Abstract
Division of labor (DOL) is a characteristic trait of insect societies, where tasks are generally performed by specialized individuals. Inside workers focus on brood or nest care, while others take risks by foraging outside. Theory proposes that workers have different thresholds to perform certain tasks when confronted with task-related stimuli, leading to specialization and consequently DOL. Workers are presumed to vary in their response to task-related cues rather than in how they perceive such information. Here, we test the hypothesis that DOL instead stems from workers varying in their efficiency to detect stimuli of specific tasks. We use transcriptomics to measure mRNA expression levels in the antennae and brain of nurses and foragers of the ant Temnothorax longispinosus. We find seven times as many genes to be differentially expressed between behavioral phenotypes in the antennae compared to the brain. Moreover, half of all odorant receptors are differentially expressed, with an overrepresentation of the 9-exon gene family upregulated in the antennae of nurses. Nurses and foragers thus apparently differ in the perception of their olfactory environment and task-related signals. Our study supports the hypothesis that antennal sensory filters predispose workers to specialize in specific tasks.
Similar content being viewed by others
Introduction
Division of labor (DOL) is an important organizing principle of complex biological systems that arose independently during three of the major evolutionary transitions1. DOL was originally formulated in the context of the production process in human societies2, but specialization to specific tasks is also found within cells and across many organisms1. Examples range from bacteria, where clonal populations are divided into subpopulations focusing on different activities3,4,5,6 to multicellular organisms with differentiation of cells into different tissues and organs, and individuals performing specific roles in animal societies7,8. To understand the evolution of complex life, it is therefore essential to investigate the mechanisms that underlie DOL.
DOL in insect societies results from individuals specializing in the performance of specific tasks. In addition to the reproductive DOL between fertile queens and functionally sterile workers, there is a behavioral DOL among workers that specialize in tasks such as brood care, foraging, nest building, and defense9,10,11. Several factors can affect task specialization, including age12,13, nutrition14,15, morphology16, genotype17,18,19, experience20, and colony size21,22,23. In leafcutter ant species, among others, behavioral specialization of morphologically distinct groups of workers contributes to the DOL24. In most social insect species, younger workers tend to perform intranidal tasks, while older individuals perform risky activities such as nest defense and foraging outside the nest25,26. Yet, such specialization among workers remains flexible, as foragers can revert to perform brood care when needed27,28.
Several molecular mechanisms have been implicated in the regulation of DOL. Task specialization is associated with transcriptional changes in the worker brain29,30,31,32,33,34. Molecular pathways such as the insulin/insulin-like signaling (IIS), vitellogenin (Vg), and juvenile hormone (JH) pathways are involved in the regulation of worker behavior35,36,37,38,39,40. Functional manipulations have confirmed that the expression of key genes in the worker brain controls task specialization41,42,43. Behavioral variation among workers is also associated with signaling of biogenic amines (e.g., dopamine, octopamine, tyramine, serotonin), which act as neurotransmitters or neuromodulators involved in the modulation of the responsiveness to task-associated stimuli44,45,46,47.
Self-organization and collective behavior in insect societies are maintained via the exchange of chemical information48. Social insects communicate primarily through glandular pheromones and complex mixtures of long-chain hydrocarbons on their cuticle. These cuticular hydrocarbons (CHC) facilitate recognition of nestmates, developmental stages, castes, sexes, and species49,50. Social insects perceive chemical information via different types of sensilla in the antennae, small receptor organs embedded in the integument that are connected to sensory neurons51,52,53. Decoding the identities of chemical compounds relies on odorant receptors (ORs) located within each sensillum54,55. ORs are transmembrane proteins expressed in the dendrites of olfactory receptor neurons (ORN). The largely conserved OR coreceptor (Orco) of insects56 is required for odorant recognition in the dendritic membrane: it forms an ion channel with specific OR, which determines the sensitivity and specificity of the ORN57. Odorant molecules penetrate through the antenna cuticular pores and are transported by odorant-binding proteins to the ORN membrane, where they interact with receptors, leading to the generation of action potentials58,59. ORN axons relay signals from the sensilla to the glomeruli of the antennal lobes in the insect brain, which are the first processing unit for olfactory information. Then, ORN make synaptic contact with the projection neurons and local neurons, which transfer information to the central brain60,61,62.
Several lines of evidence indicate that OR genes play central roles in the regulation of social life of insects. First, social insect species typically harbor large numbers of OR genes63,64,65,66,67. Second, some OR gene families have specifically expanded during social evolution, such as the 9-exon subfamily in ants, which appears to serve an important function in the perception of CHC63,66,68,69,70,71. Third, species that evolved socially parasitic strategies resulting in reduced behavioral repertoires show a strong and convergent reduction in the number of OR genes72. Finally, experimentally produced mutant ants that lack the Orco gene (coding for the co-receptor necessary for OR to properly function) show impaired social behavior73,74.
Current DOL models bring together the chemical nature of social insect communication and variation among workers in their response to chemical cues. They posit that flexible response thresholds to task-related chemical stimuli serve as regulators of worker specialization75. Workers take on a particular task when the stimulus intensity exceeds their individual threshold for this task. Therefore, individuals with lower thresholds for a given task are more likely to perform it than those with higher thresholds76. Individual response decisions and task performance are modulated via numerous parameters on different time scales7,77,78,79, and despite extensive research on DOL and task allocation, these mechanisms are not fully understood.
As the name suggests, response threshold models are based on an individual’s response to certain stimuli and the variation in their responsiveness over time. However, these studies do not include in their models how these cues are processed65,79,80,81,82,83,84,85. The fact that the processing of signal information has been primarily described in the insect brain (see ref. 86) may suggest that thresholds and associated responses are set in the central nervous system, possibly regulated via molecular pathways in the brain that correlate with behavioral variation. We propose that DOL models would benefit from considering odor sensitivity as a potential upstream sensory filter that may affect task specialization. Along these lines, we hypothesize that behavioral variation among workers may also stem from their ability and/or efficiency in detecting different signals. For example, we propose that individuals that specialize in brood care do so because they are more sensitive to brood cues, rather than (or in addition to) being more likely to respond to similar levels of brood cues. To test the hypothesis that a sensory filter regulates inter-individual behavioral variation, and thus the DOL in social insects, we investigated transcriptional signals in the brain and antennae of workers that specialize in either brood care or foraging behavior in the ant Temnothorax longispinosus. We found that (i) behavioral variation was associated with more extensive transcriptomic differences in the antennae than in the brain, (ii) these differences included a large proportion of the OR gene repertoire, and (iii) individuals specializing in brood care overexpressed an OR gene family putatively involved in detecting social cues. These findings support our hypothesis that the peripheral nervous system, acting as a sensory filter, plays an important role in regulating behavioral differences between workers and thus in the DOL of social insects.
Results
Identification of Temnothorax longispinosus behavioral phenotypes
Task specialization in the ant T. longispinosus is neither genetically fixed nor rigid, but can change with age and in response to colony needs39. We conducted behavioral observations of seven T. longispinosus laboratory colonies to identify individuals that specialize in brood care behavior (hereafter referred to as nurses), and others that specialize in foraging (hereafter referred to as foragers) (Fig. 1A; see “Methods” section for more details). The grouping of workers into the two behavioral categories was based on the frequency of their location inside or outside the colony and on their behavior, especially whether they showed brood care or foraging behavior (Dataset S1). Individuals identified as nurses interacted with the brood in 54% ± 22% (mean ± sd) of the observations, and were recorded outside the nest in 1% ± 3% of the observations. On the contrary, foragers were found outside the nest in 42% ± 26% of the observations but interacted with the brood in only 2% ± 4% of the observations.
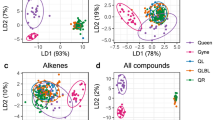
A Boxplot showing behavioral differences between ants selected for transcriptomic analysis. Each black dots represent individual ants. For differential expression analysis, each sample contains the pooled RNA of seven ants of the respective behavioral phenotype. B Venn diagrams showing the number of DEGs that were upregulated in nurses (red) and foragers (turquoise) for both antennae and brain tissues. Principal component analysis (PCA) plots based on all expressed genes for C antenna and D brain samples with 95% confidence level presented as ellipses. The color of each sample represents task (red = nurse, turquoise = forager), and the shape the colony of origin.
