Abstract
CRISPR-Cas systems endow the bacterial and archaeal species with adaptive immune mechanisms to fend off invading phages and foreign plasmids. The class 2 type VI CRISPR/Cas effector Cas13d has been harnessed to confer the protection against RNA viruses in diverse eukaryotic species. However a vast number of different viruses can potentially infect the same host plant resulting in mixed infection, thus necessitating the generation of crops with broad-spectrum resistance to multiple viruses. Here we report the repurposing of CRISPR/Cas13d coupled with an endogenous tRNA-processing system (polycistronic tRNA-gRNA, PTG) to target the multiple potato RNA viruses. Expression of Cas13d and four different gRNAs were observed in transgenic potato lines expressing the Cas13d/PTG construct. We show that the Cas13d/PTG transgenic plants exhibit resistance to either PVY, PVS, PVX or PLRV alone or two/three viruses simultaneously by reducing viral accumulation in plant cells. In sum, our findings provide an efficient strategy for engineering crops that can simultaneously resist infection by multiple RNA viruses.
Similar content being viewed by others
Introduction
Plant viruses are obligate intracellular parasites replicating themselves by using the molecular machinery of their hosts. Plant viral diseases can cause severe yield loss and continuously threaten crop production worldwide1,2,3. Diverse approaches of controlling RNA viruses in plants have been attempted including conventional breeding, genetic engineering and RNA interference4. However, due to the high rate of mutation and recombination of viruses, the virus-resistant plants could be overcome in a few years5. In addition, many plant viruses evolve and often develop various counter-defense mechanisms, such as viral suppressors of RNA silencing, nullifying the current antiviral approaches6,7.
Potato (Solanum tuberosum L.) is the world’s most important non-grain crop8 which is also sensitive to various biotic and abiotic stresses. Viruses are among the most significant biotic constraints in potato production. Potato is clonally propagated by planting tubers, further increasing the risk of virus infection. Until now, more than 50 different viruses have been reported to be able to infect potatoes worldwide. Among them, potato leaf roll virus (PLRV), potato virus Y (PVY), potato virus X (PVX) and potato virus S (PVS) have been recognized as the major potato viruses3,9. These four RNA viruses are all positive-sense single-stranded RNA (+ssRNA). PLRV is a type species of the genus Polerovirus, belonging to the family Solemoviridae10,11. An intriguing feature of PLRV is the phloem restriction. It can be delivered into phloem tissues by aphids, agro-inoculation or grafting, while not be transmitted by mechanical inoculation. Besides, PVY is a member of the genus Potyvirus in the family Potyviridae and is transmitted by aphids in a non-persistent manner or through potato tubers12. PVY is prone to high mutation and recombination rates, existing as a complex of strain groups, or variants13,14. On the other hand, PVS belongs to genus Carlavirus in the family Flexiviridae15,16. PVS can be transmitted by plant contact or several aphid species in a non-persistent manner17. Apart from this, PVX is a type species of the genus Potexvirus in the family Alphaflexiviridae18. The symptoms induced by PVY or PLRV are visible when infected with single virus, such as mosaic, mottled and crinkled leaves as well as leaf and vein necrosis in PVY infection14,19, and leaf rolling and stunting in PLRV infection10. Upon infection with a specific virus, PVY and PLRV are among the most damaging viruses of potato worldwide9,20,21. However, in the field, potato is usually challenged by multiple species of RNA viruses, which result in more significant loss of tuber quality and quantity22. For example, PVX or PVS causes varying degrees of tuber yield losses in the single infection, while the symptom and production loss can be more severe in the mixed infection3,23.
The CRISPR/Cas system (Clustered Regularly Interspaced Short Palindromic Repeats associated proteins) is emerging as a promising tool for gene editing and sequence-specific regulation of gene expression24,25,26. The Cas9 and Cas12 of Class II were employed to combat DNA viruses in eukaryotes27,28. The type VI-A effector Cas13a (previously known as C2c2) has been characterized for cleaving single-stranded RNA29 and designed to interfere with viral RNA replication against the turnip mosaic virus via transient transformation assays30. Consistent with this, the CRISPR/Cas13a system can be engineered to target PVY genome and confer resistance to PVY in the transgenic potato plants by the stable transformation31. Similarly, CRISPR/Cas13a system could enable the plant to acquire potent defense against viral infection in both tobacco and rice plants32.
