to give desired ɤ-alkynylated carbonyl compounds. However, several highly competitive reactions have to be suppressed. First, radical B is facile to undergo atom transfer39,40,41,42 or single electron oxidation43,44,45,46 by transition metal or photocatalyst (k = 105−109 M−1 s−1)58,59. Moreover, A would directly undergo metal-catalyzed (Pd, Cu) cross-coupling to give α-alkynylated carbonyl derivatives C in the presence of alkyne 60,61.
Results
Reaction conditions evaluation
With these concerns in mind, we set out to explore the possibility of direct incorporating radical intermediate B into copper-catalyzed Csp3–sp bond-forming to furnish ɤ-alkynylated carbonyl derivatives using ethyl α-bromoacetate 1a, styrene 2a, and trimethylsilylacetylene. After some initial trials, we found the use of alkynyl silicate 3a significantly improve the radical cascade coupling efficiency. Upon intensive examining a wide range of reaction parameters, we determined that CuI (10 mol%), a tridendate ligand L1 (10 mol%) and a photocatalyst Ir(ppy)3 (1 mol%) can accomplish the desired reaction in the presence of Cs2CO3 using DCM as solvent with blue LED irradiation, affording ɤ-ethynyl ester 4a in 78% isolated yield (Table 1, entry 1). No desired product was obtained in the absence of copper or light (entries 2 and 3). The absence of photocatalyst dramatically decreased the reaction efficiency, albeit delivering 4a in 35% yield (entry 4). The selection of ligand has significant impact on the radical cascade process. The employment of tridentate ligand is crucial for the success (entries 5–7). The use of bidentate ligand gave 4a in low yields (entries 8–11). When CuTc was used, 4a was obtained in 66% yield (entry 12). When the reaction was carried out with other bases, such as potassium carbonate or lithium tert-butoxide, no substantial amount of 4a was detected (entries 13 and 14). The use of other chlorine-containing solvents led to diminished yields (entries 15 and 16).
Reaction scope
With the optimal conditions in hand, we turned to test the generality of this reaction. We first evaluated different types of carbonyl derivatives (Fig. 2a). The reaction is applicable to a variety of α-bromomethyl carbonyl functional groups, affording ɤ-alkynylated esters, ketones, secondary and tertiary amides, which are inaccessible otherwise. ɤ-Alkynylated esters with two or one enolizable proton can be obtained in good yields (4a–4e). ɤ-Alkynylated lactone could be isolated in moderate yield (4e). Notably, this method is applicable to synthesize α,α-disubstituted ɤ-alkynylated esters in good yields (4f and 4g). It is delighting that ɤ-alkynylated alkyl or aryl ketones could be furnished in good yields (4h–4j). This reaction also tolerates secondary and tertiary amides to afford corresponding ɤ-alkynylated amides in synthetically useful yields (4k and 4l). This protocol tolerates a wide variety of carbonyl derivatives, providing a unified directing-group-free alternative to access ɤ-alkynylation of esters, ketones, amides with diverse substitution patterns under mild conditions.
Scope of different carbonyl derivatives and alkynes. a Scope of carbonyl derivatives. b Scope of alkynes. For reaction conditions, see Table 1, entry 1. Ar = 4-MeOC6H4
Next, we tested the scope of alkyne precursors using ethyl α-bromoacetate 1a (Fig. 2b). The reaction tolerates silyl and alkyl alkynes, giving corresponding ɤ-alkynylated esters in good yields (5a–5d). Aryl alkynes with electron-donating or -withdrawing group proceeded smoothly under the reaction conditions, affording desired product in good yields (5e–5g).