Larger task-associated transcriptomic changes in the antennae than in the brain
To investigate transcriptomic variation between nurses and foragers, we used RNA-seq to generate seven nurse and seven forager brain and antenna samples, each consisting of pooled tissue from seven workers of a single colony. Of the 14,837 genes annotated in the T. longispinosus genome, we found 91% (13,494) and 92% (13,683) to be expressed (FPKM > 0) in the brain and the antennae, respectively. To investigate gene expression differences between nurses and foragers, we compared full models that included task as an explanatory variable to reduced models that did not using the likelihood ratio test (LRT) method implemented in DEseq2. The influence of colony identity was controlled for by including it as an explanatory variable in both full and reduced models. A Benjamini–Hochberg adjusted p value of 0.05 was set as a threshold to obtain genes whose variation was significantly explained by behavioral specialization in each tissue. We detected 339 differentially expressed genes (DEGs) in the brain (223 upregulated in nurses, 116 in foragers), and 2267 in the antennae (1241 upregulated in nurses, 1026 in foragers; Dataset S2 and Fig. 1B). We found an overlap of 162 DEGs between the two tissues, including 143 DEGs that showed differences in the same direction across tissues. Principal component analyses (PCA) for both brain and antenna data reveal that most samples clustered by behavioral phenotype rather than colony of origin (Fig. 1C, D).
OR gene expression differs between antennae of nurses and foragers
To investigate whether odorant perception differs between nurses and foragers, we focused our attention on the expression of OR genes in the antennae. We found that all 419 previously annotated OR genes in the genome of T. longispinosus72 were expressed in the antennae, and that 50% (209/419) of them were differentially expressed between nurses and foragers. Specifically, 64 OR genes were upregulated in nurses (15% of all OR genes), and 145 in foragers (35% of all ORs) (Fig. 2). Then, we studied which OR subfamilies were preferentially expressed in nurses and foragers. The 64 OR genes overexpressed in nurses belonged to three OR subfamilies, while the 145 OR genes upregulated in foragers were distributed among 19 OR subfamilies. Foragers overexpressed 27, 19, 8, and 8 OR genes from the L, V, P, and H subfamilies, respectively, while no OR genes from these subfamilies were upregulated in nurses (Dataset S3). We found that 63% (27/43) of the genes from the L subfamily and 73% (8/11) from the P subfamily were overexpressed in foragers, which represents a significant overrepresentations (L subfamily: Fisher’s test, odds ratio = 3.67, p value < 0.001; P subfamily: Fisher’s test, odds ratio = 5.25, p value < 0.02). For the V and H subfamilies we did not find such an overrepresentation, likely due to lower gene numbers (V subfamily: Fisher’s test, odds ratio = 1.64, p value = 0.17; H subfamily: Fisher’s test, odds ratio = 1.71, p value = 0.30). On the other hand, we found that 80% (51/64) of the OR genes overexpressed in nurses belong to the 9-exon subfamily. This results in an overrepresentation of the 9-exon subfamily in genes that were overexpressed in nurses (Fisher’s test, odds ratio = 22.18, p value < 0.001), with 45% (51/114) of this subfamily being overexpressed in nurses. In contrast, only 6% (8/137) of the 9-exon subfamily was overexpressed in foragers, which is less than expected by chance (Fisher’s test, odds ratio = 0.09, p value < 0.001). We also found that nurses overexpressed Orco compared to foragers (FDR p value = 0.001).
A Among the DEGs overexpressed in nurses and foragers, the most represented OR subfamilies are indicated by different colors. The red dotted line represents the significance threshold of our differential expression analysis. B, C Boxplots representing the expression of B Orco and OR257, the gene with the highest expression difference in terms of log2 FoldChange in the 9-exon subfamily, and C OR041, OR045 and OR318, the genes with the highest expression difference in terms of log2 FoldChange in the H, L, and P subfamilies, respectively.
Genes in biogenic amine pathways vary in expression between nurses and foragers
Biogenic amines have been implicated in the regulation of behavior, and they may affect sensory perception46,87,88,89,90. We therefore screened our lists of brain and antennal DEGs for biogenic amine pathway genes. We detected five differentially expressed genes in the antennae that are associated with biogenic amine signaling. Genes in the serotonin (5-hydroxytryptamine, DBV15_11483), tyramine (tyramine beta-hydroxylase, DBV15_00422) and octopamine (octopamine receptor, LOC112465659) pathways were upregulated in the antennae of foragers, while nurses showed a higher expression of genes in dopamine (dopamine 1-like receptor 2, DBV15_07611) and octopamine (octopamine beta2 receptor, DBV15_10418) pathways (Fig. 3A–E). These five genes were also expressed in the brain, but not differentially expressed between nurses and foragers. Also, no other biogenic amine pathway genes were found to be differentially expressed in the brain.
Boxplots showing the expression of A–E some genes from different biogenic amines pathways, while F one behavioral candidate gene (Table S1). Gene identity is shown in parentheses.
Association between behavioral variation and molecular pathways in brain and antennae
Our analysis revealed expression differences of genes expressed in the brains of nurses and foragers involved in the regulation of task specialization in social insects. Previous studies have found that Vg genes and the associated JH and IIS/TOR pathways are important endocrine networks that play central roles in the regulation of lifespan, fertility and behavior in bees and ants36,91,92,93,94,95,96. Therefore, we searched and found evidence in our RNA-seq data for task-associated expression of genes involved in the metabolism, biosynthesis, and regulation of these pathways in the brain and antennae (Table S1). These genes included conventional Vg (LOC112466671), Vg-like A (named/classified as per;43 DBV15_03138), venom carboxylesterase-6-like (DBV15_11528), and protein takeout (DBV15_08771) overexpressed in the brain of nurses. Meanwhile, allatostatin A-like (LOC112454443) and insulin-like growth factor I (IGF1) (LOC112454447) were found overexpressed in the brain of foragers. Remarkably, venom carboxylesterase-6-like and IGF1 were overexpressed in the antennae of nurses and foragers respectively. Additionally, high expression levels of the zinc-finger transcription factor Krüppel homolog-1 (Kr-h1) were detected in the antennae of foragers (DBV15_06330) (Fig. 3F). The expression of this gene has been correlated with caste and behavioral differences in the brain of social insects93,97,98,99,100, but little is known about its function and gene targets in the antennae.
We performed a GO enrichment analysis to gain a deeper understanding of the biological processes represented in the lists of DEGs. This analysis detected many enrichments based on single one or few genes, and we mention below a few interesting processes with the number of genes driving the enrichment in parentheses (see Table S2 for complete list). Genes that were upregulated in the brain of nurses were enriched for biological processes such as translation (19 genes), cellular iron ion homeostasis (2 genes), and catabolic process (6 genes); while translation (22 genes), endocytosis (6 genes), glucose metabolic process (4 genes), and regulation of cell cycle (3 genes) were enriched in the antennae. In the list of genes that were overexpressed in the brain of foragers, we found that enriched processes included peptide metabolic process (4 genes), methionyl-tRNA aminoacylation (1 gene), positive regulation of type I interferon production (1 gene), and cell redox homeostasis (1 gene); while phosphatidylinositol phosphorylation (4 genes), inositol phosphate dephosphorylation (3 genes), carbohydrate metabolic process (17 genes), innate immune response (2 genes), and nucleotide catabolic process (3 genes) were enriched in the antennae.
Discussion
In this study, we found evidence supporting the hypothesis that variation among social insect workers in their ability to perceive different chemical signals could contribute to the regulation of task allocation, and thus to DOL in insect societies. To do so, we analyzed brain and antenna transcriptomes of nurses and foragers of the ant T. longispinosus. We report several lines of evidence that support our hypothesis. First, we found almost seven times as many genes to be differentially expressed between nurses and foragers in the antennae as in the brain, indicating that peripheral sensory organs may have an important function in the task specialization process of social insect workers. Second, we found that half of all OR genes of the T. longispinosus genomes are differentially expressed between the antennae of nurses and foragers, suggesting that behavioral specialization is associated with different sensory filters that result in specific perceptions of the chemical environment. Third, our analyses revealed that nurses and foragers upregulated distinct families of OR genes, indicating that their sensory filters may target different types of chemical cues, possibly adjusted to the tasks they perform. Finally, we detected several genes in multiple biogenic pathways to be differentially expressed between the antennae of nurses and foragers, potentially involved in fine-tuning the sensitivity of the odorant filters.
