Abstract
Tobacco is an important commercial crop and a rich source of alkaloids for pharmaceutical and agricultural applications. However, its yield can be reduced by up to 70% due to virus infections, especially by a potyvirus Potato virus Y (PVY). The replication of PVY relies on host factors, and eukaryotic translation initiation factor 4Es (eIF4Es) have already been identified as recessive resistance genes against potyviruses in many plant species. To investigate the molecular basis of PVY resistance in the widely cultivated allotetraploid tobacco variety K326, we developed a dual guide RNA CRISPR/Cas9 system for combinatorial gene editing of two clades, eIF4E1 (eIF4E1-S and eIF4E1-T) and eIF4E2 (eIF4E2-S and eIF4E2-T) in the eIF4E gene family comprising six members in tobacco. We screened for CRISPR/Cas9-induced mutations by heteroduplex analysis and Sanger sequencing, and monitored PVYO accumulation in virus challenged regenerated plants by DAS-ELISA both in T0 and T1 generations. We found that all T0 lines carrying targeted mutations in the eIF4E1-S gene displayed enhanced resistance to PVYO confirming previous reports. More importantly, our combinatorial approach revealed that eIF4E1-S is necessary but not sufficient for complete PVY resistance. Only the quadruple mutants harboring loss-of-function mutations in eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T showed heritable high-level resistance to PVYO in tobacco. Our work highlights the importance of understanding host factor redundancy in virus replication and provides a roadmap to generate virus resistance by combinatorial CRISPR/Cas9-mediated editing in non-model crop plants with complex genomes.
Similar content being viewed by others
Introduction
Tobacco (Nicotiana tabacum) is one of the widely cultivated nonfood cash crops, a natural pesticide and plant of pharmaceutical importance. It is also used as a model plant for studying gene functions and metabolic processes, and to produce high value chemicals, such as plant-based vaccines. However, the yield and quality of tobacco are heavily affected by virus infections, especially by Potato virus Y (PVY). PVY belongs to the Potyvirus genus in the Potyviridae, the largest family of plant-infecting RNA viruses1, and has a wide host range in the Solanaceae family including tomato, potato and tobacco2,3,4. According to the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA), PVY infection can result in up to 70% yield loss globally, which is associated with reduced plant performance (i.e., height, leaf size) and altered chemical composition of the infected leaves5. Thus, producing PVY resistant tobacco is of high importance.
The first PVY resistant tobacco line carrying mutations at the va locus on chromosome 21 was generated by X-ray mutagenesis6. Subsequently, three allelic forms (0, 1 and 2) of the recessive resistance gene va, conferring varying degrees of resistance to PVY, had been introduced into Nicotiana tabacum cultivars by traditional breeding methods7. Molecular characterization of the va locus revealed that va encodes for a eukaryotic translation initiation factor 4E (eIF4E)8, which is a component of the eIF4F pre-initiation complex. eIF4E can bind the 5′ m7G cap of mRNAs and other proteins including the 40S ribosomal subunit to promote the translation of endogenous gene transcripts9. In PVY-infected cells, eIF4E can also interact with a virus-derived and viral genome-linked protein called VPg to initiate the translation of viral RNAs9,10. Thus, eIF4E acts as a host factor for PVY infection. Recently, both eIF4E and its isoform eIF(iso)4E have been identified as recessive resistance genes against different potyvirus isolates in diverse plant species9,10,11,12,13,14,15. The lack of functional interactions between the viral VPg and the plant eIF4E/eIF(iso)4Es is the primary cause of potyvirus resistance in most cases. Conversely, the compatibility of these interactions likely determines the PVY virus host range16.
The expression of host factors can be suppressed by RNA interference (RNAi), and a recent work demonstrated that RNAi-mediated down regulation of eIF4E and eIF(iso)4E genes can result in a reduced susceptibility to PVY and resistance breaking (RB)-PVY strains in tobacco17. However, the efficacy of RNAi-based virus resistance is heavily influenced by several internal and external factors such as the level of transgene-derived short interfering RNAs (siRNA)18,19, the degree of homology between the RNAi trigger sequence and the target virus20,21, and the cell types22. In addition, the transgenic RNAi plants meet heavy regulatory challenges and limited public acceptance in many countries23. Field studies revealed that va0, va1 and va2-mediated potyvirus resistance can also be overcome by different potyvirus strains24,25,26. Thus, a novel approach is required to generate a broad and long-lasting resistance against the most prevalent potyviruses.
The latest gene editing technology referred to as CRISPR/Cas has been extensively employed to improve agronomic traits including virus resistance27,28. CRISPR/Cas can be expressed as a transgene and subsequently programmed to recognize and cleave invading virus genomes having complementary sequences to Cas guide RNAs29,30. Alternatively, CRISPR/Cas can be used to generate loss of function mutations in viral host factors, such as eIF4E or eIF(iso)4E31,32,33,34,35,36,37,38. The latter strategy has already been successfully employed to create potyvirus resistance in different plant species including cucumber12, Arabidopsis13, tomato35, cassava39 and barley40.
In this study, we used a dual sgRNA-expressing CRISPR/Cas9 construct to knock out multiple copies of the eIF4E gene family in the tobacco cultivar K326. We found that the simultaneous deletion of four eIF4E genes including eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T resulted in durable resistance to PVY both in T0 and T1 generations.