We also examined the scope of alkenes (Fig. 3), which corresponds to the substitution patterns of ɤ- and β-position of the esters using ethyl α-bromoacetate (1a) and trimethylethynyl silicate (3a). Styrenes with electron-donating or -withdrawing substituents at para-, meta- or ortho-position could be all applied in the reaction conditions, affording corresponding ɤ-alkynylated esters in good yields with diverse aryl substitutions at ɤ-position (4a, 4b, 6a–6l). Electron-deficient styrenes, which are challenging for radical involved cross-coupling due to the propensity of radical oligomerization, are also good substrates for this reaction (6d and 6e). Vinylpyridine is tolerated in this reaction, furnishing ɤ-pyridinyl substituted ɤ-alkynylester 6m in 52% yield. Cyclic and acyclic internal alkenes could be transformed into the desired products with ɤ- and β-substitutions in moderate yields (6n and 6o). Emamine could be transformed to ɤ-alkynyl ɤ-aminoester 6p in 33% yield. To further demonstrate the potential utility of this protocol, late-stage functionalization of natural product derivatives was examined. Isoflavone, estrone, tocopherol ocurring alkenes could be tolerated under the reaction conditions and be further modified to deliver natural product containing ɤ-alkynylesters in good yields (6q–6s).
Scope of alkenes. For reaction conditions, see Table 1, entry 1. When it is applicable, 1:1 dr is observed. [a]Isolated yield on 1.0 mmol scale
Synthetic application
We further demonstrated the synthetic application potential of this reaction using 4a as the model substrate (Fig. 4). Trimethylsily group could be removed under basic conditions, delivering ɤ-alkynyl ester (7a) or ɤ-allenyl ester (7b) in a controllable manner in 82% and 70% yield, respectively. In the presence of sodium hydroxide, 4a was deprotected as well as saponificated, giving the formal ɤ-ethynyl carboxylic acid (7c) in 60% yield, which is the precursor for an inhibitor of serine proteases62. ɤ-Triazole substituted ester (7d) could be obtained in 54% yield via copper catalyzed [3+2] reaction. The triple bond could also be cleaved via ruthenium catalysis to deliver 1,4-dicarboxylic acid (7e) in 70% yield. In the presence of DIBAL-H, δ-alkenyl or δ-alkynyl alcohol (7f and 7g) could be obtained in good yields depending on the reaction conditions, providing a divergent method to functionalize δ-position of alcohol which is inaccessible otherwise. It is noteworthy that ɤ-alkynylated aldehyde (7h) could be obtained in 62% yield, rendering this three-component radical cascade protocol accessible to ɤ-alkynylated acids, esters, amides, ketones, and aldehydes.
Synthetic applications of the ɤ-alkynylated ester. 4a Reaction conditions: (i) K2CO3 (3.0 equiv), MeOH (0.05 M), rt, 2 h. (ii) TBAF (3.0 equiv), THF (0.05 M), rt, 10 h. (iii) NaOH (2.0 M aq.), MeOH (0.1 M), rt, 3.5 h. (iv) K2CO3 (3.0 equiv), MeOH (0.05 M), rt, 2 h, then CuTc (10 mol%), TsN3 (1.0 equiv), toluene, rt, 4 h. v) RuCl3 (5 mol%), NaIO4 (4.0 equiv), CCl4/CH3CN/H2O = 2/2/3, rt, 2 h. (vi) DIBAL-H (5.0 equiv), Et2O, 40 °C, 20 h. (vii) DIBAL-H (3.0 equiv), Et2O, −78 oC, 2 h. (viii) DIBAL-H (1.0 equiv), Et2O, −78 oC, 2 h
Mechanistic investigation
To further understand the reaction process, we carried out a series of experiments to probe the reaction mechanism (Fig. 5). In the presence of TEMPO, the reaction was completely shut down with the formation TEMPO adduct 8 in 60% yield, indicating the formation of radical intermediate M2. The use of 2-cyclopropyl styrene under the standard conditions delivered the radical ring-opening and cyclization product 9, which further suggested the presence of radical intermediate M4. When phenyethynyl copper was used as the catalyst, the desired product 10 was obtained in 66% yield, proving alkynylcopper M1 as one of the intermediates in the catalytic cycle.