Many organisms have evolved sensory filters to focus on only a subset of all environmental cues101,102,103,104. In insects, the peripheral olfactory filtering system enables individuals to detect and discriminate odors that convey ecologically relevant information used to mediate important behaviors such as courtship, locomotion and navigation to avoid predators and locate food or nesting sites105. For example, the mosquito Anopheles gambiae strongly responds to odorant components of its vertebrate hosts that provide meals106. Sensory filters have been selected for because they limit the amount of information perceived by the organism, and thus the energy and time required by the brain to process it105. Within insect colonies, individuals typically exhibit many morphological and physiological traits associated with increased efficiency in task specialization. These can be understood as adaptations that allow individuals to become more proficient at their task due to learning, training, or the perception of valuable task-related information, allowing the colony to avoid the cost of task switching107. In this context, it is interesting to find that T. longispinosus workers may have different sensory filters that would serve as a basis for their task specialization, as workers would mostly perceive chemical cues that pertain to their tasks. Our findings indicate that the sensory filter of ant workers is dynamic, and its changes may underlie their behavioral maturation.
In this study, we report a higher number of DEGs between nurses and foragers in the antennae than in the brain. The brain is a heterogeneous tissue in which different cell types perform very specific functions, which differ greatly in their gene expression108,109,110. Therefore, using the whole brain for transcriptome analyses could make it more difficult to identify genes that are differentially expressed only in specialized parts of the brains of nurses or foragers. In comparison, the antennae might have a more uniform cell composition, which could facilitate the identification of DEGs. In contrast to this prediction, a transcriptome comparison of two different behavioral phenotypes of worker honey bees revealed that transcriptional differences are much more pronounced in the antenna than in the separately studied brain parts including the mushroom body, antennal lobe and central brain111. Furthermore, Chandra et al.112. used whole-brain RNA-seq in several ant species to detect differential expression of the gene Ilp2 between castes, a gene that was later found to be expressed in only about 15 cells of the pars intercerebralis. This led us to conclude that it may be slightly more difficult to identify DEGs in the brain than in the antennae, but this unlikely explains the nearly seven-fold difference in the number of DEGs.
Age, genetic background, social environment, individual experiences and hormones influence behavioral differences between workers leading to DOL13,20,23,113,114,115. In our study, we expect that age differed between individuals identified as nurses and those identified as foragers, although we did not measure it directly. However, we know from previous studies on T. longispinosus that workers live between one to three years and switch from brood carer to forager about one year after eclosion, when new generation of workers emerged and taken over the care of the brood43,116. Previous experiments designed to disentangle gene expression associated with behavioral specialization, age and fertility showed that behavioral specialization is much more strongly associated with gene expression than age and fertility in T. longispinosus39. We propose that the molecular and physiological regulators such as JH, Vg, biogenic amines, and nutritional status known to regulate task specialization could drive different OR expression patterns, which in turn would produce behavioral variation via contrasting abilities to detect different sets of odors. This hypothesis is supported by several studies (reviewed in ref. 117) showing that the physiological condition of an animal can influence the level of receptor expression, including mating status, oviposition, feeding, circadian rhythm, experience, and aging. Alternatively, we cannot exclude that the exposure to different odors that is associated with performing different tasks may at least in part have affected OR gene expression in the antennae. However, such an effect is unlikely to explain the large-scale variation in gene expression, as there is very limited evidence that the mere exposure to odors influences the expression of the gene coding for the OR that binds this odor118,119.
According to our sensory filter hypothesis, ant workers would be expected to primarily detect chemical cues that correspond to their tasks. We found that the 9-exon subfamily of OR was overrepresented in genes that were upregulated in the antennae of nurses. Interestingly, recent studies have shown a rapid expansion of the 9-exon subfamily in ants, and several lines of evidence indicate that OR genes from this subfamily mediate complex social interactions in ant colonies67,68,69,70. First, comparisons of antennal transcriptomes revealed that 9-exon OR genes are expressed more frequently in workers than in males, suggesting a role in social communication among workers63,70. Second, representative OR from the 9-exon subfamily can detect CHC extracts from several castes71. According to McKenzie et al.70, 9-exon OR genes were first expressed in solitary ancestors of aculeate wasps and facilitated CHC discrimination, likely for prey or mate recognition, with a lineage giving rise to the ancestors of ants. Moreover, OR genes of the 9-exon subfamily were convergently lost in socially parasitic ant species that lost the ability to perform brood care or foraging72, suggesting that they are essential for the performance of these worker tasks. Our finding of an overexpression of 9-exon OR genes in the antennae of nurses is in line with these previous studies, and suggests that many of these receptors have important functions within the nest, such as sensing chemical cues from the larvae, queen, or other workers, and/or that they are less important for sensing task-related stimuli or other signals outside the nest. Among the OR genes overexpressed in nurses was also the co-receptor Orco, which is widely expressed in olfactory sensory neurons and nearly unchanged in sequence in distant insect taxa120,121,122. ORs form a unique class of heteromeric cation channels composed of two related heptahelical subunits: a divergent OR subunit that confers odor specificity, and the co-receptor Orco subunit123,124. Since those functional receptors would increase the sensitivity of the workers to odors, we propose that the overexpression of Orco may indicate higher olfactory sensitivity to odors in the antennae of nurses compared to foragers. This would be supported by previous studies that showed that changes in Orco expression can be indicative of physiological conditions and sensory receptivity125,126.
The behavioral transition from nursing to foraging may be triggered by a lower efficiency in detecting brood cues via the downregulation of specific OR genes (e.g., from the 9-exon subfamily). This would result in ants moving farther away from the brood, and this change in spatial location may trigger the behavioral transition to outside tasks127,128. In addition to being less efficient at detecting brood cues, the sensory filter of foragers may also become fine-tuned to detect a more diverse set of odors. Foragers overexpressed a greater number of OR gene subfamilies compared to nurses (19 and 3 for foragers and nurses, respectively), which may indicate that the olfactory system of foragers could perceive the more diverse chemical environment outside the nest. Similar to the 9-exon subfamily, the L subfamily has also been expanded in social insects64,66,67, and along with the P and H subfamilies, it has been lost in socially parasitic ants72,129. Interestingly, the OR genes from the L, P, V, and H subfamilies have been upregulated in foragers, and thus may have a task-specific function, such as recognition of chemical cues related to environmental perception or recruitment cues outside the nest. OR genes belonging to subfamilies L, H and V have been shown to be highly responsive to long-chain hydrocarbons and are overexpressed in the antennae of males and workers of the ant Harpegnathos saltator130. In addition, several ORs of the H subfamily have been proposed to act as putative floral odorant detectors in the antennae of honey bees131.
Finding task-specific variation in OR gene expression raises the question as to which molecular mechanisms regulate those changes. Variation in biogenic amines levels have been identified as one of the leading causes of behavioral plasticity and specialization of social insects to different tasks (reviewed in ref. 132). Functional manipulation of biogenic amines has led to changes in behavior, dominance status and reproductive activity, as well as shifts in worker task performance133,134,135,136. Previous studies on insects revealed that olfaction-guided behavior is mediated by biogenic amine receptors in the antenna, and their expression is involved in fine-tuning the sensitivity of the olfactory system89,117. Signal transduction of biogenic amine receptors is mediated by G protein-coupled receptors (GPCRs) located on the cell membrane, which trigger different signaling cascades that lead to increased or decreased cAMP level and Ca2+ release137,138,139,140. For example, modified concentration cAMP and intracellular Ca2+ levels due to octopamine-induced signal transduction in the moth Manduca sexta141 activate Orco, leading to changes in ORN sensitivity142,143. Our results suggest that the biogenic amine signaling pathway may modulate the sensory filtering function of insect antennae and alter sensitivity to various signals. We found that genes encoding tyramine and its precursor, octopamine, are upregulated in forager antennae, similar to genes involved in serotonin signaling. Tyramine and dopamine (which was upregulated in nurses) have been implicated in modulating taste and olfactory receptor neurons, while serotonin may serve as a neurotransmitter and neurohormone in antennal vessels and mechanosensory organs89. Serotonin influences foraging activity88 and regulates food intake in many animals144,145,146. Dopamine signaling also plays an important role in controlling the insect circadian clock and mediating clock-controlled behavioral phenotypes such as locomotion147,148. In our focal species T. longispinosus, inside workers were found to exhibit a stronger circadian rhythmicity than foragers, which may be regulated via differences in the acetylation of histone proteins149.