Recently, a new Cas13 subtype, type VI-D (Cas13d), has been identified. Cas13d effector is relatively smaller compared to previously reported subtypes. Moreover, Cas13d was shown to have more robust RNA virus interference applications compared to LwaCas13a and PspCas13b variants in Nicotiana benthamiana33, making it suitable for diverse RNA viruses targeting or detection34.
It has been demonstrated that numerous gRNAs can be generated by employing the endogenous tRNA-processing system of plant cells, enabling the simultaneous targeting of multiple genomic loci35. To produce multi-virus-resistant plants, we sought to develop a robust multiple RNA-targeting CRISPR/Cas13d system conferring resistance to different RNA viruses. We show that CRISPR/Cas13d assembled with a PTG gene can reduce the multiplexed viral accumulation in transgenic potato plants, providing a new strategy for conferring broad-spectrum resistance to viral diseases in crops.
Result
Engineering a tRNA-processing System for Producing Four gRNAs
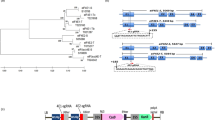
To target multiple RNA viruses simultaneously, we constructed a binary vector (pPTG) harboring a synthetic Cas13d gene (fusion with a 3 × HA tag sequence) from Ruminococcus flavefaciens XPD3002 (CasRx) and a polycistronic tRNA-gRNA (PTG) gene for simultaneous production of four gRNAs (Fig. 1a; Supplementary Fig. 1), targeting the CP genes of four potato viruses (PVS, PVY, PLRV and PVX). The expression of Cas13d and PTG genes is driven by the UBQ10 (Arabidopsis ubiquitin-10) and AtU6 promoters, respectively (Fig. 1b). Each sgRNA contains an oligo (A)-rich tail, which serves as a signal for nuclear export36.
a Schematic depiction of the Cas13d/PTG system for simultaneously targeting multiple viruses. The synthetic PTG consists of tandemly arrayed tRNA-gRNA units, with each gRNA containing a sequence-specific spacer designed for targeting one RNA virus genome (labeled as diamonds with different colors) and conserved gRNA scaffold (gray rectangle). The tRNAs are shown as red rectangles. The primary transcript of PTG is cleaved by endogenous RNase P and RNase Z (labeled as scissors) to release mature gRNAs and tRNA (lines of cloverleaf structure). The excised mature gRNAs containing four individual gRNA (PVS-sgRNA, PVX-sgRNA, PLRV-sgRNA and PVY-sgRNA), directing Cas13d to four RNA virus genomes. The oligo(A) motif of the sgRNA serves as a signal for nuclear export. b Map of the T-DNA locus in transgenic potato lines transformed with a construct for expression of Cas13d/PTG (St-PTG). PTG contains four gRNAs targeting four RNA virus genomes as shown in (a). 3 × HA: hemagglutinin epitope. c Analysis of the Cas13d expression in the transgenic potato lines by western blotting. Anti-HA antibody was used to detect the expression of HA-tagged Cas13d protein.
We next introduced the pPTG construct into potato plants by stable Agrobacterium-mediated transformation. Transgenic lines were selected on kanamycin-containing plant regeneration medium. Over 15 independent transgenic lines were obtained. The expression of Cas13d was determined by western blotting (Fig. 1c). Four gRNAs transcribed from the PTG genes were verified by qRT-PCR (Supplementary Fig. 2a–d). Two independent transgenic lines (St-PTG#18 and St-PTG#19) with relatively higher transgene expression were chosen for further experiments. Furthermore, phenotypes of transgenic lines growing on synthetic medium and soil were screened. We found that the transgenic plants expressing Cas13d/PTG were indistinguishable from wild-type plants under mixotrophic and autotrophic growth conditions (Supplementary Fig. 4a). The height, the number of aerial branches and stems of transgenic plants were similar to those of wild-type potato plants (Supplementary Fig. 4b–d).