Based on the results and literature, we proposed the following reaction mechanism (Fig. 6). First, ligand ligated copper (I) undergoes transmetalation with alkynyl silicate to generate alkynyl copper (I) species M1, which reduces α-bromomethyl carbonyl derivatives 1 to give radical intermediate M2 and copper (II) intermediate M3 by single electron transfer. M2 could be trapped by alkene 2 to give a new carbon-centered radical M439,40,41,42,43,44,45,46, which recombines with M3 to deliver intermediate M5. M5 could be activated by energy transfer from excited Ir(III) photocatalyst to generate M663, which accelerates reductive elimination to furnish streamlined three-component assembly product and regenerate Cu(I) catalyst. At this stage, the possibility of undergoing direct reductive elimination from M5 to generate final product and Cu (I) species could not be ruled out 64,65.
Discussion
In summary, we have developed a unified ɤ-alkynylation of esters, ketones, secondary and tertiary amides enabled by the combination of copper- and visible-light catalysis at room temperature. This method features with the exclusive chemoselectivity of alkenes over alkynes, and is applicable to a wide variety of carbonyl derivatives with excellent functional group compatibility. The merger of photocatalysis and copper catalysis along with judicious selection of ligand allows for the streamlined formation of Csp3-sp3 bond and Csp3-sp bond to assemble the carbon chain skeleton and alkynylate ɤ-position from alkene, α-bromomethyl carbonyl precursors, and alkyne silicates, circumventing the tedious synthesis of skeleton. Further synthetic extention provides access to ɤ-alkynylated esters, acids, amides, ketones, and aldehydes. Mechanistic investigation suggests the reaction undergoes copper catalyzed radical cascade reaction, facilitated by energy transfer from excited photocatalyst.
Methods
Synthetic procedures
Characterization
See Supplementary Methods, Supplementary Figs. 1–103 for 1H, 19F and 13C NMR spectra of synthesized products.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fürstner, A. & Davies, P. W. Alkyne metathesis. Chem. Commun. 2307–2320 (2005).
Liu, J., Lam, J. W. Y. & Tang, B. Z. Acetylenic polymers: Syntheses, structures, and functions. Chem. Rev. 109, 5799–5867 (2009).
Willis, M. C. Transition metal catalyzed alkene and alkyne hydroacylation. Chem. Rev. 110, 725–748 (2009).
Parenty, A., Moreau, X. & Campagne, J.-M. Macrolactonizations in the total synthesis of natural products. Chem. Rev. 106, 911–939 (2006).
de Armas, P., Tejedor, D. & Garcia-Tellado, F. Asymmetric alkynylation of imines by cooperative hydrogen bonding and metal catalysis. Angew. Chem. Int. Ed. 49, 1013–1016 (2010).
Dudnik, A. S. & Gevorgyan, V. Formal inverse Sonogashira reaction: direct alkynylation of arenes and heterocycles with alkynyl halides. Angew. Chem. Int. Ed. 49, 2096–2098 (2010).
Brand, J. P. & Waser, J. Electrophilic alkynylation: the dark side of acetylene chemistry. Chem. Soc. Rev. 41, 4165–4179 (2012).
Wang, M., Wang, Z., Shang, M. & Dai, H. Transition-metal-catalyzed C–H alkynylation. Chin. J. Org. Chem. 35, 570–577 (2015).
Kaschel, J. & Werz, D. B. Ethynyl benziodoxolone (EBX): Installing alkynes the reversed way. Angew. Chem. Int. Ed. 54, 8876–8878 (2015).
He, J., Wasa, M., Chan, K. S. L. & Yu, J.-Q. Palladium (0)-catalyzed alkynylation of C(sp 3)–H bonds. J. Am. Chem. Soc. 135, 3387–3390 (2013).
Zhang, J. et al. Cobalt-catalyzed cyclization of aliphatic amides and terminal alkynes with silver-cocatalyst. J. Am. Chem. Soc. 137, 12990–12996 (2015).
Ye, X. et al. Silver-free palladium-catalyzed sp 3 and sp 2 C–H alkynylation promoted by a 1,2,3-triazole amine directing group. Org. Lett. 18, 2970–2973 (2016).
