Abstract
Low-frequency (<4 Hz) neural activity, particularly in the delta band, is generally indicative of loss of consciousness and cortical down states, particularly when it is diffuse and high amplitude. Remarkably, however, drug challenge studies of several diverse classes of pharmacological agents—including drugs which treat epilepsy, activate GABAB receptors, block acetylcholine receptors, or produce psychedelic effects—demonstrate neural activity resembling cortical down states even as the participants remain conscious. Of those substances that are safe to use in healthy volunteers, some may be highly valuable research tools for investigating which neural activity patterns are sufficient for consciousness or its absence.
Similar content being viewed by others
Introduction
Neural oscillations in the delta (1–4 Hz) and slow (<1 Hz) frequency bands are generally indicators of unconsciousness1,2,3 or severely reduced consciousness4, especially when activity is diffuse and of high amplitude. Nonetheless, some pharmacological agents are known to enhance activity at these frequencies while sparing consciousness. Such paradoxical pharmacological dissociations (PPDs) are useful for falsifying putative spectral electroencephalogram (EEG) markers of conscious and unconscious states, e.g., if a substance can induce cortical slow waves during wakeful consciousness, then slow waves are not a universal indicator of loss of consciousness. Furthermore, understanding how such PPDs are possible is crucial for understanding how neural activity relates to consciousness.
A recent review5 described a range of conditions in which high amplitude delta oscillations (HADOs) appear in the awake and conscious state including, most notably, Angelman syndrome, a rare genetic condition characterized by diffuse HADOs during conscious wakefulness in children6. However, the above review focused largely on neurological conditions and less so on pharmacological manipulations. Here, we continue reviewing paradoxical EEG patterns by describing pharmacological drug challenges that induce HADOs without loss of consciousness. Our mini-review comprehensively covers literature in both non-human animals and human participants from over 70 years ago to the present day. By doing so, we illuminate PPDs in which the oscillatory regime exhibited by the EEG may be safely and reversibly switched independently of consciousness in the laboratory, e.g., as a means of validating candidate biomarkers of consciousness. Given their mechanistic similarities and the lack of meaningful boundaries between frequency bands7, we treat both delta and slow oscillations as belonging to the delta band for the purpose of this mini-review; thus, our principal focus is on oscillations occurring at frequencies at or below 4 Hz. Due to the inverse relationship between oscillatory frequency and spatial extent7, delta activity is often extensive across the scalp. For this reason, our mini-review does not particularly emphasize the scalp location of delta (but see the column “Spatial topography” in Table 1).
Depending on the location of the reference electrode, either the peak or trough of each oscillatory cycle in the delta band corresponds to a down state of cortical silence8. During these periodic down states, cortical neurons switch to a more hyperpolarized resting membrane potential, increasing the difficulty of spiking and communicating with other neurons9,10. Conversely, up states result in a widespread spiking of cortical neurons at the opposite phase of the delta cycle. This dynamical regime can be represented by a simplified two-state model (described as the “monolithic brain” by Tononi11) in which the cortex is either up (spiking) or down (not-spiking).
Theories that attribute consciousness to the information-richness of cortical dynamics, such as integrated information theory in both its strong12 and weak13 formulations, explain the loss of consciousness that typically occurs under these conditions in terms of the loss of information integration. According to this view, HADOs may cause such loss of information by restricting the number of possible states visited by the brain. Yet even in rare genetic disorders where EEG oscillations are incongruent with the typical oscillatory regime seen during consciousness, informational measures (such as permutation entropy) reliably indicate the presence or absence of consciousness14. To learn how consciousness and complexity may persist under such unusual condition, one may use pharmacological manipulations that reversibly induce abnormal EEG patterns in healthy adults, thus allowing for comparisons of normal and abnormal cortical dynamics within the same individuals. The aim of such research should be to find a common denominator of consciousness14 across both normal and abnormal cortical dynamics.
Below, we review pharmacological manipulations that might be used for this purpose. Although the level consciousness is not entirely unimpaired by some substances (e.g., tiagabine) reviewed below, participants do not lose consciousness all together in the studies described, and in some cases, certain dimensions of consciousness are even enhanced (e.g., using psychedelic tryptamines). In the latter context, recent evidence15 shows that awake EEG delta activity correlates with global functional connectivity as assessed by concurrent functional magnetic resonance imaging (fMRI). Some substances which we review have potential as valuable experimental tools for revealing the common denominator of consciousness which persists even as an abnormal oscillatory regime emerges.