Changes in behavior and olfactory sensitivity in insects could be related to the expression of genes involved in IIS, target of rapamycin (TOR), JH and Vg pathways, according to age, circadian rhythm, mating and feeding status117. For example, appetite state in D. melanogaster is signaled by insulin, which upregulates a peptide receptor on the olfactory receptor cells that innervate the DM1 glomerulus. Activation of the DM1 glomerulus is enough to drive the fly to reach for food150. Recent studies have shown that pheromone release and odor sensitivity appear to be under JH control in Schistocerca gregaria and Locusta migratoria, which could lead to behavioral changes151,152,153,154,155. Finally, an experimental downregulation of Vg-like A in T. longispinosus workers resulted in decreased brood care behavior and a lower sensitivity to brood-related chemical cues, suggesting changes in odor perception and olfactory-driven decision making43. Our results reveal that genes associated with all these pathways were differentially expressed in the brain and antennae between nurses and foragers, predicting a link between their role in task-associated behavioral changes and the regulation of odor perception. Given the central role of IIS, Vg, JH, and TOR pathways in regulating division of labor in social insects, and our finding of task-associated patterns of the antennal expression of genes from multiple biogenic amines, we hypothesize that these modulators and hormones could be involved in the regulation of the olfactory filter. How the detailed molecular mechanisms of these pathways in the brain are causally linked to the complex changes in olfactory perception in the antennae should therefore be investigated next.
Conclusion
Our transcriptomic analyses of the brain and antennae of T. longispinosus nurses and foragers provide support to our hypothesis that behavioral variation and task specialization in ant workers are regulated via differences in olfactory perception. We predict that antennal physiology acts as sensory filters that limit the type and amount of chemical information passed to the brain. This would allow workers to target relevant chemical information from the environment and discriminate signal from noise without using energetically costly processing by the central nervous system. We argue that this sensory filter is flexible and regulated through changes in physiological conditions such as age, nutrition, and hormones. Variation among workers in their efficiency to detect specific chemical cues would result in task specialization and division of labor. The information perceived by the peripheral ORs is transmitted to the primary brain center of the olfactory pathway, the glomeruli of the antennal lobes. The question now arises whether there are differences between nurses and foragers in the morphology or physiology of the antennal lobes. A limited subset of active ORs and glomeruli might be easier to process and less energy consuming. Our study opens novel avenues of research to better understand the role of sensory filters in controlling DOL in insect societies.
Methods
Sample collection and behavioral determination
A total of seven colonies of the ant T. longispinosus were selected with an average colony size of 110 ± 31.5 workers (mean ± sd, Dataset S4) at the moment when the workers were sampled. The ants were collected in the forests of the Edmund Niles Huyck Preserve, Renssellearville, NY, USA (42°31′41.0′′N 74°09′38.8′′W), in June of 2018 with permission. Upon collection, we housed each colony in a plaster-floored nesting box (43 cm × 28 cm × 10 cm) divided into three chambers containing a single slide nest, in which the colony relocated. A slide nest is an artificial nesting site comprised of a small Plexiglas cavity sandwiched between two glass microscope slides. Colonies were established at the Johannes Gutenberg University in Mainz, Germany, under a 14 h:10 h light:dark photoperiod at 18 °C to a 22 °C temperature. We provided honey and water ad libitum and fed crickets to the colony twice a week. To allow for visible behavioral division of labor between workers of the two behavioral phenotypes, we marked, observed and recaptured ants from inside and outside the nest. We defined foragers as workers that perform outside-nest tasks, including gathering and searching for food and water and exploring the environment surrounding the nest, while nurses remained inside the dark nest and cared for the ant brood. A total of 69 workers inside (from the brood pile) and 76 workers outside the nest were marked with fine colored metal wires (0.02 mm Elektrisola, Eckenhagen, Germany). To immobilize the workers, they were placed with their heads and part of the thorax in a notch of a soft sponge without prior anesthesia. Then we marked the ants with a very thin loop around the petiole. It was then checked that the wires did not interfere with the ants’ movement. We performed behavioral observations every 2 h, four times a day for 5 days (total = 20 scans), in which we noted down how many times an individual performed brood care and foraging behavior and the position in the nest (Table S3). Based on these behavioral observations, the marked individuals found outside the nest, exploring the surroundings for food or water, were identified as foragers. These workers usually do not care for the brood and do not frequently reside on brood piles, as ant colonies organize themselves spatially in a way that reduces contact between foragers and brood156. We identified nurses as workers that remain inside the nest in direct contact with brood and were unlikely to leave the nest. Foragers were found in 42% ± 26% of the observations outside of the nest, whereas nurses spend only 1% ± 3% outside. In contrast, nurses were interacting with the brood in 54% ± 22% of the observations, whereas we found that foragers only did this only in 2% ± 4% of the observations. We scanned the behavior of workers over 20 observations, albeit earlier studies have shown that a single observation makes it possible to group T. longispinosus and other ants reliably into nurses and foragers that differ in behavior116,156, gene expression39 and CHC composition43. Furthermore, spatial location can alone can predict behavior in Temnothorax workers157. We focused in this study on individuals highly specialized on either foraging or brood care. Workers that performed both tasks regularly were not included in this study. After all observations were completed, the marked nurses and foragers were collected, directly frozen in liquid nitrogen and stored at -80 °C until further processing for dissection and pooling according to behavioral state and colony.
RNA extraction and sequencing
For RNA extraction, we removed both antennae and stored them in a 1.5 ml Eppendorf tube containing 50 μl TRIzol (Invitrogen), cut the head off and fixed it on a slide with melted dental wax. We then made an incision around the head with a surgical scalpel and removed the head capsule with forceps to expose the intact brain. Finally, we carefully pulled the brain out of the head capsule and removed the remains of other tissues that were connected to it. The dissected brain was transferred to a 1.5 ml Eppendorf tube containing 20 μl PBS. Each dissection was completed in less than 5 min to prevent RNA degradation. We dissected brain and antennae tissues from 48 nurses and 49 foragers. We pooled the brains and antennae from seven workers from each behavioral state and colony. The only exception was the “GO” colony (NY18 E110), for which we pooled only 6 brains and 12 antennae from 6 nurses (Dataset S4) due to the loss of one sample during the dissection process. Immediately after dissection of each brain and antennae, the Eppendorf tubes were kept on dry ice while we dissected the remaining individuals. Brain and antennae tissues were homogenized with a pestle. Sample brains were transferred separately to a 1.5 ml Eppendorf tube containing 50 μl of TRIzol. We added 50 μl chloroform to each brain and antenna samples, gently inverted for 30 s and then centrifuged samples at 12,000 × g for 15 min at 4 °C. We collected the resulting supernatant and precipitated RNA with 25 μl 70% ethanol. We conducted the subsequent RNA extraction with the RNeasy Mini Kit (Qiagen), following the manufacturer’s instruction. The resulting 28 samples (14 brains and 14 antennae) were stored at −80 °C until library preparation.
RNA-seq libraries were prepared by Novogene Company Limited, Cambridge, UK, using the NEBNext Ultra RNA Library Prep Kit for Illumina according to the manufacturer’s protocol. After amplification and purification, 28 libraries were sequenced on an Illumina NovaSeq 6000 S4 flow cell platform using a paired-end 150 bp. Approximately 43 million raw reads were generated from each library (Dataset S4).