Results
Analysis of the eIF4E gene family in tobacco variety K326 for CRISPR/Cas9 target selection
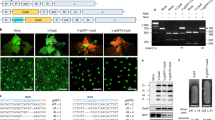
Tobacco is an allotetraploid plant, which inherited its genome from the diploid Nicotiana sylvestris (S-genome) and Nicotiana tomentosiformis (T-genome)41. Detailed characterization of eIF4E and its isoform eIF(iso)4E in tobacco revealed that five of these genes are derived from the N. tomentosiformis (T) genome [T015277 (Genbank KM202067), T021658 (Genbank KM202068), T021287 (Genbank KM202069), T025160 (Genbank KM202070) and T024242 (Genbank KM202065)], and three are originated from the N. sylvestris (S) genome [S10760 (Genbank KF155696), S05588 (Genbank KM202071), and S10809 (Genbank KM202066)]8. To identify the eIF4E homologues in the N. tabacum K326 genome (https://solgenomics.net/organism/Nicotiana_tabacum/genome), we performed a TBlastN search using the amino acid sequence of a tobacco IF4E domain (GenBank accession number QNT12790.1) as a protein query. This exercise returned eight candidate sequences, all of which contained a typical IF4E domain as confirmed by Pfam analysis (http://pfam.xfam.org/). Phylogenic analysis revealed that the eIF4E genes form three well-separated clusters/clades in K326 (Fig. 1a). The first cluster corresponds to the eIF4E1 group and includes four genes: one from N. sylvestris designated as eIF4E1-S and three from N. tomentosiformis named as eIF4E1-T, eIF4E1-Tb and eIF4E1-Tc. The second eIF4E2 cluster contains one orthologous gene from each ancestor: eIF4E2-S and eIF4E2-T. The third cluster includes two eIF(iso)4E sequences, eIF(iso)4E-S and eIF(iso)4E-T.
Tobacco eIF4E diversity, sgRNAs selection and CRISPR/Cas9 vector map. (a) Phylogenetic analysis of the eIF4E family in tobacco cultivar K326. The phylogenetic tree was constructed by the maximum likelihood method using the nucleotide alignment of genes encoding eukaryotic initiation factors in tobacco variety K326 along with reference sequences including the N. tomentosiformis (T) genome [T015277 (Genbank KM202067), T021658 (Genbank KM202068), T021287 (Genbank KM202069), T025160 (Genbank KM202070) and T024242 (Genbank KM202065)]; the N. sylvestris (S) genome [S10760 (Genbank KF155696), S05588 (Genbank KM202071), and S10809 (Genbank KM202066)]; and the K326 eIF4E/eIF(iso)4E genes [eIF4E1-S; eIF4E1-T; eIF4E1-Tc; eIF4E1-Tb; eIF4E2-T; eIF4E2-S, eIF(iso)4E-S; eIF(iso)4E-T]. Bootstrap values are shown above the corresponding branches. (b) Schematic of the eIF4E gene structures and the CRISPR/Cas9 target sites. The position and sequence of sgRNA target sites are indicated by a red arrow and a dashed rectangle, respectively. The sequences of the protospacer adjacent motifs (PAM) are underlined. The position of the sgRNA targeted region relative to the translation start site of the corresponding ORF is indicated by a black arrow. E1-E5, exons of eIF4E genes. (c) Schematic of the CRISPR/Cas9-sgRNA construct used for tobacco transformation. KanR Kanamycin resistant gene, Cas9 Maize-codon-optimized Cas9 gene driven by the 35S promoter (Cauliflower Mosaic Virus promoter); two sgRNAs (4E1-sgRNA and 4E2-sgRNA) regulated by the Arabidopsis U6 promoter (AtU6p), U6ter Arabidopsis U6 terminator, E9ter Pea RuBisCO small subunit E9 terminator, NLS Nuclear localization signal, LB/RB left and right border.
Redundancy in host/susceptibility factor genes, such as eIF4E, is a major obstacle in generating virus resistance because the invading viruses could recruit a plethora of paralog gene products to support their life cycle42. Consequently, mutations in only one member of the eIF4E gene family may result in a non-durable, and limited virus resistance spectrum to PVY17,43. In contrast, deletion of all eIF4E genes could be associated with severe developmental defects or lethality due to the fundamental role of eIF4Es in the translation of endogenous mRNAs44,45. We speculated that knocking out multiple, but not all eIF4E genes could result in a durable and broad resistance against PVY without jeopardizing plant performance. In the eIF4E1 clade (Fig. 1a), eIF4E1-Tb has not been implicated in PVY resistance yet. However, the expression level of eIF4E1-Tc has been shown to positively correlate with durable PVY resistance in tobacco cultivars43. RNAi-mediated suppression or targeted mutagenesis of the remaining eIF4E1 members eIF4E1-S and eIF4E1-T, and the eIF4E2 clade members eIF4E2-S and eIF4E2-T were associated with PVY resistance17,43,46 but the additive and combined effects of the above genes on PVY susceptibility have not been investigated. Hence, we decided to simultaneously knock out eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T by CRISPR/Cas9, and subsequently investigate the effect of the combinatorial mutations on PVY susceptibility in tobacco K326. To this end, we designed two Cas9 guide RNAs referred to as 4E1-sgRNA and 4E2-sgRNA, which can independently target the eIF4E1 (eIF4E1-S and eIF4E1-T) and eIF4E2 (eIF4E2-S and eIF4E2-T) orthologues, respectively (Fig. 1b, Supplementary Fig. S1), but not the eIF4E1-Tb and eIF4E1-Tc (Supplementary Fig. S2). We then generated a dual CRISPR/Cas9 construct harboring both the 4E1-sgRNA and 4E2-sgRNA expression cassettes for tobacco transformation (Fig. 1c).