Cas13d/PTG-mediated interference with PLRV, PVY, PVS or PVX
We first determined whether engineering of Cas13d/PTG system in potato plants could confer the resistance against individual RNA virus. To this end, transgenic (St-PTG#18 and St-PTG#19) and wild-type (St-wt) plants were challenged with PLRV or PVYO. Accumulation of PLRV or PVYO was assessed at 15 and 25 days post-inoculation (dpi) by enzyme-linked immunosorbent assays (ELISA) and qRT-PCR assays, respectively. Resistance of St-PTG transgenic plants to PLRV or PVYO was evidenced by a strongly reduced accumulation of PLRV and PVYO in the systemic leaves (Fig. 2a–d). Similarly, transgenic and wild-type (St-wt) plants were exposed to PVS or PVX and the virus accumulation was determined at 15 and 20 dpi by qRT-PCR. We showed that, compared to wild-type control plants, Cas13d/PTG expression strongly inhibited PVS or PVX accumulation in the systemic leaves of the virus-infected transgenic plants (Fig. 2e, f, Supplementary Fig. 5). To further evaluate the resistance level, the S. chacoense accession 40-3 containing the Rychc gene that could confer extreme resistance to PVY37 was chose for comparison. Although the resistance level to PVY of Cas13d/PTG transgenic plants was relatively lower than that of PVY-resistant line 40-3, they were additionally resistant to PLRV, PVS and PVX (Supplementary Fig. 6). These results demonstrate that Cas13d/PTG transgenic plants could reduce the accumulation of four individual viruses, respectively.
PLRV accumulation in transgenic potato plants was assessed at 15 dpi by ELISA (a) and qRT-PCR (b). PVYO accumulation in transgenic plant was assessed at 25 dpi by ELISA (c) and qRT-PCR (d). PVX (e) and PVS (f) accumulation in transgenic potato plants was assessed at 20 dpi by qRT-PCR. Data are means ± SD, and represent five biological replicates (n = 5). The letters above the bar indicate the significant differences as determined by one-way ANOVA (P < 0.05).
Cas13d/PTG exhibits multiplexed virus-targeting activity
To investigate whether the Cas13d/PTG is capable of targeting multiple RNA viruses simultaneously, we performed mechanical inoculation with mixed PVY/PVX to infect transgenic (St-PTG#18 and St-PTG#19) and wild-type (St-wt) plants. A PVY-resistant line (St-CP#7) expressing a single guide RNA targeting the CP gene of PVY31 (Supplementary Fig. 3a) was used for comparison. The virus titer was assessed and evaluated at 25 dpi by qRT-PCR. We found that transgenic plants expressing Cas13d/PTG exhibited significantly reduced accumulation of both PVY and PVX compared to wild-type and St-CP transgenic plants (Fig. 3a, b). When infected with individual PVY or PVX, transgenic plants expressing Cas13d/PTG can also interfere with PVY and PVX replication. In contrast, the PVY-resistant line (St-CP#7) can only reduce the accumulation and disease symptoms of PVY, but not PVX (Fig. 3c, d, g). These results indicated engineering of Cas13d/PTG system in potato plants was capable of targeting two viruses simultaneously, while the transgenic plant expressing single guide RNA (St-CP#7) failed to attenuate the accumulation of mixed viruses (Fig. 3a). Moreover, the PVY accumulation in St-CP#7 transgenic plants was shown to be similar to those of wild-type plants when infected with mixed PVY/PVX, suggesting the mixture of PVY/PVX could breakdown the PVY resistance of transgenic plants expressing a single guide RNA. Next, we challenged the transgenic and wild-type plants with mixed PVS/PVY, a typical mixture in the field. The qRT-PCR assays were performed to detect the virus accumulation at 25 dpi. Similarly, we observed a lower accumulation of PVS and PVY in the Cas13d/PTG transgenic plants compared to wild-type and St-CP#7 transgenic plants (Fig. 3e, f). While severe mosaic, mottled and crinkled leaves were observed in the infected leaves of wild-type and St-CP#7 transgenic plants, almost no disease symptoms were observed in the Cas13d/PTG transgenic plants in the mixed infection of PVY/PVX or PVS/PVY (Fig. 3h, i). Finally, three mixed viruses infection of PVY/PVX/ PVS was also assayed. Similar to the observation of two mixed viruses, a significant decrease of PVY, PVX and PVS accumulation in Cas13d/PTG transgenic plants was verified compared to wild-type and St-CP#7 transgenic plants (Fig. 4a–c). The disease symptom was presented in the systemic leaves in wild-type and St-CP#7 transgenic plants, while no obvious symptom was observed in transgenic plants expressing Cas13d/PTG (Fig. 4d). Thus, these results indicated that the Cas13d/PTG system has a high potential to produce numerous gRNAs and target multiple virus genomes simultaneously.