Gene expression analyses
Raw data obtained from Novogene were checked using FastQC v.0.11.9158, and Illumina adapters were removed using Trimmomatic v.0.36159. The protein-coding genes of T. longispinosus together with the manual OR annotations (GCA_004794745.1)160; and the congener T. curvispinosus (GCA_003070985.1) were retrieved from the NCBI database and we used Liftoff v.1.6.1 tool161 to assign these annotations to the recently published T. longispinosus genome72. In total, 10,029 of 13,061 (~77%) annotated protein-coding genes were assigned from the original T. longispinosus assembly (genes identified as “DBV15”) and 4808 were assigned from T. curvispinosus (genes identified as “LOC”), for a total of 14,837. For gene expression analysis, reads were mapped to our T. longispinosus genome assembly, and the read counts table was generated using STAR 2.7.0162 with default settings. Detailed mapping statistics for each sample is available in Dataset S4. We used the deseq2 v1.16.1 package for R to identify differentially expressed genes163. To avoid biased results due to low read counts, we removed from the counting matrix those genes for which less than 10 of the reads mapped to at least 6 of our 14 samples (n − 1 of the smallest sample size). Then, we conducted a differential gene expression analysis with DESeq2164. We began with comparisons between nurses and foragers using the ~Colony+Task model, followed by a likelihood ratio test (LRT) approach, with colony ID as a fixed factor. Genes were considered differentially expressed if the false discovery rate (FDR), using Benjamini–Hochberg procedure, had an adjusted p value of ≤0.05. The resulting lists of DEGs refer to genes that are overexpressed and underexpressed in foragers compared to nurses. We used the online tool Venny v.2.1 (https://bioinfogp.cnb.csic.es/tools/venny) to generate a Venn diagram containing the DEGs associated with task and tissues. Separation of differentially expressed genes by task was visualized by performing principal component analysis (PCA) with a 95% confidence ellipse using the ggplot2 v3.4.2 package for R165. For PCA, we used the transformed reads of filtered transcriptomes from all contigs using the plotPCA function provided by DESeq2. Samples “GO”, “BG” and “YY” showed a divergent expression pattern. To ensure that our results were not influenced by these deviating samples, we re-run the DEseq2 analyses repeatedly removing sample after sample. This resulted show slight shifts in the number of DEGs in the antennae (2267 with all samples vs. 1960 without “GO”; 1861 without “BG”; and 1862 without “YY”) and in the brain (339 with all samples vs. 227 without “GO”; 272 without “BG”; and 346 without “YY”). However, the main findings remained similar and a large number of differentially expressed ORs were always found in all analyses (209 with all samples vs. 195 without “GO”; 209 without “BG”; and 179 without “YY”). Finally, OR genes that were upregulated in each behavioral phenotype were visualized in a volcano plot using ggplot2. All statistical tests and graphical visualizations were performed in RStudio v.1.4.1106166.
Identification of behavior candidate genes and ORs
We used gene annotations based on a BlastX search of the T. longispinosus transcriptome compared to a list of different invertebrate proteomes (i.e., Acromyrmex echinatior, Apis mellifera, Camponotus floridanus, Drosophila melanogaster, Harpegnathos saltator, Odontomachus brunneus, Temnothorax curvispinosus) downloaded from the NCBI database with an E-value of 1e−5 and below. Clusters containing more than one sequence match per species were reduced to a single specimen based on the highest blast score. We constructed orthogroups across all of the above species using OrthoFinder167, including amino acid sequences from the T. longispinosus proteome160, and retained orthogroups containing caste DEGs (Dataset S5) to again compare potential behavioral candidate genes previously identified as involved in regulating the division of labor in social insects39,42,94,100,155,168,169,170,171. GO enrichment analysis was performed with TopGO v.2.44.0 for R using a Fisher’s exact test for the different gene sets compared to the whole genome with the weight01 algorithm172. Only annotated GO terms with a p value of ≤0.05 were considered significantly enriched.
OR protein sets were clustered across multiple ant species using OrthoFinder to derive orthologous groups and identify subfamilies for each OR in T. longispinosus. To associate orthogroups with previously identified OR subfamilies, we used OR annotation in Atta cephalotes, Acromyrmex echinatior from Engsontia et al.67, and Camponotus floridanus, Harpegnathos saltator, and Solenopsis invicta from Zhou et al.63,66. Missing subfamily information was labeled as “unassigned” (Dataset S3).
Statistics and reproducibility
The experiments were performed in seven replicates. Each sample contains the pooled RNA from either the antennae or brain of seven ant workers of the respective behavioral phenotype belonging to the same colony, with the colony representing the level of replicates. Bar graphs show next to median and quartiles, the individual data points. Statistical analyses were performed in RStudio v.1.4.1106, bioinformatics analyses in Bash, and scripts for both are available on Mendeley. Gene expression analyses were performed using DEseq2, and to exclude the influence of outliers, these were removed individually, and results presented without them.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Numerical source data found in Supplementary Data S1 was used to create Fig. 1A and Data S2 for Figs. 2B, C and 3. Raw sequencing reads generated for this study have been deposited in NCBI under BioProject PRJNA926589. Any remaining information can be obtained from the corresponding author upon request.
Code availability
Data analysis and visualization for this study was done using code written in R and Bash, which can be found on Mendeley Data (https://doi.org/10.17632/yyg46xmph6.3).
References
Maynard Smith, J. & Szathmáry, E. The Major Evolutionary Transitions in Evolution (Oxford University Press, 1995).
Smith, A. An Inquiry into the Nature and Causes of the Wealth of Nations (University Chicago Press, 1776).
Veening, J. W. et al. Transient heterogeneity in extracellular protease production by Bacillus subtilis. Mol. Syst. Biol. 4, 1–15 (2008).
Marlow, V. L. et al. The prevalence and origin of exoproteaseproducing cells in the Bacillus subtilis biofilm. Microbiology 160, 56–66 (2014).
van Gestel, J., Vlamakis, H. & Kolter, R. From cell differentiation to cell collectives: bacillus subtilis uses division of labor to migrate. PLoS Biol. 13, 1–29 (2015).
West, S. A. & Cooper, G. A. Division of labour in microorganisms: an evolutionary perspective. Nat. Rev. Microbiol. 14, 716–723 (2016).
Oster, G. F. & Wilson, E. O. Caste and ecology in the social insects. Acta Biotheor. 28, 234–235 (1979).
Wilson, E. O. The ergonomics of caste in the social insects. Am. Nat. 102, 41–66 (1968).
Wilson, E. O. Behavioral discretization and the number of castes in an ant species. Behav. Ecol. Sociobiol. 1, 141–154 (1976).
Herbers, J. M. Social social organization in Leptothorax ants: within-and between-species patterns. Psyche J. Entomol. 90, 52489 (1983).
Pinter-Wollman, N., Hubler, J., Holley, J. A., Franks, N. R. & Dornhaus, A. How is activity distributed among and within tasks in Temnothorax ants? Behav. Ecol. Sociobiol. 66, 1407–1420 (2012).
Seeley, T. D. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav. Ecol. Sociobiol. 11, 287–293 (1982).
Tripet, F. & Nonacs, P. Foraging for work and age-based polyethism: the roles of age and previous experience on task choice in ants. Ethology 110, 863–877 (2004).
Blanchard, G. B., Orledge, G. M., Reynolds, S. E. & Franks, N. R. Division of labour and seasonality in the ant Leptothorax albipennis: worker corpulence and its influence on behaviour. Anim. Behav. 59, 723–738 (2000).
Toth, A. L. & Robinson, G. E. Worker nutrition and division of labour in honeybees. Anim. Behav. 69, 427–435 (2005).
Wetterer, J. K. The ecology and evolution of worker size-distribution in leaf-cutting ants (Hymenoptera: Formicidae). Sociobiology 34, 119–144 (1999).
Fewell, J. H. & Page, R. E. Genotypic variation in foraging responses to environmental stimuli by honey bees, Apis mellifera. Experientia 49, 1106–1112 (1993).
Graham, S., Myerscough, M. R., Jones, J. C. & Oldroyd, B. P. Modelling the role of intracolonial genetic diversity on regulation of brood temperature in honey bee (Apis mellifera L.) colonies. Insectes Soc. 53, 226–232 (2006).
Gove, R., Hayworth, M., Chhetri, M. & Rueppell, O. Division of labour and social insect colony performance in relation to task and mating number under two alternative response threshold models. Insectes Soc. 56, 319–331 (2009).
Ravary, F., Lecoutey, E., Kaminski, G., Châline, N. & Jaisson, P. Individual experience alone can generate lasting division of labor in ants. Curr. Biol. 17, 1308–1312 (2007).
Gautrais, J., Theraulaz, G., Deneubourg, J. L. & Anderson, C. Emergent polyethism as a consequence of increased colony size in insect societies. J. Theor. Biol. 215, 363–373 (2002).
Merkle, D. & Middendorf, M. Dynamic polyethism and competition for tasks in threshold reinforcement models of social insects. Adapt Behav. 12, 251–262 (2004).
Jeanson, R., Fewell, J. H., Gorelick, R. & Bertram, S. M. Emergence of increased division of labor as a function of group size. Behav. Ecol. Sociobiol. 62, 289–298 (2007).
Wilson, E. O. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behav. Ecol. Sociobiol. 7, 157–165 (1980).