Generation of transgenic tobacco lines and the analysis of CRISPR/Cas9-induced mutations
To generate eIF4E mutants, we used tobacco leaf explants and the AGL1 strain harboring the pKSE401/4E1-sgRNA/4E2-sgRNA plasmid for Agrobacterium-mediated transformation. After shoot and subsequent root induction, we screened the regenerated plants for the presence of cas9 and nptII genes by PCR (Table S2). Of the thirty transgenic T0 lines referred to as eFs, twenty-six yielded the expected PCR products for both cas9 (711 bp) and nptII (766 bp), which were then subjected to heteroduplex analyses. We observed DNA band shifts for at least one of the Cas9-targeted eIF4E loci in each plant indicating that our CRISPR reagents were highly efficient in making independent DNA edits at multiple loci. We then selected five plants for detailed analysis (Fig. 2). Our data revealed that eF-19 carried mutations only in the eIF4E1-S gene. In contrast, eF-4 and eF-7 had mutations in both paralogs (T and S) of eIF4E1 and eIF4E2, respectively. Importantly, eF-10 and eF-31 showed induced mutations at all four tested eIF4E genes by heteroduplex analysis (Fig. 2).
Identification of CRISPR/Cas9-induced mutations in the eIF4E genes by heteroduplex analysis. (a–d) PAGE-based heteroduplex analysis of eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T, respectively. eF-3–eF-31: T0 tobacco mutant lines. WT wild type plant, M marker 100 bp, Thermo Scientific. Red triangles denote heteroduplex bands.
Next, we characterized the CRISPR/Cas9-induced mutations at each target loci by Sanger sequencing (Fig. 3). In agreement with the literature, we found both insertions and deletions at the CRISPR cut sites. All detected insertions were limited to 1 bp, however, deletions ranged from − 1 to − 8 bp. Interestingly, we observed biallelic and homozygous mutations at high frequency in the T0 lines (Fig. 3 and Table 1) suggesting that the mutations occurred very early, presumably at the single-cell stage of the regeneration process. Based on the deduced amino acid sequence analysis, most biallelic and homozygous mutations result in frameshifts with premature stop codons, and consequently produce truncated non-functional eIF4E proteins. Our sequencing data revealed that the eF-19 line harbors biallelic nonsense mutations in the eIF4E1-S gene only (Table 1). The eF-4 line carries biallelic null mutations in the eIF4E1-T gene and it is heterozygous for eIF4E1-S. eF-7 contains loss-of-function mutations both in the eIF4E2-S and eIF4E2-T genes in biallelic forms. In the eF-31 line, the Cas9-induced mutations disrupted both paralogs of the eIF4E2 and eIF4E1-T genes, but eIF4E1-S gene may still be functional due to a 3 bp deletion resulting in a single amino acid loss in the eIF4E protein sequence. Importantly, eF-10 harbors null mutations in all four Cas9-targeted eIF4E genes (Table 1).
Alignment of eIF4E sequences isolated from the selected T0 lines. (a) eIF4E1-S, (b) eIF4E1-T, (c) eIF4E2-S, (d) eIF4E2-T loci. The sgRNA target sequences and the PAM motifs are highlighted in yellow and blue, respectively. Red letters indicate inserted nucleotides. Red dashed lines represent deleted nucleotides. Δ refers to changes in the CRISPR/Cas9 targeted sequence: 0, no change; − deletion; + insertion. “Clones” indicate the number of clones used for sequencing.
Assessing PVY resistance in the eIF4E edited T0 tobacco lines
To investigate the impact of eIF4E gene redundancy on PVY susceptibly, we decided to challenge the T0 lines carrying a variety of homo- and heterozygous mutations in the eIF4E gene family including eF-19 (E1-ssTT/E2-SSTT), eF-4 (E1-Sstt/E2-SSTT), eF-31 (E1-sstt/E2-SsTt), eF-7 (E1-SSTT/E2-sstt) and eF-10 (E1-sstt/E2-sstt) with a local PVYO isolate. We also added a tobacco line harboring null mutations in the eIF(iso)4E gene family (iso4E-sstt)47 as control (Supplementary Table S1, Fig. 4a). To assess PVY resistance in the selected tobacco lines, we employed DAS-ELISA, a widely used and highly accurate technique for immunodetection of virus infections15,35,48,49. Two weeks after rub inoculation, PVY accumulation was detected in the systemic leaves of eF-7, eF-4 and isoE (iso4E-sstt) lines at similar level to WT plants (Fig. 4a, Supplementary Table S1). In contrast, the eF-19 and eF-31 lines showed lower PVYO titer, even 4 weeks post infection (Fig. 4a). However, only a small fraction of eF19 plants displayed systemic accumulation throughout the experiment (Supplementary Table S1) suggesting that PVY resistance is more durable in eF19 then in eF-31. More importantly, no PVYO infection was detected in the eF-10 mutant lines at any time points tested (Fig. 4a,b and Supplementary Table S1).
Effect of eIF4E/eIF(iso)4E mutations on PVY accumulation in T0 tobacco plants. (a) PVYO accumulation in the systemic leaves of infected plants was assessed by DAS-ELISA at 2, 3 and 4 weeks post-inoculation (wpi); WT wild type plant, isoE eIF(iso)4E-S and eIF(iso)4E-T double mutant line, eF-7 eIF4E2-S and eIF4E2-T double mutant line, eF-4 eIF4E1-T mutant line, eF-19 eIF4E1-S mutant line, eF-31 eIF4E1-S and eIF4E1-T double mutant line; eF-10 eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T quadruple mutant line; negative control: non-inoculated plant; Mean and standard errors of 405 nm absorbance (A405) values were calculated for 5 independent plants per line, *P < 0.05 versus corresponding positive control (WT). (b) PVYO-infected Nicotiana tabacum K326 plants at 10 wpi.