In the mixed infection with PVY/PVX mixture, PVY (a) and PVX (b) accumulation in the indicated transgenic plants at 25 dpi was determined by qRT-PCR. Accumulation of infection with individual PVY (c) and PVX (d) in the indicated transgenic plant at 25 dpi was determined by qRT-PCR. Similarly, the mixture of PVY/PVS was exposed to wild type and transgenic plants. PVY (e) and PVS (f) accumulation in the indicated transgenic plant at 25 dpi were shown by qRT-PCR. A transgenic line only targeting the CP gene of PVY (St-CP#7) (Zhan et al., 2019) was served as a control. Data represent five biological replicates (n = 5). The letters above the bars indicate the significant differences as determined by one-way ANOVA (P < 0.05). Disease symptoms in response to individual infection (only PVY) (g) and mixed infection (PVY/PVX) (h). Symptoms of transgenic and wild-type potato plants challenged with mixed viruses (PVY/PVS) at 25 dpi (i).
Transgenic and wild type potato plants were challenged with the mixture of PVY/PVX/PVS. The lower virus accumulation of PVY (a), PVX (b) and PVS (c) were verified in transgenic plants expressing Cas13d/PTG (St-PTG#18 and St-PTG#19) by qRT-PCR at 25 dpi. Data are means ± SD, and represent five biological replicates (n = 5). The significant differences were determined by one-way ANOVA (P < 0.05). d The symptoms such as mosaic, mottled and crinkled leaves were displayed in wild type and St-CP#7 transgenic plants, while transgenic plants expressing Cas13d/PTG (St-PTG#18 and St-PTG#19) had no obvious symptom.
Discussion
In this study, we engineered a novel Cas13d/PTG system to efficient protection of transgenic potato plants from four RNA viruses either individually or simultaneously by producing four gRNAs from a single synthetic PTG gene (Figs. 1–4). Previous study has demonstrated the proof-of-concept of simultaneously expressing several gRNAs to interfere with PVX-GFP and TRBO-BFP RNA replication in the model plant Nicotiana benthamiana by the transient transformation assays33. Recently, the CRISPR/Cas13 has been reported to recover the RNA silencing activity in host cell by targeting SPCSV-RNase3, improving the SPVD resistance of sweet potato38. Differing from these findings, we confirmed the potential of the CRISPR/Cas13d system to confer the stable resistance to multiple important viral disease in potato plants by directly targeting multiple virus genomes (Figs. 3, 4).
It is worth highlighting that a PVY-resistant line (St-CP#7) can only reduce the accumulation of PVY (Supplementary Fig. 3a), but not PLRV, PVX and PVS (Supplementary Fig. 3b–d). Many groups of viruses (such as PLRV, PVY, PVS, PVM, PVX and PVA) have been found in the potato field39. The mixed infection of potato viruses (usually two or three different viruses) commonly occurred with diverse combinations3,22. For example, PVX, PVY and PVS are mostly found to make interactions, the percentage of PVX and PVY co-infection was equivalent with PVY and PVS co-infection in potato plants40. Our results indicated that the expression of four gRNAs (St-PTG) exhibited high interference with two or three mixed viruses. While interfere with the PVY accumulation when infected with PVY alone (Fig. 3c; Supplementary Fig. 3a), transgenic plant expressing a single gRNA (St-CP#7) has no effect on reduction of PVY accumulation in mixed infection (Figs. 3a, e, 4a). Mixed infection is often associated with an increase in symptom severity and virus accumulation in comparison with the plants infected by a single virus40,41. Thus the interference of PVY accumulation in mixed viruses could be covered by synergistic effects of two or three viruses in transgenic plant expressing a single gRNA, while Cas13d/PTG transgenic plants may outperform in the mixed infection by targeting two or three RNA viruses.