Luo, F.-X. et al. Nickel-vatalyzed oxidative voupling of unactivated C(sp 3)–H bonds in aliphatic amides with terminal alkynes. Organometallics 36, 18–21 (2017).
Han, Y.-Q. et al. Pd(II)-catalyzed enantioselective alkynylation of unbiased methylene C(sp 3)–H bonds using 3,3′-fluorinated-BINOL as a chiral ligand. J. Am. Chem. Soc. 141, 4558–4563 (2019).
Ano, Y., Tobisu, M. & Chatani, N. Palladium-catalyzed direct ethynylation of C(sp 3)–H bonds in aliphatic carboxylic acid derivatives. J. Am. Chem. Soc. 133, 12984–12986 (2011).
Fu, H. et al. Ligand-enabled alkynylation of C(sp 3)-H bonds with Pd (II) catalysts. Angew. Chem. Int. Ed. 56, 1873–1876 (2017).
Xu, Y., Wu, Z., Jiang, J., Ke, Z. & Zhu, C. Merging distal alkynyl migration and photoredox catalysis for radical trifluoromethylative alkynylation of unactivated olefins. Angew. Chem. Int. Ed. 56, 4545–4548 (2017).
Tang, X. & Studer, A. α-Perfluoroalkyl-β-alkynylation of alkenes via radical alkynyl migration. Chem. Sci. 8, 6888–6892 (2017).
Yu, J., Wang, D., Xu, Y., Wu, Z. & Zhu, C. Distal functional group migration for visible-light induced carbo-difluoroalkylation/monofluoroalkylation of unactivated alkenes. Adv. Synth. Catal. 360, 744–750 (2018).
Jia, K., Zhang, F., Huang, H. & Chen, Y. Visible-light-induced alkoxyl radical generation enables selective C(sp 3)–C(sp 3) bond cleavage and functionalizations. J. Am. Chem. Soc. 138, 1514–1517 (2016).
Pintauer, T. & Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 37, 1087–1097 (2008).
Cao, M.-Y., Ren, X. & Lu, Z. Olefin difunctionalizations via visible light photocatalysis. Tetrahedron Lett. 56, 3732–3742 (2015).
Tang, S., Liu, K., Liu, C. & Lei, A. Olefinic C–H functionalization through radical alkenylation. Chem. Soc. Rev. 44, 1070–1082 (2015).
Studer, A. & Curran, D. P. Catalysis of radical reactions: a radical chemistry perspective. Angew. Chem. Int. Ed. 55, 58–102 (2016).
Koike, T. & Akita, M. Fine design of photoredox systems for catalytic fluoromethylation of carbon–carbon multiple bonds. Acc. Chem. Res. 49, 1937–1945 (2016).
Wang, X. & Studer, A. Iodine(III) reagents in radical chemistry. Acc. Chem. Res. 50, 1712–1724 (2017).
Wang, F., Chen, P. & Liu, G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Derosa, J., Tran, V. T., van der Puyl, V. A. & Engle, K. M. Carbon–carbon π-bonds as conjunctive reagents in cross-coupling. Aldrichimica Acta 51, 21–32 (2018).
Gu, J.-W., Min, Q.-Q., Yu, L.-C. & Zhang, X. Tandem difluoroalkylation-arylation of enamides catalyzed by nickel. Angew. Chem. Int. Ed. 55, 12270–12274 (2016).
García-Domínguez, A., Li, Z. & Nevado, C. Nickel-catalyzed reductive dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 139, 6835–6838 (2017).
Gao, P., Chen, L.-A. & Brown, M. K. Nickel-catalyzed stereoselective diarylation of alkenylarenes. J. Am. Chem. Soc. 140, 10653–10657 (2018).
KC, S. et al. Ni-catalyzed regioselective alkylarylation of vinylarenes via C(sp 3)–C(sp 3)/C(sp 3)–C(sp 2) bond formation and mechanistic studies. J. Am. Chem. Soc. 140, 9801–9805 (2018).