Investigating PVY resistance in the segregating T1 offspring of eF-10
To further confirm the genotype of eF-10 and examine the effect of eIF4E mutations on PVYO resistance in tobacco, we analyzed the inheritance of Cas9-induced mutations and PVY resistance in the segregating T1 progeny. First, we genotyped twelve randomly selected seedlings by heteroduplex analysis (Fig. 5). No band shift was detected for both eIF4E2-S and eIF4E2-T in the offspring (Fig. 5b), which confirms that eF-10 harbored homozygous mutations for eIF4E2s in T0. As expected, we observed segregating DNA bands for the eIF4E1-S (eF-10–5 and eF-10–9, Fig. 5a) and eIF4E1-T (eF-10–5, eF-10–11, eF-10–1 and eF-10–8; Fig. 5a) genes indicating that both eIF4E1s indeed contained biallelic mutations in T0. Sequencing of the corresponding loci from selected eF-10 T1 plants (Supplementary Fig. S5) revealed that eF-10–5 and eF-10–9 carried one of the insertion (+ 1 bp) and deletion (-1 bp) alleles of the eIF4E1-S gene, respectively, while eF-10–1 and eF-10–8 inherited both mutant alleles of eIF4E1-T from the T0 generation (Supplementary Fig. S5a). Similarly, biallelic mutations (− 2 and + 1 bp) of eIF4E1-T were inherited from T0 plants in eIF-10–2 and eIF-10–6, whereas eF-10–5, eF-10–11, eF-10–1 and eF-10–8 were homozygous for the eIF4E1-T mutant alleles (Supplementary Fig. S5b). In addition, 1 bp insertion was detected in the eIF4E2-S and eIF4E2-T genes in all sequenced T1 plants (Supplementary Fig. S5c,d). All in all, our sequencing data were in line with the heteroduplex analysis and confirmed the presence of loss of function mutations in eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T in eF-10 and its progeny.
To evaluate PVY resistance, we randomly selected thirty T1 offspring of eF-10 and rub inoculated them with PVYO. We then monitored the phenotype of virus infected plants and subsequently analyzed the virus load at 4 weeks post inoculation by DAS-ELISA in the newly emerging (systemic) leaves since this timepoint gave us the most reliable results in T0 (Fig. 4). As expected, all WT plants showed a typical PVY phenotype. However, none of the T1 progeny of eF-10 developed PVY symptoms. In agreement with the phenotypic observation, the vast majority of eF-10 T1s (27 out of 30, 90%) had similar DAS-ELISA values to the mock inoculated plants (Fig. 6). From these experiments we conclude that null mutations in all four eIF4E genes are associated with highly efficient and durable resistance to PVYO in tobacco.
Analysis of PVY accumulation in T1 generation of the eF-10 line. PVYO accumulation in the systemic leaves of infected plants was assessed by DAS-ELISA at 4 weeks post-inoculation; WT wild type plant, T1 eF-10 T1 progeny of eF-10 line; negative control: non-inoculated plant. Mean and standard errors of 405 nm absorbance (A405) values were calculated for 30 (T1 eF-10) or 10 (WT and negative control) independent plants per line; ****P < 0.0001 versus corresponding positive control (WT).
Off-target analysis
Five potential off-target sites of 4E1-sgRNA and 4E2-sgRNA were predicted by CCTop in the N. tabacum genome (Supplementary Table S3). However, no Cas9-induced mutations were identified in T0 and its progeny by PAGE-based heteroduplex analysis and sequencing of the corresponding loci (Supplementary Fig. S6).
Discussion
Both single and multiple sgRNA expressing CRISPR/Cas9 vectors can be utilized to edit several genes simultaneously that share high sequence complementarity with the first 20 nucleotide (nt) of sgRNA(s). In a recent report, a single sgRNA construct was designed to target a highly conserved region in the berberine bridge enzyme-like (BBL) gene family which is involved in nicotine biosynthesis, and nicotine‐free tobacco plants carrying loss-of-function mutations in all six BBL genes were obtained in the T3 generation50. Similarly, members of the rbcS gene family displaying high sequence homology were knocked out by a dual sgRNA-CRISPR/Cas9 vector in Nicotiana tabacum cv. Petit Havana51. Moreover, multiple sgRNA expressing constructs were also employed to simultaneously edit non-homologous genes (phytoene desaturase (PDS) and PDR-type transporter (PDR6)) in tobacco52. In this study, we developed a dual sgRNA CRISPR/Cas9 system to induce mutations in two separate clades of the eIF4E gene family in the tobacco variety K326 (Figure S1), namely eIF4E1 (eIF4E1-S and eIF4E1-T) and eIF4E2 (eIF4E2-S and eIF4E2-T), respectively. We identified regenerated plants with loss-of-function mutations in the combinations of one, two, three and all four targeted genes in the T0 generation, indicating that CRISPR/Cas9 can be efficiently harnessed for precision combinatorial gene editing applications in tobacco.
eIF-mediated potyvirus resistance has been extensively studied in many plants12,48,53,54, which is based on the interaction of the viral genome-linked protein (VPg) with the eukaryotic translation initiation factor eIF4E or its orthologue eIF(iso)4E to initiate viral RNA translation and subsequent virus replication55,56. Consequently, down regulation of the above translation initiation factors or mutations that disrupt the binding of VPg to the corresponding eIFs could result in resistance to potyviruses57,58. Interestingly, we found that simultaneous loss-of function mutations in eIF4E2s and eIF(iso)4Es (eF-7 and isoE, respectively; Fig. 4a and Supplementary Table S1) and partial knock out of eIF4E1s (E1-Sstt’ in eF-4, Fig. 4a and Supplementary Table S1) had no impact on PVYO resistance. In contrast, systemic accumulation of PVYO was significantly reduced in the eF-31 line harboring partially disrupted eIF4E1-S and knocked out eIF4E1-T (Fig. 4a and Supplementary Table S1). In the eF-19 line, which carries null mutation only in eIF4E1-S, PVYO was detected in the fraction of infected plants (20% and 40% at 2 and 4 wpi, respectively; Supplementary Table S1). Thus, our data indicate that eIF4E1-S is required but not sufficient for durable PVYO resistance in K326. This is in line with previous reports revealing that tobacco plants carrying natural frameshift or nonsense mutations in eIF4E1-S were only partially resistant to a large collection of PVY isolates from the O and C clades43, and tobacco cultivars VAM and TN90, lacking eIF4E1-S, showed limited resistance to PVYNTN38.