CRISPR/PTG system has been first applied in simultaneously expressing several gRNAs (up to eight) by employing an endogenous tRNA-processing system in rice protoplasts to efficiently guide Cas9 to multiple chromosomal targets35. We showed that the Cas13d/PTG system could exhibit targeting multiple potato virus genomes, suggesting the PTG technology could be adapted to wide organisms for multiple genome targeting. It would also be reasonable that the Cas13d/PTG system could target more than four different viruses. In this case, multiple gRNAs could be embedded in PTG system matching with different virus genomes. It was known that RNase P and RNase Z precisely recognize RNA substrates with tRNA-like structures42. Since there are number of tRNA genes with variable sequences, these could be embedded in PTG gene for generation of multiple gRNAs. In addition, the establishment of the Cas13d/PTG system in plants would provide a platform to dissect the functions of multiple endogenous gene at RNA levels.
To engineer multi-resistance to RNA viruses, multiplexed gRNA expression would be crucial. Therefore, the use of PTG would be necessary for engineering to resistance to multiple viruses. Except for the endogenous tRNA system used in this study, exogenous co-expression of Csy4 or self-cleaving ribozymes such as Hammerhead or HDV ribozymes flanking by each gRNA are another alternative methods to produce multiple gRNAs simultaneously43,44,45. These strategies would allow the expression of multiple gRNAs from a single transcript.
Although the expression of PVY-sgRNA in St-PTG transgenic plants was significantly lower compared to that of St-CP#7 transgenic plants (Supplementary Fig. 2d), the PVY accumulation levels in St-PTG transgenic plants were similar to those in St-CP#7 transgenic plants (Fig. 3c). Thus, besides conferring board-spectrum resistance to RNA viruses, PTG technology would also efficiently target single virus without impairing the RNA interfering efficiency. Moreover, although the resistance levels to PVY is lower in Cas13d/PTG transgenic plants compared to that in PVY-resistant line 40-3, the Cas13d/PTG transgenic plants can additionally generate stable broad-spectrum resistance to other RNA viruses (Supplementary Fig. 6a, c–d), while the PVY-resistant line 40-3 only specifically resistant to PVY (Supplementary Fig. 6b).
In sum, we developed a Cas13d/PTG system for engineering broad-spectrum resistance to multiple RNA viruses in potato. The Cas13d/PTG system enables programmable RNA virus interference for targeting either one virus alone or two/three mixed RNA viruses simultaneously, thereby extending the applicability of the CRISPR system to crop protection against multiple RNA viruses.
Materials and methods
Plant material and viral strains
Potato (Solanum tuberosum cv. Désirée) plants were grown under standard greenhouse conditions. Four virus isolates PVYO-FL (HM367075), PLRV (MT264739.1), PVX (AB056718.1) and PVS (KU896946) were maintained in tobacco or potato plants as inoculated hosts. S. chacoense accession 40-3 containing the Rychc gene that confers extreme resistance to PVY37 was grown under standard greenhouse conditions for comparison.
Vector construction for potato transformation
Cas13d (Ruminococcus flavefaciens XPD3002, CasRx) gene sequence was codon optimized for expression in S. tuberosum (Supplementary Note 1). Four tandemly arrayed tRNA-gRNA units, with each gRNA targeting the CP genes (encoding virus coat protein) of four different RNA viruses (PVS, PVX, PLRV and PVY) (Supplementary Note 2), were designed and named as PTG gene. Cas13d and PTG genes were synthesized by GeneCreate (Wuhan, China). Subsequently, PTG gene with a poly(T) sequence (as a terminator) was cloned into under the Arabidopsis U6 promoter using the restriction enzymes BsaI. Cas13d sequence was cloned as EcoRI/KpnI fragment into the similarly cut vector pJZH131, generating plasmid pEV. Arabidopsis U6 promoter was amplified using pJZH3 as template, and inserted as SacII/SbfI fragment into the pEV vector digested with SacII and SbfI, generating plasmid pXH9. Finally, the PTG sequence was cloned into pXH9 digested with BsaI, producing plasmid pPTG. Sanger sequencing was used to confirm the sequence accuracy of all the clones.