Wakano, J. Y., Nakata, K. & Yamamura, N. Dynamic model of optimal age polyethism in social insects under stable and fluctuating environments. J. Theor. Biol. 193, 153–165 (1998).
Iwasa, Y. & Yamaguchi, S. Task allocation in a cooperative society: specialized castes or age-dependent switching among ant workers. Sci. Rep. 10, 1–9 (2020).
Robinson, E. J. H., Feinerman, O. & Franks, N. R. Flexible task allocation and the organization of work in ants. Proc. R. Soc. B Biol. Sci. 276, 4373–4380 (2009).
Shimoji, H., Kasutani, N., Ogawa, S. & Hojo, M. K. Worker propensity affects flexible task reversion in an ant. Behav. Ecol. Sociobiol. 74, 92 (2020).
Ben-Shahar, Y., Robichon, A., Sokolowski, M. B. & Robinson, G. E. Influence of gene action across different time scales on behavior. Science 296, 741–744 (2002).
Ben-Shahar, Y., Leung, H. T., Pak, W. L., Sokolowski, M. B. & Robinson, G. E. cGMP-dependent changes in phototaxis: a possible role for the foraging gene in honey bee division of labor. J. Exp. Biol. 206, 2507–2515 (2003).
Whitfield, C. W., Cziko, A. M. & Robinson, G. E. Gene expression profiles in the brain predict behavior in individual honey bees. Science 302, 296–299 (2003).
Ingram, K. K., Oefner, P. & Gordon, D. M. Task-specific expression of the foraging gene in harvester ants. Mol. Ecol. 14, 813–818 (2005).
Lucas, C. & Sokolowski, M. B. Molecular basis for changes in behavioral state in ant social behaviors. Proc. Natl Acad. Sci. USA 106, 6351–6356 (2009).
Zayed, A. & Robinson, G. E. Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee. Annu. Rev. Genet. 46, 591–615 (2012).
Sullivan, J. P., Jassim, O., Fahrbach, S. E. & Robinson, G. E. Juvenile hormone paces behavioral development in the adult worker honey bee. Horm. Behav. 37, 1–14 (2000).
Ament, S. A., Corona, M., Pollock, H. S. & Robinson, G. E. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl Acad. Sci. USA 105, 4226–4231 (2008).
Dolezal, A. G., Brent, C. S., Hölldobler, B. & Amdam, G. V. Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. J. Exp. Biol. 215, 454–460 (2012).
Corona, M. et al. Vitellogenin underwent subfunctionalization to acquire caste and behavioral specific expression in the harvester ant Pogonomyrmex barbatus. PLoS Genet. 9, e1003730 (2013).
Kohlmeier, P., Alleman, A. R., Libbrecht, R., Foitzik, S. & Feldmeyer, B. Gene expression is more strongly associated with behavioural specialization than with age or fertility in ant workers. Mol. Ecol. 28, 658–670 (2019).
Libbrecht, R. et al. Interplay between insulin signaling, juvenile hormone, and vitellogenin regulates maternal effects on polyphenism in ants. Proc. Natl Acad. Sci. USA 110, 11050–11055 (2013).
Marco Antonio, D. S., Guidugli-Lazzarini, K. R., Do Nascimento, A. M., Simões, Z. L. P. & Hartfelder, K. RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften 95, 953–961 (2008).
Gospocic, J. et al. The neuropeptide corazonin controls social behavior and caste Identity in ants. Cell 170, 748.e12–759.e12 (2017).
Kohlmeier, P., Feldmeyer, B. & Foitzik, S. Vitellogenin-like A–associated shifts in social cue responsiveness regulate behavioral task specialization in an ant. PLoS Biol. 16, 1–26 (2018).
Kamhi, J. F. & Traniello, J. F. A. Biogenic amines and collective organization in a superorganism: neuromodulation of social behavior in ants. Brain Behav. Evol. 82, 220–236 (2013).
Schulz, D. & Robinson, G. Biogenic amines and division of labor in honey bee colonies: behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J. Comp. Physiol. A 184, 481–488 (1999).
Mercer, A. R. & Menzel, R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J. Comp. Physiol. 145, 363–368 (1982).
Scheiner, R., Entler, B. V., Barron, A. B., Scholl, C. & Thamm, M. The effects of fat body tyramine level on gustatory responsiveness of honeybees (Apis mellifera) differ between behavioral castes. Front. Syst. Neurosci. 11, 1–8 (2017).
Ferguson, S. T., Bakis, I. & Zwiebel, L. J. Advances in the study of olfaction in eusocial ants. Insects 12, 252 (2021).
Martin, S. & Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 35, 1151–1161 (2009).
van Wilgenburg, E., Symonds, M. R. E. & Elgar, M. A. Evolution of cuticular hydrocarbon diversity in ants. J. Evol. Biol. 24, 1188–1198 (2011).
Slifer, E. H. The structure of arthropod chemoreceptors. Annu. Rev. Entomol. 15, 121–142 (1970).
Altner, H. & Prillinger, L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 67, 69–139 (1980).
Hallberg, E. & Hansson, B. S. Arthropod sensilla: morphology and phylogenetic considerations. Microsc. Res. Tech. 47, 428–439 (1999).
Wicher, D. & Miazzi, F. Functional properties of insect olfactory receptors: ionotropic receptors and odorant receptors. Cell Tissue Res. 383, 7–19 (2021).
Leal, W. S. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391 (2013).
Sánchez‐Gracia, A., Vieira, F. G., Almeida, F. C. & Rozas, J. Comparative genomics of the major chemosensory gene families in arthropods. Encyclopedia Life Sci. https://doi.org/10.1002/9780470015902.a0022848 (2011).
Breer, H., Fleischer, J., Pregitzer, P. & Krieger, J. in Olfactory Concepts of insect Control-Alternative to Insecticides (ed. Picimbon, J.-F.) 93–114 (Springer, 2019).
Jacquin-Joly, E. & Lucas, P. Pheromone reception and transduction: mammals and insects illustrate converging mechanisms across phyla. Curr. Top. Neurochem. 4, 75–105 (2005).
Wilson, R. K. & Mainen, Z. F. Early events in olfactory processing. Annu. Rev. Neurosci. 29, 163–201 (2006).
Anton, S. & Homberg, U. in Insect Olfaction (ed. Hansson, B.S.) 97–124 (Springer, 1999).
Hansson, B. S. & Anton, S. Function and morphology of the antennal lobe: new developments. Annu. Rev. Entomol. 45, 203–231 (2000).
de Belle, J. S. & Kanzaki, R. in Insect Olfaction (ed. Hansson, B.S.) 243–281 (Springer, 1999).
Zhou, X. et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 8, e1002930 (2012).
Saad, R., Cohanim, A. B., Kosloff, M. & Privman, E. Neofunctionalization in ligand binding sites of ant olfactory receptors. Genome Biol. Evol. 10, 2490–2500 (2018).
Oxley, P. R. et al. The genome of the clonal raider ant Cerapachys Biroi. Curr. Biol. 24, 451–458 (2014).
Zhou, X. et al. Chemoreceptor evolution in Hymenoptera and its implications for the evolution of eusociality. Genome Biol. Evol. 7, 2407–2416 (2015).
Engsontia, P., Sangket, U., Robertson, H. M. & Satasook, C. Diversification of the ant odorant receptor gene family and positive selection on candidate cuticular hydrocarbon receptors. BMC Res. Notes 8, 1–13 (2015).
Smith, C. D. et al. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl Acad. Sci. USA 108, 5673–5678 (2011).
Smith, C. R. et al. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc. Natl Acad. Sci. USA 108, 5667–5672 (2011).
McKenzie, S. K., Fetter-Pruneda, I., Ruta, V. & Kronauer, D. J. C. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc. Natl Acad. Sci. USA 113, 14091–14096 (2016).
Pask, G. M. et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun. 8, 1–10 (2017).
Jongepier, E. et al. Convergent loss of chemoreceptors across independent origins of slave-making in ants. Mol. Biol. Evol. 39, 1–14 (2022).
Trible, W. et al. orco Mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170, 727.e10–735.e10 (2017).
Yan, H. et al. An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170, 736.e9–747.e9 (2017).
Beshers, S. & Fewell, J. H. Models of division of labor in social insects. Annu. Rev. Entomol. 46, 413–440 (2001).