Recent studies suggested that eIF4Es could act redundantly in PVY translation17,38,43. Conversely, more than one eIF4E orthologues may need to be deleted/mutated to generate efficient and stable PVY resistance. Indeed, in our study, the eF-10 line harboring quadruple nonsense mutations in all four eIF4E genes (eIF4E1-S, eIF4E1-T, eIF4E2-S and eIF4E2-T) exhibited complete and durable resistance to PVYO both in the T0 and in the T1 generation (Figs. 4 and 6). Thus, our findings provide evidence for the above hypothesis. Since random mutations generated during virus replication may yield to novel VPg variants that can selectively recruit eIF4E orthologues or isoforms, simultaneous deletion of redundant viral host factors could prevent or at least reduce the efficacy of virus replication, and consequently suppress/delay the emergence of resistance breaking virus strains. Hence, our work may also establish a framework to create broad spectrum virus resistance in tobacco and in other crop species. Interestingly, a recent study by Udagawa et al.38 showed that simultaneous knock out of eIF4E1-S and eIF(iso)4E1-T resulted in high-level resistance against both PVY and RB-PVY in tobacco, which indicates that eIF(iso)4E1-T can also be recruited by PVY variants. Thus, further studies may be performed to evaluate PVY resistance in tobacco lines carrying mutations in all 5 genes eIF4E1-S, eIF4E1-T, eIF4E2-S, eIF4E2-T and eIF(iso)4E1-T.
Materials and methods
Identification and phylogenetic analysis of the eIF4E gene family in N. tabacum K326
To identify the members of the eIF4E gene family in N. tabacum K32641, the IF4E domain (pfam01652) of the tobacco eIF4E amino acid sequence QNT12790.1 (GenBank) was used as a protein query in TblastN search using the Sol Genomics website (https://solgenomics.net/). The returned sequences were then subjected to Pfam analysis (http://pfam.xfam.org/) using the default parameters to validate the eIF4E genes. Next, a phylogenetic analysis was conducted to investigate the evolutionary relationship among the eIF4E gene family members in N. tabacum K326 and in its Nicotiana ancestors, N. sylvestris and N. tomentosiformis. To this end, the coding DNA Sequences (CDS) were aligned by ClustalW (MEGA 6.0 software)59 and the phylogenetic tree was then generated by the maximum-likelihood method based on the JTT model using 1000 replicate bootstrap support.
Single guide RNA (sgRNA) design and CRISPR/Cas9 binary vector construction
To generate Cas9‐induced mutations in all four eIF4E homologs, two independent Cas9 target sites referred to as 4E1-gRNA and 4E2-gRNA were identified by CCTop (https://cctop.cos.uni-heidelberg.de:8043/)60. Next, complementary single-stranded DNA oligos corresponding to 4E1-gRNA and 4E2-gRNA (Supplementary Table S2) were annealed and subsequently ligated into the Bsa I-digested binary vector pKSE401 (Addgene, #62202)61 to generate two single guide RNA constructs pKSE401/4E1-sgRNA and pKSE401/4E2-sgRNA, respectively. Finally, the sgRNA expression cassettes were recombined into a single construct pKSE401/4E1-sgRNA/4E2-sgRNA by Golden Gate cloning62. The sequence of the pKSE401/4E1-sgRNA/4E2-sgRNA plasmid was confirmed by Sanger sequencing, which was subsequently introduced into A. tumefaciens strain AGL1 for tobacco transformation.
Agrobacterium-mediated tobacco transformation and transgene confirmation
N. tabacum cultivar K326 (provided by Tobacco Institute One Member Comapy Limited, Vietnam) was used for Agrobacterium-mediated transformation following the protocol by Topping et al.63. Briefly, a single Agrobacterium colony harboring the pKSE401/4E1-sgRNA/4E2-sgRNA binary plasmid was cultured in 50 mL of LB medium containing appropriate antibiotics at 200 rpm at 28 °C for 14–16 h until OD600 reached 0.6–0.8. The bacterial cells were then harvested by centrifugation at 5000 rpm for 10 min at room temperature and then resuspended in ½ MS liquid medium supplemented with 0.02 µM acetosyringone. The leaves of in vitro grown tobacco plants were cut into small pieces (1 × 1 cm) and immersed in the Agrobacterium suspension for 30 min with gentle agitation. The infected explants were then placed onto the co-cultivation medium and kept in the dark for 2 days at 25 ± 2 °C. Next, the explants were washed with sterile water supplemented with 500 mg/L cefotaxime. After removing the excess water by sterile filter papers, the leaf pieces were cultured on selection medium containing 150 mg/L kanamycin and 500 mg/L cefotaxime. Healthy shoots regenerated from transformed explants were transferred to rooting medium. Rooted tobacco plants with 3–4 leaves were used to identify the presence of the CRISPR/Cas9 construct by PCR using primer pairs specific for cas9 and nptII genes (Supplementary Table S2).
Identification and characterization of CRISPR/Cas9-induced mutations
Genomic DNA was extracted from leaves of wild-type (WT) and transgenic tobacco plants using a CTAB-based extraction method64. DNA spanning the Cas9 gRNA target sites was amplified by PCR using eIF4E-specific primer pairs listed in Table S2. CRISPR/Cas9-induced mutations were detected by heteroduplex analysis using native polyacrylamide gel electrophoresis (PAGE) as previously described65. Briefly, the mixture containing equal amounts of PCR products amplified from WT and the corresponding transgenic tobacco line was denatured at 95 °C for 10 min, which was then subsequently cooled down to room temperature for renaturation. Next, DNA was separated in 15% native polyacrylamide gels. Note, CRISPR/Cas9-induced indels result in shifted DNA bands due to heteroduplex formation with WT DNA65. To further characterize the Cas9-induced mutations, the PCR amplicons were purified by the ProNex® Size-Selective Purification System (Promega, USA) and then ligated into the pJET1.2/blunt cloning vector (Thermo Fisher Scientific, USA). Seven to ten clones from each line were sequenced by the Sanger method using the Applied Biosystems 3500 Genetic Analyzer (Thermo Fisher Scientific, USA). The sequencing data were analyzed by BioEdit (v. 7.2.5, Ibis Biosciences, Carlsbad, CA, USA)66. The stability of the Cas9-induced mutations was assessed at three time points in every transgenic tobacco line, first in in vitro culture and then 2 and 4 weeks after transferring the rooted plants into soil.