Generation of the transgenic potato plants
Agrobacterium tumefactions strain GV3101 harboring the pPTG construct was employed to transform the potato plants (S. tuberosum cv. Désirée) using a protocol described previously46. Transgenic plants were screened by kanamycin (50 mg L−1) selection and initially tested for the presence of the transgene by PCR assays. Cas13d and gRNA expression levels were subsequently determined by qRT-PCR.
Protein extraction and Western blot analysis
Total proteins were extracted from ~100 mg of leaf samples using protein extraction buffer (Na2S2O5, 50 mM; Tris-HCl, 125 mM, pH8.8; SDS, 1% (w/v); Glycerol, 10% (v/v)). Proteins were separated on an 8% polyacrylamide gel. Cas13d expression was detected by western blot analysis. An anti-HA antibody (ABclonal, Oxfordshire, UK) was used as primary antibody (1:3000 dilution). The signals on the membrane were visualized using the enhanced chemiluminescence substrate (SuperSignal West Pico; Pierce, Rockford, I L) following the manufacturer’s instructions.
RNA extraction and qRT-PCR analysis of viral RNA genomes
Total RNA was extracted from virus-infected plants using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. For virus expression analysis, cDNA was synthesized using reverse transcriptase and oligo(dT) primer (TaKaRa, Japan). qRT-PCR assays were carried out in a CFX96 Touch TM real-time PCR detection system (Bio-Rad, Hercules, CA) using SYBR Premix Ex Taq TM II (TaKaRa). Three technical replicates were performed for each biological replicate. The potato TUBULIN2 gene was used as a reference. Primer sequences for qRT-PCR are listed in Supplementary Table 1.
ELISA assay
Double-antibody sandwich enzyme-linked immunosorbent assays (DAS-ELISA) with virus-specific antibodies (Agdia, Elkhart, IN) were conducted to measure virus accumulation at the protein levels in the systemic leaves according to the manufacturer’s protocol17.
Virus inoculation
To determine the response of transgenic plants to various viral strains or isolates, wild-type and transgenic plants were grown under long-day conditions (16-h light: 8-h dark) in the greenhouse at 20–25 °C. Aphid transmission was used for single virus infection except for PVX, which is generally considered transmitted by mechanical inoculation. Aphids were reared on virus-infected wild-type tobacco plants under controlled growth conditions at 20–25 °C. 25 adults were transferred onto wild-type and transgenic potato plants to transmit viruses from the aphids to the plants for 2 days, and then the aphids were removed.
For mechanical inoculation, five to eight plants of each line at the 6–8 leaf stage were inoculated with wild-type virus inocula or isolates (leaf extract: 1 g leaf tissue homogenized in 10 ml 0.1 M potassium phosphate buffer, pH 7.4) by mechanical wounding as described previously47. The mixed infection were also performed by mechanical inoculation using the inocula of the two or three viruses mixed at 1:1 or 1:1:1 titer.
Statistics and reproducibility
The data for virus expression analysis including ELISA and qRT-PCR were analyzed using one-way analysis of variance (ANOVA) coupled with Tukey or Dunnett’s test for multiple comparisons. All the virus assays were repeated at least three independent experiments. Three technical replicates were performed for each biological replicate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this article (and its supplementary information files). Uncropped and unedited blot/gel images is provided as Supplementary Fig. 7. Source data underlying figures are presented in Supplementary Data 1.
References
Boualem, A., Dogimont, C. & Bendahmane, A. The battle for survival between viruses and their host plants. Curr. Opin. Virol. 17, 32–38 (2016).
Rashid, M. O., Wang, Y. & Han, C. G. Molecular detection of potato viruses in Bangladesh and their phylogenetic analysis. Plants 9, E1413 (2020).