Derosa, J., Tran, V. T., Boulous, M. N., Chen, J. S. & Engle, K. M. Nickel-catalyzed β,γ-dicarbofunctionalization of alkenyl carbonyl compounds via conjunctive cross-coupling. J. Am. Chem. Soc. 139, 10657–10660 (2017).
Shrestha, B. et al. Ni-catalyzed regioselective 1,2-dicarbofunctionalization of olefins by intercepting Heck intermediates as imine-stabilized transient metallacycles. J. Am. Chem. Soc. 139, 10653–10656 (2017).
Ouyang, X.-H. et al. Intermolecular dialkylation of alkenes with two distinct C(sp 3)−H bonds enabled by synergistic photoredox catalysis and iron catalysis. Sci. Adv. 5, eaav9839 (2019).
Nakamura, K., Hara, R., Sunada, Y. & Nishikata, T. Radical-organometallic hybrid reaction system enabling couplings between tertiary-alkyl groups and 1-alkenyl groups. ACS Catal. 8, 6791–6795 (2018).
Li, Z., García-Domínguez, A. & Nevado, C. Nickel-catalyzed stereoselective dicarbofunctionalization of alkynes. Angew. Chem. Int. Ed. 55, 6938–6941 (2016).
Shu, W., Merino, E. & Nevado, C. Visible light mediated, redox neutral remote 1,6-difunctionalizations of alkenes. ACS Catal. 8, 6401–6406 (2018).
Nguyen, J. D., Tucker, J. W., Konieczynska, M. D. & Stephenson, C. R. J. Intermolecular atom transfer radical addition to olefins mediated by oxidative quenching of photoredox catalysts. J. Am. Chem. Soc. 133, 4160–4163 (2011).
Wallentin, C.-J., Nguyen, J. D., Finkbeiner, P. & Stephenson, C. R. J. Visible light-mediated atom transfer radical addition via oxidative and reductive quenching of photocatalysts. J. Am. Chem. Soc. 134, 8875–8884 (2012).
Xu, T. & Hu, X. Copper-catalyzed 1,2-addition of α-carbonyl iodides to alkynes. Angew. Chem. Int. Ed. 54, 1307–1311 (2015).
Tucker, J. W., Nguyen, J. D., Narayanam, J. M. R., Krabbe, S. W. & Stephenson, C. R. J. Tin-free radical cyclization reactions initiated by visible light photoredox catalysis. Chem. Commun. 46, 4985–4987 (2010).
Liu, C. et al. Nickel-catalyzed Heck-type alkenylation of secondary and tertiary α-carbonyl alkyl bromides. Angew. Chem. Int. Ed. 51, 3638–3641 (2012).
Liu, Q. et al. Visible-light photocatalytic radical alkenylation of α-carbonyl alkyl bromides and benzyl bromides. Chem. Eur. J. 19, 5120–5126 (2013).
Ouyang, X.-H., Song, R.-J., Hu, M., Yang, Y. & Li, J.-H. Silver-mediated intermolecular 1,2-alkylarylation of styrenes with α-carbonyl alkyl bromides and indoles. Angew. Chem. Int. Ed. 55, 3187–3191 (2016).
Yong, X., Han, Y.-F. & Li, J.-H. Alkylarylation of styrenes via direct C(sp 3)–Br/C(sp 2)–H functionalization mediated by photoredox and copper cooperative catalysis. Chem. Commun. 54, 12816–12819 (2018).
Zhang, W., Chen, P. & Liu, G. Copper-catalyzed arylation of benzylic C–H bonds with alkylarenes as the limiting reagents. J. Am. Chem. Soc. 139, 7709–7712 (2017).
Wang, D., Zhu, N., Chen, P., Lin, Z. & Liu, G. Enantioselective decarboxylative cyanation employing cooperative photoredox catalysis and copper catalysis. J. Am. Chem. Soc. 139, 15632–15635 (2017).