The selected T0 plants harboring mutations in the CRISPR/Cas9-targeted eIF4E genes were grown in the greenhouse. After self-pollination, the seeds were collected and subsequently germinated on a substrate containing perlite and vermiculite (1:3 v/v) and the seedlings were then transferred to the greenhouse. The segregating T1 plants were characterized for the presence of Cas9-induced mutations in the eIF4E genes as described above.
Potential off-target analysis
Potential off-target sites were predicted by CCTop. To assess the frequency of off-target mutations, the genomic DNA isolated from the T0 mutant lines and their T1 progeny were pooled together, and then used to amplify and sequence the putative off-target sites using locus-specific primers (Supplementary Table S2).
PVY isolation and multiplication
To obtain PVY isolates for the virus challenge experiments, potato plants showing PVY symptoms were collected from different locations and the presence of PVY was confirmed by RT-PCR using PVY capsid protein (CP)-specific primers (Supplementary Table S2). Sequencing and phylogenetic analysis of the collected PVY isolates (Supplementary Fig. S3) revealed that the majority of potato plants were infected with PVYO—the most common PVY strain with a wide host range67. The selected PVY isolate was multiplied and maintained in virus-free Nicotiana benthamiana plants (a gift from Dr. H.T. Phan, Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany) as described by Korbecka-Glinka68 with minor modifications. Briefly, PVY-infected potato leaves were homogenized in ten volumes of 0.01 M phosphate-buffered saline (PBS) pH 7.4 using a cold mortar and pestle. The homogenate was then gently rubbed onto N. benthamiana leaves, which were dusted with silicon carbide powder prior infection. Next, the excess carborundum and sap were removed by rinsing the leaves with distilled water. Inoculated plants were kept in the greenhouse at 22 °C (± 2 °C) under long day conditions, which were sheltered from direct sunlight for 48 h. Two weeks post infection, PVY symptoms were observed on inoculated N. benthamiana plants (Supplementary Fig. S4). The presence of PVYO was confirmed by RT-PCR as described above.
PVY susceptibility test of the eIF4E mutant tobacco lines
Selected T0 lines harboring mutations in eIF4E genes were propagated and multiplied in vitro and then transferred to the greenhouse for virus infection. Sap collected from PVYO-infected N. benthamiana leaves was rubbed onto the top leaves of at least five plants from each T0 line (genotype) in 5–6-leaf developmental stage as described above. Mock inoculated plants were used as negative controls. In T1 generation, up to 30 in vitro propagated plants were challenged with PVYO from each segregating genotype. The presence and stability of the Cas9-induced mutations in eIF4E genes were investigated prior virus infection as described above. PVYO accumulation was analyzed at two-, three- and four-weeks post-inoculation (wpi). Systemic leaves were sampled and subjected to double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) using a commercially available kit (BIOREBA, Germany) according to the manufacturer’s instruction. The absorbance at OD405 was measured by a Benchmark microplate reader (Bio‐Rad). Data were plotted by the GraphPad Prism software (version 7.04; LA Jolla, California, USA). For statistical analysis, a one-way analysis of variance (ANOVA) was performed (P values < 0.05). Plant growth and PVY symptoms were continuously monitored up to 10 weeks post inoculation.
Ethics declarations
We had obtained permission to collect plants. All the experiments were performed in accordance with relevant guidelines and regulations.
Data availability
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/, Reference Number KM202067, KM202068, KM202069, KM202070, KM202065, KF155696, KM202071, and KM202066.
References
Pasin, F., Daròs, J.-A. & Tzanetakis, I. E. Proteome expansion in the Potyviridae evolutionary radiation. FEMS Microbiol. Rev. 46, 011. https://doi.org/10.1093/femsre/fuac011 (2022).
Karasev, A. V. & Gray, S. M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 51, 571–586 (2013).
Quenouille, J., Vassilakos, N. & Moury, B. Potato virus Y: A major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol. Plant Pathol. 14, 439 (2013).
Scholthof, K. G. et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 12, 938–954 (2011).
Verrier, J. L. & Doroszewska, T. Tobacco virus collaborative study (1996–2011). Virus disease sub-group. Technical Report. CORESTA (2018). https://www.coresta.org/tobacco-viruscollaborative-study-1996-2011-31554.html
Koelle, G. Versucher zur vererbung der krankheitsresistenz bei tabak. 2 Mitt. Eine rippen-braune-resistante virgin a mutante nach anwendung kunstlicher mutations auslosung durch rontgenstrahlen. Tabak-Forschung 24, 83–84 (1958).
Anon, G., Blancard, D. & Cailleteau, B. Mise au point sur la résistance récessive aux souches nécrotiques du virus Y de la pomme de terre (PVY) présente chez Nicotiana tabacum. Ann. Tabac 27, 35–42 (1995).
Julio, E. et al. A eukaryotic translation initiation factor 4E (eIF4E) is responsible for the “va” tobacco recessive resistance to Potyviruses. Plant Mol. Biol. Rep. 33, 609–623 (2015).
Sanfaçon, H. Plant translation factors and virus resistance. Viruses 7, 3392–3419 (2015).