Lichocki, P., Tarapore, D., Keller, L. & Floreano, D. Neural networks as mechanisms to regulate division of labor. Am. Nat. 179, 391–400 (2012).
Naug, D. From division of labor to collective behavior: behavioral analyses at different levels. Behav. Ecol. Sociobiol. 70, 1113–1115 (2016).
Jeanson, R. & Weidenmüller, A. Interindividual variability in social insects - proximate causes and ultimate consequences. Biol. Rev. 89, 671–687 (2014).
Ulrich, Y. et al. Response thresholds alone cannot explain empirical patterns of division of labor in social insects. PLoS Biol. 19, 1–21 (2021).
Yamanaka, O., Shiraishi, M., Awazu, A. & Nishimori, H. Verification of mathematical models of response threshold through statistical characterisation of the foraging activity in ant societies. Sci. Rep. 9, 1–8 (2019).
Shiraishi, M., Yamanaka, O. & Nishimori, H. Effect of interaction network structure in a response threshold model. Artif. Life Robot. 27, 743–750 (2022).
Theraulaz, G., Bonabeau, E. & Deneubourg, J. L. Response threshold reinforcement and division of labour in insect societies. Proc. R. Soc. B Biol. Sci. 265, 327–332 (1998).
Bonabeau, E., Theraulaz, G. & Deneubourg, J. L. Quantitative study of the fixed threshold model for the regulation of division of labour in insect societies. Proc. R. Soc. B Biol. Sci. 263, 1565–1569 (1996).
Bonabeau, E., Theraulaz, G. & Deneubourg, J. L. Fixed response thresholds and the regulation of division of labor in insect societies. Bull. Math. Biol. 60, 753–807 (1998).
Bonabeau, E., Sobkowski, A., Theraulaz, G. & Deneubourg, J. L. Adaptive task allocation inspired by a model of division of labor in social insects. In: Proc. BCEC97 36–45 (ACM, 1997).
Thiagarajan, D. & Sachse, S. Multimodal information processing and associative learning in the insect brain. Insects 13, 332 (2022).
Raza, M. F. et al. Biogenic amines mediate learning success in appetitive odor conditioning in honeybees. J. King Saud. Univ. Sci. 34, 0–6 (2022).
Schulz, D. J., Elekonich, M. M. & Robinson, G. E. Biogenic amines in the antennal lobes and the initiation and maintenance of foraging behavior in honey bees. J. Neurobiol. 54, 406–416 (2003).
Zhukovskaya, M. I. & Polyanovsky, A. D. Biogenic amines in insect antennae. Front. Syst. Neurosci. 11, 1–9 (2017).
Cook, C. N. et al. Individual differences in learning and biogenic amine levels influence the behavioural division between foraging honeybee scouts and recruits. J. Anim. Ecol. 88, 236–246 (2019).
Azevedo, D. O., De Paula, S. O., Zanuncio, J. C., Martinez, L. C. & Serrão, J. E. Juvenile hormone downregulates vitellogenin production in Ectatomma tuberculatum (Hymenoptera: Formicidae) sterile workers. J. Exp. Biol. 219, 103–108 (2016).
Rodrigues, M. A. & Flatt, T. Endocrine uncoupling of the trade-off between reproduction and somatic maintenance in eusocial insects. Curr. Opin. Insect Sci. 16, 1–8 (2016).
Jedlicka, P., Ernst, U. R., Votavová, A., Hanus, R. & Valterová, I. Gene expression dynamics in major endocrine regulatory pathways along the transition from solitary to social life in a bumblebee, Bombus terrestris. Front. Physiol. 7, 1–20 (2016).
Opachaloemphan, C. et al. Early behavioral and molecular events leading to caste switching in the ant Harpegnathos. Genes Dev. 35, 410–424 (2021).
Norman, V. C. & Hughes, W. O. H. Behavioural effects of juvenile hormone and their influence on division of labour in leaf-cutting ant societies. J. Exp. Biol. 219, 8–11 (2016).
Robinson, G. E. Regulation of honey bee age polyethism by juvenile hormone. Behav. Ecol. Sociobiol. 20, 329–338 (1987).
Fussnecker, B. & Grozinger, C. Dissecting the role of Kr-h1 brain gene expression in foraging behavior in honey bees (Apis mellifera). Insect Mol. Biol. 17, 515–522 (2008).
Grozinger, C. M. & Robinson, G. E. Endocrine modulation of a pheromone-responsive gene in the honey bee brain. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 461–470 (2007).
Shpigler, H. et al. The transcription factor Krüppel homolog 1 is linked to hormone mediated social organization in bees. BMC Evol. Biol. 10, 120 (2010).
Gospocic, J. et al. Kr-h1 maintains distinct caste-specific neurotranscriptomes in response to socially regulated hormones. Cell 184, 5807–5823.e14 (2021).
Warrant, E. J. Sensory matched filters. Curr. Biol. 26, 976–980 (2016).
Ponnath, A., Ryan, M. J., Fang, Z. & Farris, H. E. Tuned in to communication sounds: neuronal sensitivity in the túngara frog midbrain to frequency modulated signals. PLoS ONE 17, e0268383 (2022).
Hempel De Ibarra, N., Holtze, S., Bäucker, C., Sprau, P. & Vorobyev, M. The role of colour patterns for the recognition of flowers by bees. Philos. Trans. R. Soc. B Biol. Sci. 377, 20210284 (2022).
Patullo, B. W. & Macmillan, D. L. Making sense of electrical sense in crayfish. J. Exp. Biol. 213, 651–657 (2010).
von der Emde, G. & Warrant, E. The Ecology of Animal Senses Matched Filters for Cconomical Sensing 1st edn (Springer International Publishing, 2016).
Carey, A. F., Wang, G., Su, C. Y., Zwiebel, L. J. & Carlson, J. R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 (2010).
Dornhaus, A. Specialization does not predict individual efficiency in an ant. PLoS Biol. 6, 2368–2375 (2008).
Koch, C. & Laurent, G. Complexity and the nervous system. Science 284, 96–98 (1999).
McCaffrey, J. B. The brain’s heterogeneous functional landscape. Philos. Sci. 82, 1010–1022 (2015).
Ito, M., Masuda, N., Shinomiya, K., Endo, K. & Ito, K. Systematic analysis of neural projections reveals clonal composition of the Drosophila brain. Curr. Biol. 23, 644–655 (2013).
Kennedy, A. et al. Use of waggle dance information in honey bees is linked to gene expression in the antennae, but not in the brain. Mol. Ecol. 30, 2676–2688 (2021).
Chandra, V. et al. Social regulation of insulin signaling and the evolution of eusociality in ants. Science 361, 398–402 (2018).
Libbrecht, R. & Keller, L. Genetic compatibility affects division of labor in the argentine ant Linepithema humile. Evolution 67, 517–524 (2013).
Schulz, D. J., Sullivan, J. P. & Robinson, G. E. Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm. Behav. 42, 222–231 (2002).
Geva, S., Hartfelder, K. & Bloch, G. Reproductive division of labor, dominance, and ecdysteroid levels in hemolymph and ovary of the bumble bee Bombus terrestris. J. Insect Physiol. 51, 811–823 (2005).
Kohlmeier, P. et al. Intrinsic worker mortality depends on behavioral caste and the queens’ presence in a social insect. Sci. Nat. 104, 34 (2017).
Gadenne, C., Barrozo, R. B. & Anton, S. Plasticity in insect olfaction: to smell or not to smell? Annu. Rev. Entomol. 61, 317–333 (2016).
Zhou, S., Stone, E. A., Mackay, T. F. C. & Anholt, R. R. H. Plasticity of the chemoreceptor repertoire in Drosophila melanogaster. PLoS Genet. 5, e1000681 (2009).
Von Der Weid, B. et al. Large-scale transcriptional profiling of chemosensory neurons identifies receptor-ligand pairs in vivo. Nat. Neurosci. 18, 1455–1463 (2015).
Benton, R., Sachse, S., Michnick, S. W. & Vosshall, L. B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4, 240–257 (2006).
Krieger, J., Klink, O., Mohl, C., Raming, K. & Breer, H. A candidate olfactory receptor subtype highly conserved across different insect orders. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 189, 519–526 (2003).
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004).
Guo, H., Kunwar, K. & Smith, D. Odorant receptor sensitivity modulation in Drosophila. J. Neurosci. 37, 9465–9473 (2017).