Wang, B. et al. Potato viruses in China. Crop Prot. 30, 1117–1123 (2011).
Bhushan, K. CRISPR/Cas13a targeting of RNA virus in plants. Plant Cell Rep. 37, 1707–1712 (2018).
Nicaise, V. Crop immunity against viruses: outcomes and future challenges. Front. Plant Sci. 5, 660 (2014).
Incarbone, M. & Dunoyer, P. RNA silencing and its suppression: novel insights from in planta analyses. Trends Plant Sci. 18, 382–392 (2013).
Simón-Mateo, C. & García, J. A. Antiviral strategies in plants based on RNA silencing. Biochim. Biophys. Acta 1809, 722–731 (2011).
Hardigan, M. A. et al. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proc. Natl. Acad. Sci. USA 114, E9999–E10008 (2017).
Kreuze, J. F. et al. Viral Diseases in Potato (ed. Campos, H. & Ortiz, O.) 389–430 (Springer International Publishing, Cham Switzerland, 2020).
Taliansky, M., Mayo, M. A. & Barker, H. Potato leafroll virus: a classic pathogen shows some new tricks. Mol. Plant Pathol. 4, 81–89 (2003).
Walker, P. J. et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 167, 2429–2440 (2022).
Sangeetha, E. & Jebasingh, T. Plant virus-host interaction 2nd edn, (eds. Gaur, R.K., Khurana, S.M.P., Sharma, P. & Hohn, T.) 169-189 (Boston, 2021).
Davie, K., Holmes, R., Pickup, J. & Lacomme, C. Dynamics of PVY strains in field grown potato: impact of strain competition and ability to overcome host resistance mechanisms. Virus Res. 241, 95–104 (2017).
Karasev, A. V. & Gray, S. M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 51, 571–586 (2013).
Lin, Y. H., Abad, J. A., Maroon-Lango, C. J., Perry, K. L. & Pappu, H. R. Molecular characterization of domestic and exotic potato virus S isolates and a global analysis of genomic sequences. Arch. Virol. 159, 2115–2122 (2014).
Matousek, J., Schubert, J. & Dedic, P. Complementation analysis of triple gene block of Potato virus S (PVS) revealed its capability to support systemic infection and aphid transmissibility of recombinant Potato virus X. Virus Res. 146, 81–88 (2009).
Wang, J. et al. RT-PCR differentiation, molecular and pathological characterization of Andean and ordinary strains of Potato virus S in potatoes in China. Plant Dis. 100, 1580–1585 (2016).
Kutnjak, D. et al. Complete genome sequences of new divergent potato virus X isolates and discrimination between strains in a mixed infection using small RNAs sequencing approach. Virus Res. 191, 45–50 (2014).
Faurez, F., Baldwin, T., Tribodet, M. & Jacquot, E. Identification of new Potato virus Y (PVY) molecular determinants for the induction of vein necrosis in tobacco. Mol. Plant Pathol. 13, 948–959 (2012).
Quenouille, J., Vassilakos, N. & Moury, B. Potato virus Y: a major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol. Plant Pathol. 14, 439–452 (2013).
Rahman, M. S. & Akanda, M. Infection pattern of PVY and PLRV in potato tubers obtained from potato plants infected with both the viruses. Agriculturists 6, 118 (2010).
Wu, X., Zhang, H., Shi, Y., Sun, Q. & Chen, S. Survey of important potato viruses in some potato planting areas in China. J. Henan Agric. Sci. 42, 84–87 (2013).
Nyalugwe, E., Wilson, C., Coutts, B. & Jones, R. Biological properties of Potato Virus X in potato: effects of mixed infection with Potato virus S and resistance phenotypes in cultivars from three continents. Plant Dis. 96, 43–54 (2012).
Dominguez, A. A., Lim, W. A. & Qi, L. S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 17, 5–15 (2016).
Koonin, E. V., Makarova, K. S. & Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 37, 67–78 (2017).
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017).
Mahas, A. & Mahfouz, M. Engineering virus resistance via CRISPR-Cas systems. Curr. Opin. Virol. 32, 1–8 (2018).