Robaglia, C. & Caranta, C. Translation initiation factors: A weak link in plant RNA virus infection. Trends Plant Sci. 11, 40–45 (2006).
Hwang, J. et al. Double mutations in eIF4E and eIFiso4E confer recessive resistance to Chilli veinal mottle virus in pepper. Mol. Cells 27, 329–336 (2009).
Chandrasekaran, J. et al. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 17, 1140–1153 (2016).
Pyott, D. E., Sheehan, E. & Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 17, 1276–1288 (2016).
Gutierrez Sanchez, P. A. et al. Overexpression of a modified eIF4E regulates potato virus Y resistance at the transcriptional level in potato. BMC Genomics 21, 18 (2020).
Yoon, Y.-J. et al. Genome editing of eIF4E1 in tomato confers resistance to Pepper Mottle Virus. Front. Plant Sci. 11, 1098 (2020).
Martínez-Turiño, S., Calvo, M., Bedoya, L. C., Zhao, M. & García, J. A. Virus host jumping can be boosted by adaptation to a bridge plant species. Microorganisms 9, 805 (2021).
Takakura, Y., Udagawa, H., Shinjo, A. & Koga, K. Mutation of a Nicotiana tabacum L. eukaryotic translation-initiation factor gene reduces susceptibility to a resistance-breaking strain of Potato virus Y. Mol. Plant Pathol. 19, 2124–2133 (2018).
Singh, K., Dardick, C. & Kumar Kundu, J. RNAi-mediated resistance against viruses in perennial fruit plants. Plants 8, 359 (2019).
Vanderschuren, H., Alder, A., Zhang, P. & Gruissem, W. Dose-dependent RNAi-mediated geminivirus resistance in the tropical root crop cassava. Plant Mol. Biol. 70, 265–272 (2009).
Tabein, S. et al. The induction of an effective dsRNA-mediated resistance against Tomato spotted wilt virus by exogenous application of double-stranded RNA largely depends on the selection of the viral RNA target region. Front. Plant Sci. 11, 533338 (2020).
Gaba, V. et al. Hairpin-based virus resistance depends on the sequence similarity between challenge virus and discrete, highly accumulating siRNA species. Eur. J. Plant Pathol. 128, 153–164 (2010).
Marshall, W. F. Modeling recursive RNA interference. PLoS Comput. Biol. 4, e1000183 (2008).
Zhao, Y., Yang, X., Zhou, G. & Zhang, T. Engineering plant virus resistance: From RNA silencing to genome editing strategies. Plant Biotechnol. J. 18, 328–336 (2020).
Acosta-Leal, R. & Xiong, Z. Intrahost mechanisms governing emergence of resistance-breaking variants of Potato virus Y. Virology 437, 39–47 (2013).
Lacroix, C., Glais, L., Kerlan, C., Verrier, J.-L. & Jacquot, E. Biological characterization of French Potato virus Y (PVY) isolates collected from PVY-susceptible or -resistant tobacco plants possessing the recessive resistance gene va. Plant Pathol. 59, 1133–1143 (2010).
Masuta, C., Nishimura, M., Morishita, H. & Hataya, T. A single amino acid change in viral genome-associated protein of Potato virus Y correlates with resistance breaking in ‘virgin a mutant’ tobacco. Phytopathology 89, 118–123 (1999).
Arora, L. & Narula, A. Gene editing and crop improvement using CRISPR-Cas9 system. Front. Plant Sci. 8, 1932 (2017).
Borrelli, V. M. G., Brambilla, V., Rogowsky, P., Marocco, A. & Lanubile, A. The enhancement of plant disease resistance using CRISPR/Cas9 technology. Front. Plant Sci. 9, 1245 (2018).
Ali, Z. et al. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 16, 238 (2015).
Ali, Z., Ali, S., Tashkandi, M., Zaidi, S.S.-A. & Mahfouz, M. M. CRISPR/Cas9-mediated immunity to Geminiviruses: Differential interference and evasion. Sci. Rep. 6, 26912 (2016).
Li, F., Cao, Y., Zhou, H. & Zhou, X. Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Front. Microbiol. 11, 593700. https://doi.org/10.3389/fmicb.2020.593700 (2020).
Atarashi, H. et al. Artificially edited alleles of the eukaryotic translation initiation factor 4E1 gene differentially reduce susceptibility to cucumber mosaic virus and potato virus Y in tomato. Front. Microbiol. 11, 564310 (2020).
Liu, H. et al. CRISPR/Cas9-mediated resistance to cauliflower mosaic virus. Plant Direct. 2, e00047 (2018).
Macovei, A. et al. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 16, 1918–1927 (2018).
Moury, B. et al. Knock-out mutation of eukaryotic initiation factor 4E2 (eIF4E2) confers resistance to pepper veinal mottle virus in tomato. Virology 539, 11–17 (2020).
Pramanik, D. et al. CRISPR/Cas9-mediated generation of pathogen-resistant tomato against tomato yellow leaf curl virus and powdery mildew. Int. J. Mol. Sci. 22, 1878 (2021).
Schmitt-Keichinger, C. Manipulating cellular factors to combat viruses: A case study from the plant eukaryotic translation initiation factors eIF4. Front. Microbiol. 10, 17 (2019).
Udagawa, H., Koga, K., Shinjo, A., Kitashiba, H. & Takakura, Y. Loss-of-function of Nicotiana tabacum L. eukaryotic translation initiation factors eIF4E1-S and eIF(iso)4E-T synergistically confers high-level resistance to both Potato virus Y (PVY) and resistance-breaking PVY. Breed. Sci. 71, 193–200 (2021).
Gomez, M. A. et al. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 17, 421–434 (2019).