Antiepileptic drugs
Antiepileptic drugs carbamazepine and tiagabine are reported to induce delta oscillations during wakeful consciousness, albeit while inducing mild sedation. Carbamazepine’s main mechanism of action is inhibition of voltage-gated sodium channels16. An early report of the delta-enhancing effects of carbamazepine came from a small study of patients who took a 400 mg dose over a period of 35 days, which reported increases in EEG delta and theta power relative to baseline17. Soon after, a similar effect was also inferred from an observational study of schizophrenia patients, many of whom regularly took carbamazepine with their antipsychotic medications and showed diffuse delta activity18. Although delta activity was diffuse, the authors noted that it was sometimes frontally predominant, similar to an earlier report19 of intermittent frontal rhythmic delta activity in eight schizophrenia patients taking antipsychotic drugs (exact medications not specified). However, a more recent study of acute carbamazepine (600 mg) in 15 healthy volunteers only found significant increases in resting EEG power in the theta, alpha, and beta bands20, and it is thus unclear if carbamazepine’s effect on EEG delta power is seen outside of chronic dosing.
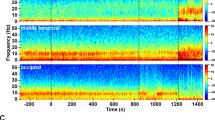
Unlike carbamazepine, tiagabine is a gamma-aminobutyric acid (GABA) reuptake inhibitor21. Besides enhancing delta power during wakefulness in young adult volunteers, as seen diffusely both in sensor space22 and source space23, tiagabine also induces episodes of hypersynchronous delta activity during wakefulness in rodents24, and in elderly humans, it increases delta power not only during NREM sleep, but also during REM sleep, i.e., when episodes of dreaming, and therefore some degree of consciousness, are common25. The degree of delta power enhancement induced by tiagabine during wakefulness in healthy volunteers, referenced to placebo, is highly variable, with some individuals exhibiting magnetoencephalogram (MEG) delta power increases >1000%26—comparable to the degree of delta enhancement in the Angelman syndrome EEG27—while others show much more modest changes (Fig. 1). Tiagabine is thus an attractive drug for studying PPDs that resemble the Angelman syndrome EEG phenotype, but its usefulness may be limited by tolerability issues28 (see below).
Data are from publicly available26 source-localized resting-state MEG recordings23. The experiment was approved by the UK National Research Ethics Service (South East Wales) and all participants gave written informed consent. One outlier participant was excluded due to an extremely large power increase for which we could not confirm that tiagabine was the sole cause. a Tiagabine induces clear increases in cortical oscillatory delta activity, as evidenced by the MEG power changes relative to placebo. The mean power change (red) induced by tiagabine is greatest at 3.3 Hz, featuring a more than 5-fold (430%) power increase from placebo. The largest power change we observed (after excluding one outlier, not shown here) was a 1200% power increase at 3.1 Hz. b The majority of participants (8/14) showed maximum power changes in the delta band (<4 Hz). The mean frequency of the maximum power change was 4.3 Hz, and the median frequency was 3.4 Hz. Source data supporting panel b are presented in Supplementary Data 1.
A recent small study by Darmani et al.20 investigated the “degraded state of consciousness” induced by tiagabine. Prior to each of four transcranial magnetic stimulation (TMS)-EEG sessions, 15 healthy volunteers were administered tiagabine, carbamazepine, brivaracetam (another antiepileptic drug), or placebo, experiencing each condition once. Similar to another report of partial sedation, confusion, disorientation, hallucinations, and amnesia caused by 15 mg tiagabine28, three participants had difficulty tolerating tiagabine and did not complete the subsequent TMS-EEG session. Tiagabine was found to significantly increase resting power in all frequency bands examined (delta, theta, alpha, and beta). Using the perturbational complexity index (PCI), a highly accurate measures of one’s level of consciousness based on the cortical response to TMS29,30,31, tiagabine was found to induce a PCI value (computed using the state transition algorithm30) intermediate between consciousness and unconsciousness. Similarly, other studies have shown that ketamine (not an antiepileptic, but rather an N-methyl-D-aspartate receptor antagonist) yields a PCI value that is intermediate between that typically seen in wakeful consciousness and unconsciousness, though it should be emphasized that PCI values obtained with ketamine were not significantly different from wakefulness in a small n = 6 sample31,32 (see the “other substances” section below for further discussion). By comparison, the other drugs investigated by Darmani et al.20 (brivaracetam and the ostensible delta-enhancer carbamazepine) did not significantly alter delta power or PCI values, though carbamazepine significantly increased power in other bands (theta, alpha, and beta). While both carbamazepine and tiagabine are generally known to increase delta EEG power (as shown in Fig. 1 for tiagabine), it is unclear whether results in the Darmani et al. study truly corresponded to delta-specific effects or rather non-specific increases in broadband power.