Castillo, P., Husseneder, C. & Sun, Q. Molecular characterization and expression variation of the odorant receptor co-receptor in the Formosan subterranean termite. PLoS ONE 17, e0267841 (2022).
Sato, K. et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (2008).
del Mármol, J., Yedlin, M. A. & Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 597, 126–131 (2021).
Mersch, D. P., Crespi, A. & Keller, L. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340, 1090–1093 (2013).
Richardson, T. O. et al. Ant behavioral maturation is mediated by a stochastic transition between two fundamental states. Curr. Biol. 31, 2253–2260.e3 (2021).
Schrader, L. et al. Relaxed selection underlies genome erosion in socially parasitic ant species. Nat. Commun. 12, 1–13 (2021).
Slone, J. D. et al. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. Proc. Natl Acad. Sci. USA 114, 8586–8591 (2017).
Claudianos, C. et al. Odor memories regulate olfactory receptor expression in the sensory periphery. Eur. J. Neurosci. 39, 1642–1654 (2014).
Barbero, F., Mannino, G. & Casacci, L. P. The role of biogenic amines in social insects: with a special focus on ants. Insects 14, 386 (2023).
Penick, C. A., Brent, C. S., Dolezal, K. & Liebig, J. Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J. Exp. Biol. 217, 1496–1503 (2014).
Fussnecker, B. L., Smith, B. H. & Mustard, J. A. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera). J. Insect Physiol. 52, 1083–1092 (2006).
Yakovlev, I. K. Effects of octopamine on aggressive behavior in red wood ants. Neurosci. Behav. Physiol. 48, 279–288 (2018).
Yaguchi, H., Inoue, T., Sasaki, K. & Maekawa, K. Dopamine regulates termite soldier differentiation through trophallactic behaviours. R. Soc. Open Sci. 3, 150574 (2016).
Ohta, H. & Ozoe, Y. in Advances in Insect Physiology 1st edn, Vol. 46 (ed, Cohen, E.) 73–166 (Elsevier Inc., 2014).
Beggs, K. T., Tyndall, J. D. A. & Mercer, A. R. Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 6, e26809 (2011).
Blenau, W. & Baumann, A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. Physiol. 48, 13–38 (2001).
Vleugels, R., Verlinden, H. & Broeck, J. V. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2, e314 (2015).
Dacks, A. M., Dacks, J. B., Christensen, T. A. & Nighorn, A. J. The cloning of one putative octopamine receptor and two putative serotonin receptors from the tobacco hawkmoth, Manduca sexta. Insect Biochem. Mol. Biol. 36, 741–747 (2006).
Getahun, M. N., Olsson, S. B., Lavista-Llanos, S., Hansson, B. S. & Wicher, D. Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors. PLoS ONE 8, 1–9 (2013).
Stengl, M. & Funk, N. W. The role of the coreceptor Orco in insect olfactory transduction. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 897–909 (2013).
Blundell, J. E. & Halford, J. C. G. Serotonin and appetite regulation: implications for the pharmacological treatment of obesity. CNS Drugs 9, 473–495 (1998).
French, A. S. et al. The role of serotonin in feeding and gut contractions in the honeybee. J. Insect Physiol. 61, 8–15 (2014).
Voigt, J. P. & Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 277, 14–31 (2015).
Beninger, R. J. The role of dopamine in locomotor activity and learning. Brain Res. Rev. 6, 173–196 (1983).
Liang, X. et al. Morning and evening circadian pacemakers independently drive premotor centers via a specific dopamine relay. Neuron 102, 843.e4–857.e4 (2019).
Libbrecht, R., Nadrau, D. & Foitzik, S. A role of histone acetylation in the regulation of circadian rhythm in ants. iScience 23, 100846 (2020).
Root, C. M., Ko, K. I., Jafari, A. & Wang, J. W. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–144 (2011).
Anton, S., Dufour, M. C. & Gadenne, C. P. Plasticity of olfactory-guided behaviour and its neurobiological basis: lessons from moths and locusts. Entomol. Exp. Appl. 123, 1–11 (2007).
Tawfik, A. I., Osir, E. O., Hassanali, A. & Ismail, S. H. Effects of juvenile hormone treatment on phase changes and pheromone production the desert locust, Schistocerca gregaria (Forskal) (Orthoptera: Acrididae). J. Insect Physiol. 43, 1177–1182 (1997).
Tawfik, A. I., Treiblmayr, K., Hassanali, A. & Osir, E. O. Time-course haemolymph juvenile hormone titres in solitarious and gregarious adults of Schistocerca gregaria, and their relation to pheromone emission, CA volumetric changes and oocyte growth. J. Insect Physiol. 46, 1143–1150 (2000).
Wiesel, G., Tappermann, S. & Dorn, A. Effects of juvenile hormone and juvenile hormone analogues on the phase behaviour of Schistocerca gregaria and Locusta migratoria. J. Insect Physiol. 42, 385–395 (1996).
Guo, W. et al. Juvenile hormone suppresses aggregation behavior through influencing antennal gene expression in locusts. PLoS Genet. 16, 1–18 (2020).
Pamminger, T., Foitzik, S., Kaufmann, K. C., Schützler, N. & Menzel, F. Worker personality and its association with spatially structured division of labor. PLoS ONE 9, 1–8 (2014).
Sendova-Franks, A. & Franks, N. R. Task allocation in ant colonies within variable environments (a study of temporal polyethism: experimental). Bull. Math. Biol. 55, 75–96 (1993).
Andrews, S. A quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kaur, R. et al. Ant behaviour and brain gene expression of defending hosts depend on the ecological success of the intruding social parasite. Philos. Trans. R. Soc. B Biol. Sci. 374, 1769 (2019).
Shumate, A. & Salzberg, S. L. Liftoff: accurate mapping of gene annotations. Bioinformatics 37, 1639–1643 (2021).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Love, M. I., Anders, S. & Huber, W. Differential analysis of count data - the DESeq2 package. Genome Biol. 15, 550 (2014).
Wickham, H. ggplot2: Elegant graphics for data analysis. https://ggplot2.tidyverse.org (2016).
RStudio Team. RStudio: integrated development for R. http://www.rstudio.com/ (2020).
Emms, D. M. & Kelly, S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015).
Das, B. & de Bekker, C. Time-course RNASeq of Camponotus floridanus forager and nurse ant brains indicate links between plasticity in the biological clock and behavioral division of labor. BMC Genomics. 23, 1–23 (2022).
Alleman, A., Stoldt, M., Feldmeyer, B. & Foitzik, S. Tandem-running and scouting behaviour are characterized by up-regulation of learning and memory formation genes within the ant brain. Mol. Ecol. 28, 2342–2359 (2019).
Korb, J. et al. Comparative transcriptomic analysis of the mechanisms underpinning ageing and fecundity in social insects. Philos. Trans. R. Soc. B Biol. Sci. 376, 20190728 (2021).
Qiu, B. et al. Canalized gene expression during development mediates caste differentiation in ants. Nat. Ecol. Evol. 6, 1753–1765 (2022).
Alexa, A. & Rahnenfuhrer, J. topGO: enrichment analysis for gene ontology. R package version 2.50.0. https://bioconductor.org/packages/release/bioc/html/topGO.html (2018).
Acknowledgements
We thank Diego Páez-Moscoso for many helpful discussions and comments throughout the study, Marah Stoldt and Carlotta Martelli for providing helpful comments on the manuscript, Marion Kever and Jenny Fuchs for technical assistance, and Mark Harrison for providing the manual OR annotations. This project was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) to SF – GRK2526/1 – Projectnr. 407023052. M.A.C. thanks the E.N. Huyck Preserve for support and funding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.A.C., R.L., and S.F. conceived the study; M.A.C. conducted the research; M.A.C., M.M., R.L., and D.V.H. contributed to the development of the data analytical protocol; M.A.C. analyzed the data; and M.A.C., R.L., M.M., P.B., and S.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Ching-Han Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editors: Luke Grinham, Karli Montague-Cardoso, David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caminer, M.A., Libbrecht, R., Majoe, M. et al. Task-specific odorant receptor expression in worker antennae indicates that sensory filters regulate division of labor in ants. Commun Biol 6, 1004 (2023). https://doi.org/10.1038/s42003-023-05273-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05273-4
This article is cited by
-
Reproductive potentials of task-shifting workers in a queenless ant
Insectes Sociaux (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.