Hoffie, R. E., Otto, I., Hisano, H. & Kumlehn, J. Site-directed mutagenesis in barley using RNA-guided Cas endonucleases during microspore-derived generation of doubled haploids. Methods Mol. Biol. 2287, 199–214 (2021).
Edwards, K. D. et al. A reference genome for Nicotiana tabacum enables map-based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genomics 18, 448 (2017).
Gallois, J.-L., Moury, B. & German-Retana, S. Role of the genetic background in resistance to plant viruses. Int. J. Mol. Sci. 19, E2856 (2018).
Michel, V. et al. A complex eIF4E locus impacts the durability of va resistance to Potato virus Y in tobacco. Mol. Plant Pathol. 20, 1051–1066 (2019).
Callot, C. & Gallois, J. L. Pyramiding resistances based on translation initiation factors in Arabidopsis is impaired by male gametophyte lethality. Plant Signal. Behav. 9, e27940 (2014).
Gauffier, C. et al. A TILLING approach to generate broad-spectrum resistance to potyviruses in tomato is hampered by eIF4E gene redundancy. Plant J. 85, 717–729 (2016).
Xing, Z. et al. Development of a PVY resistant flue-cured tobacco line via EMS mutagenesis of eIF4E. Agronomy 10, 36 (2019).
Nguyen, G. T., Le, N. T., Nguyen, T. T., Chu, H. H. & Pham, N. B. Mutagenesis of the eIF(iso)4E Gene in Tobacco by CRISPR/CAS9 Genome Editing Technique (National Confence on Biotechnology, 2018).
Mazier, M., Flamain, F., Nicolaï, M., Sarnette, V. & Caranta, C. Knock-down of both eIF4E1 and eIF4E2 genes confers broad-spectrum resistance against potyviruses in tomato. PLoS ONE 6, e29595 (2011).
Miras, M., Juárez, M. & Aranda, M. A. Resistance to the emerging Moroccan watermelon mosaic virus in Squash. Phytopathology 109, 895–903 (2019).
Schachtsiek, J. & Stehle, F. Nicotine-free, nontransgenic tobacco (Nicotiana tabacum L.) edited by CRISPR-Cas9. Plant Biotechnol. J. 17, 2228–2230 (2019).
Donovan, S., Mao, Y., Orr, D. J., Carmo-Silva, E. & McCormick, A. J. CRISPR-Cas9-mediated mutagenesis of the rubisco small subunit family in Nicotiana tabacum. Front. Genome Ed. 2, 28 (2020).
Gao, J. et al. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 87, 99–110 (2015).
Rodríguez-Hernández, A. M. et al. Melon RNA interference (RNAi) lines silenced for Cm-eIF4E show broad virus resistance. Mol. Plant Pathol. 13, 755–763 (2012).
Wang, X. et al. Silencing of the host factor eIF(iso)4E gene confers plum pox virus resistance in plum. PLoS ONE 8, e50627 (2013).
Li, G. et al. Variability in eukaryotic initiation factor iso4E in Brassica rapa influences interactions with the viral protein linked to the genome of Turnip mosaic virus. Sci. Rep. 8, 13588 (2018).
Shopan, J., Lv, X., Hu, Z., Zhang, M. & Yang, J. Eukaryotic translation initiation factors shape RNA viruses resistance in plants. Hortic. Plant J. 6, 81–88 (2020).
Shopan, J. et al. Eukaryotic translation initiation factor 2B-beta (eIF2Bβ), a new class of plant virus resistance gene. Plant J. 90, 929–940 (2017).
van Schie, C. C. N. & Takken, F. L. W. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 52, 551–581 (2014).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Stemmer, M., Thumberger, T., Keyer, M. S., Wittbrodt, J. & Mateo, J. L. CCTop: An intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE 10, e0124633 (2015).
Xing, H.-L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Gao, X. et al. Modular construction of plasmids by parallel assembly of linear vector components. Anal. Biochem. 437, 172–177 (2013).
Topping, J. F. Tobacco transformation. Methods Mol. Biol. 81, 365–372 (1998).
Narzary, D., Verma, S., Mahar, K. & Rana, T. A rapid and effective method for isolation of genomic DNA from small amount of silica-dried leaf tissues. Natl. Acad. Sci. Lett. 38, 441–444 (2015).
Zhu, X. et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep. 4, 6420 (2014).
Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Ellis, P., Stace-Smith, R. & de Villiers, G. Identification and geographic distribution of serotypes of Potato virus Y. Plant Dis. 81, 481–484 (1997).
Korbecka-Glinka, G., Czubacka, A., Przybys, M. & Doroszewska, T. Resistance vs tolerance to Potato virus Y in tobacco-comparing effectiveness using virus isolates from Central Europe. Breed. Sci. 67, 459–465 (2017).
Acknowledgements
This study was carried out by facilities of Plant Cell Biotechnology, Institute of Biotechnology, VAST, Vietnam. The authors thank Dr. Van Tuong Nguyen for helpful suggestions on the manuscript.
Funding
This research was supported by the Institute of Biotechnology, Vietnam Academy of Science and Technology (CSCL08.03/22-22) and the Royal Society International Collaboration Award (IC170320).
Author information
Authors and Affiliations
Contributions
P.D., H.C. and N.P. supervised the study. P.D. and N.P. designed the experiments. N.L. and D.N. performed CRISPR/Cas9 vector constructions. G.N., D.T. and T.B. conducted tobacco transformation. N.L., D.T. and H.T. analyzed induced mutations and performed PVY resistance test. P.D., N.L. and T.B. analyzed the data and wrote the manuscript. H.C., N.P. and A.M. revised and proof-read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le, N.T., Tran, H.T., Bui, T.P. et al. Simultaneously induced mutations in eIF4E genes by CRISPR/Cas9 enhance PVY resistance in tobacco. Sci Rep 12, 14627 (2022). https://doi.org/10.1038/s41598-022-18923-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18923-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.