GABAB receptor agonists
Gamma hydroxybutyrate (GHB), sodium oxybate (i.e., the sodium salt of GHB), and baclofen are GABAB receptor agonists that induce large increases in EEG delta power during sleep and wakefulness33. Unlike baclofen, GHB is also an agonist of the eponymous GHB receptor, an endogenous neurotransmitter34, and both a precursor and a metabolite of GABA35. One of the oldest published reports of pharmacologically induced HADOs is from a 1966 study of GHB’s effects in adult volunteers36. Volunteers given large doses (35–63 mg/kg) of GHB were awakened from an unconscious state induced by GHB and displayed HADOs while behaviorally responsive36. However, consciousness could only be maintained briefly (10–15 s) due to GHB’s strongly sedating effects. More recently, in an attempt to better understand the neural underpinnings of this PPD, GHB has been studied at lower doses that allow for uninterrupted wakefulness (20–35 mg/kg) in healthy male volunteers using sophisticated analyses such as source localization, global omega complexity, and functional connectivity analysis37. Findings of this analysis suggest that low-frequency oscillations induced by GHB emerge from the posterior cingulate cortex, a region that is sometimes implicated in consciousness due to its role in the default mode network, and a later study by the same group also reported that GHB increased delta power in posterior cingulate cortex, as well as medial prefrontal cortex, parahippocampal gyrus, and fusiform gyrus, during NREM sleep38. Results of both studies also suggested that GHB sedation may be related to increased lagged phase synchronization between posterior cingulate cortex and other regions37,38.
Likely because the above study of GHB chose a relatively low GHB dose, it only detected a trend-level increase in EEG delta power induced by GHB compared to placebo37. However, other studies from the 2010s33,38 have reinforced the results of older literature36,39, reproducing GHB-induced increases in relatively low-frequency EEG power during NREM sleep, REM sleep, and wakefulness. Evidence of enhanced activity at similar frequencies has also been obtained for baclofen—a similar GABABergic compound—in both humans33,40 and mice41,42, though most studies have focused on NREM sleep and suggest an effect in the theta band more so than the delta band. As with tiagabine20, GHB and baclofen are likely to be useful pharmacological tools for validating PCI and other biomarkers of consciousness under conditions of abnormal cortical dynamics, and both drugs may be better tolerated than tiagabine28, even when TMS is combined with pharmaco-EEG to compute PCI43,44. Underscoring the potential usefulness of these substances, GABAergic inhibition is a key parameter of perturbational complexity, as has been revealed by experiments that estimated PCI using electrical stimulation in ferret cortical slices while synchronous states (low PCI) were induced using both GABAA and GABAB receptor antagonists45. Interestingly, the above study induced low-frequency cortical oscillations by blocking GABAB receptors, whereas baclofen and GHB induce low-frequency cortical oscillations while activating GABAB receptors; thus, tuning the ratio of excitation to inhibition in either direction away from its optimum may induce hypersynchronous HADOs. However, because these experiments were performed in cortical slices, effects of GABAB receptor antagonism on consciousness could not be studied.
Anticholinergics
During states of wakefulness and arousal, acetylcholine acts as a neuromodulator and desynchronizes cortical activity46, thus diminishing delta oscillations. Conversely, anticholinergic drugs enhance the presence of delta oscillations. This has been noted for decades using the muscarinic antagonist atropine, which was first observed in 1952 to induce a PPD in dogs47. Several years later, a report also noted EEG slowing with 10 mg atropine in a small group of human volunteers, although the effect was reported to be more in the 5–8 Hz theta range48. Slowing effects have been noted using the muscarinic antagonist scopolamine in two small human studies that noted increased delta power49,50,51,52. This delta band effect was, however, not replicated by at least one EEG study53. More recently, a small study of healthy elderly participants found evidence of enhanced MEG connectivity (phase locking value) in the delta band under scopolamine54.