Tashkandi, M., Ali, Z., Aljedaani, F., Shami, A. & Mahfouz, M. M. Engineering resistance against Tomato yellow leaf curl virus via the CRISPR/Cas9 system in tomato. Plant Signal. Behav. 13, e1525996 (2018).
Abudayyeh, O. O. et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016).
Aman, R. et al. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 19, 1 (2018).
Zhan, X. et al. Generation of virus-resistant potato plants by RNA genome targeting. Plant Biotechnol. J. 17, 1814–1822 (2019).
Zhang, T. et al. Establishing CRISPR/Cas13a immune system conferring RNA virus resistance in both dicot and monocot plants. Plant Biotechnol. J. 17, 1185–1187 (2019).
Mahas, A., Aman, R. & Mahfouz, M. CRISPR-Cas13d mediates robust RNA virus interference in plants. Genome Biol. 20, 263 (2019).
Konermann, S. et al. Transcriptome engineering with RNA-targeting Type VI-D CRISPR effectors. Cell 173, 665–676.e14 (2018).
Xie, K., Minkenberg, B. & Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. USA 112, 3570–3575 (2015).
Stewart, M. Nuclear export of mRNA. Trends Biochem. Sci. 35, 609–617 (2010).
Li, G. et al. Ry(chc) confers extreme resistance to potato virus Y in potato. Cells 11, 2577 (2022).
Yu, Y. et al. Targeting of SPCSV-RNase3 via CRISPR-Cas13 confers resistance against sweet potato virus disease. Mol. Plant Pathol. 23, 104–117 (2022).
Loebenstein, G., Berger, P. H., Brunt, A. & Lawson, R. H. Virus and virus-like diseases of potatoes and production of seed-potatoes. (ed. Brunt, A. A.) 65–67 (Kluwer, Dordrecht, Holland, 2001).
Hameed, A., Iqbal, Z., Asad, S. & Mansoor, S. Detection of multiple potato viruses in the field suggests synergistic interactions among potato viruses in Pakistan. Plant Pathol. J. 30, 407–415 (2014).
Hull, R. Matthews' plant virology 4th edn, (ed. Hull, R.) 75–107 (Academic Press, London, 2002).
Canino, G. et al. Arabidopsis encodes four tRNase Z enzymes. Plant Physiol. 150, 1494–1502 (2009).
Gao, Y. & Zhao, Y. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J. Integr. Plant Biol. 56, 343–349 (2014).
Nissim, L., Perli, S. D., Fridkin, A., Perez-Pinera, P. & Lu, T. K. Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/Cas toolkit in human cells. Mol. Cell 54, 698–710 (2014).
Xu, L., Zhao, L., Gao, Y., Xu, J. & Han, R. Empower multiplex cell and tissue-specific CRISPR-mediated gene manipulation with self-cleaving ribozymes and tRNA. Nucleic Acids Res. 45, e28 (2017).
Zhang, J. et al. Pest control. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 347, 991–994 (2015).
Nie, X., Singh, R. & Singh, M. Molecular and pathological characterization of N:O isolates of the Potato virus Y from Manitoba, Canada. Can. J. Plant Pathol. 26, 573–583 (2004).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (32271912 and 31600408).
Author information
Authors and Affiliations
Contributions
J.Z. designed research; X.Z. and W.L. performed research; X.Z., B.N., F.Z. and J.Z. analyzed data; F.Z. and J.Z. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Sun Jian, Erik Andreasson, and Shahid Mansoor for their contribution to the peer review of this work. Primary Handling Editors: Leena Tripathi and David Favero.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhan, X., Liu, W., Nie, B. et al. Cas13d-mediated multiplex RNA targeting confers a broad-spectrum resistance against RNA viruses in potato. Commun Biol 6, 855 (2023). https://doi.org/10.1038/s42003-023-05205-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-05205-2
This article is cited by
-
CRISPR/Cas genome editing in plants: mechanisms, applications, and overcoming bottlenecks
Functional & Integrative Genomics (2024)
-
Contemporary perspectives on the global evolution of potato virus Y pathogen
Indian Phytopathology (2024)
-
CRISPR–Cas13d in plant biology: an insight
Plant Biotechnology Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.