Ye, C. et al. Copper-catalyzed 1,4-alkylarylation of 1,3-enynes with masked alkyl electrophiles. Chem. Sci. 10, 3632–3636 (2019).
Fu, L., Zhou, S., Wan, X., Chen, P. & Liu, G. Enantioselective trifluoromethylalkynylation of alkenes via copper-catalyzed radical relay. J. Am. Chem. Soc. 140, 10965–10969 (2018).
Wu, L., Wang, F., Chen, P. & Liu, G. Enantioselective construction of quaternary all-carbon centers via copper-catalyzed arylation of tertiary carbon-centered radicals. J. Am. Chem. Soc. 141, 1887–1892 (2019).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Ye, Y. & Sanford, M. S. Visible-light photocatalysis and transition-metal catalysis in the copper-catalyzed trifluoromethylation of boronic acids with CF3I. J. Am. Chem. Soc. 134, 9034–9037 (2012).
Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp 3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Lv, X.-L., Wang, C., Wang, Q.-L. & Shu, W. Rapid synthesis of γ-arylated carbonyls enabled by the merge of copper-and photocatalytic radical relay alkylarylation of alkenes. Org. Lett. 21, 56–59 (2019).
Mathew, L. & Warkentin, J. Rate constants for abstraction of bromine from bromotrichloromethane by butyl, cyclopropylmethyl, and phenyl radicals in solution. Can. J. Chem. 66, 11–16 (1988).
Curran, D. P. & Kim, D. Iodine atom transfer addition reactions with alkynes. Part 1: alkyl iodides. Tetrahedron 47, 6171–6188 (1991).
Kang, J. Y. & Connell, B. T. Palladium-catalyzed alkynylation of secondary α-bromo carbonyl compounds via Stille coupling. J. Org. Chem. 76, 6856–6859 (2011).
Shi, W., Liu, C., Yu, Z. & Lei, A. Alkynylation of α-halocarbonyl compounds–a Stille-type cross-coupling for the formation of C(sp)–C(sp 3) bonds under neutral conditions. Chem. Commun. 2342–2344 (2007).
Sofia, M. J. & Katzenellenbogen, J. A. Enol lactone inhibitors of serine proteases. The effect of regiochemistry on the inactivation behavior of phenyl-substituted (halomethylene)tetra- and dihydrofuranones and tetrahydropyranones toward.alpha-chymotrypsin: stable acyl enzyme intermediate. J. Med. Chem. 29, 230–238 (1986).
Heitz, D. R., Tellis, J. C. & Molander, G. A. Photochemical nickel-catalyzed C–H arylation: Synthetic scope and mechanistic investigations. J. Am. Chem. Soc. 138, 12715–12718 (2016).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C-N cross-couplings induced by visible light. Science 351, 681–68 (2016).
Yu, X.-Y., Zhao, Q.-Q., Chen, J., Chen, J.-R. & Xiao, W.-J. Copper-catalyzed radical cross-coupling of redox-active oxime esters, styrenes, and boronic acids. Angew. Chem. Int. Ed. 57, 15505–15509 (2018).
Acknowledgements
Financial support from NSFC (21801126 and 21971101), Thousand Talents Program for Young Scholars, Shenzhen Nobel Prize Scientists Laboratory Project (C17783101), and SUSTech is sincerely appreciated. SUSTech-MCPC is acknowledged for instrumental support. Cong Wang (SUSTech) is thanked for initial experiments. We thank Dr. Yang Yu (SUSTech) for HRMS analysis and Jiao He (SUSTech) for reproducing the results of 4b, 6b, and 6g.
Author information
Authors and Affiliations
Contributions
X.L.L. performed the experiments and collected the data. W.S. conceived the idea, supervised the project, and prepared the paper with contributions from X.L.L.; both authors contributed to discussions and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lv, XL., Shu, W. Unified and practical access to ɤ-alkynylated carbonyl derivatives via streamlined assembly at room temperature. Commun Chem 2, 119 (2019). https://doi.org/10.1038/s42004-019-0219-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-019-0219-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.