Because anticholinergic substances derived from the genus Datura, including atropine and scopolamine, are known to produce deliriant effects, cognitive impairment, and tachycardia, human research is highly limited. For instance, one study of scopolamine50 in healthy adult volunteers noted that following a 0.75 mg dose, participants “complained about subjective symptoms which were definitely unpleasant” and “were restless and confused, with mild muscular incoordination and evident drowsiness when not adequately aroused.” Due to these concerns, anticholinergic drugs—unlike the substances reviewed in previous sections—are not recommended for validating biomarkers of consciousness in humans. However, careful research using anticholinergic substances in non-human animals may reveal useful insights into the compatibility of delta oscillations with consciousness. A recent study of atropine and scopolamine in cats55, for example, found that the EEG pattern induced by anticholinergics, while resembling NREM sleep with respect to delta oscillations and sleep-like spindles, had a distinct profile in the gamma band, featuring gamma bursts and greater gamma coherence than NREM sleep. The authors concluded55 that these gamma EEG features, sometimes associated with consciousness56,57, might “explain why the animals remain awake in spite of the presence of slow waves and spindles.”
Tryptamines
Of all substances that enhance delta oscillations while sparing consciousness, perhaps the most puzzling are the psychedelic tryptamines that actually increase the richness and intensity of visual imagery, imagination, and perceptual meaning58,59 while also inducing delta activity. This crucially demonstrates that, unlike some prior examples, delta-enhancing drugs do not necessarily induce drowsiness and partial-sedation. Most notable of these tryptamines is N,N-dimethyltryptamine (DMT), a principal ingredient in ayahuasca, a psychedelic brew made by the indigenous people of the Amazon basin60. DMT is also an endogenous neurotransmitter61, though its function as such is poorly understood. The first ever rigorous, placebo-controlled EEG study of DMT62 in humans recently revealed a surprising increase in delta power that occurred with intravenous DMT administration in 13 participants during eyes closed EEG. Moreover, this increase in delta power after subtracting the 1/f background of the EEG power spectrum correlated positively with subjective ratings of both the visual and overall intensity of the DMT experience. EEG signal complexity measured using the Lempel-Ziv algorithm63 (a common proxy for the level of consciousness, e.g., used to compute PCI) increased despite the introduction of delta oscillations which might otherwise be expected to reduce signal complexity by increasing the regularity and predictability of the signal. The above EEG findings were recently replicated by the same research team in a newer study using 17 participants each given 20 mg of intravenous DMT during simultaneous fMRI while wearing an eye mask15. Notably, increases in frontal delta power following DMT infusion correlated with increases in global fMRI connectivity. This replication also demonstrated that EEG delta power in the experiment did not significantly correlate with visual analog scales of drowsiness (in fact, the sign of the correlation coefficient was always negative). Separately, a study of inhaled DMT in naturalistic settings outside of the laboratory using 35 participants64 also reported an increase in delta power induced by DMT compared with an eyes closed resting baseline condition (as in the work by Timmerman et al.62, participants kept their eyes closed through the experiment). Interestingly, DMT enhanced gamma power and coherence relative to the baseline recording, thus suggesting a similar mechanism as that hypothesized for scopolamine55, whereby gamma-band activity sustains consciousness despite background delta activity. Finally, DMT in a naturalistic setting64 also recapitulates the laboratory finding of increased Lempel-Ziv complexity with DMT compared to the eye closed baseline EEG.
Other tryptamines have also been reported to increase delta power despite their psychedelic effects. In mice65, the DMT derivative 5-methoxy-DMT enhances theta and gamma power in medial prefrontal cortex and delta power in primary visual cortex. The latter finding supports the hypothesis5 that enhanced delta power observed in powerful psychedelic states relates to hyperpolarization of primary visual cortex when decoupled from the external sensory environment66, as may also occur in non-pharmacological dream states during REM sleep67,68,69 (though the recent findings of Timmermann et al.15 may point instead toward global hyperconnectivity). Additionally, 4-hydroxy-DMT (better known as psilocin, the pharmacologically active agent of psychedelic mushrooms) enhances 4 Hz power in mice during both wakefulness and REM sleep70; the latter effect was interpreted by the study’s authors as “a bleeding of NREM-like activities (delta waves, spindles, reduced gamma) into REM sleep.” Despite this, psilocybin (the prodrug of psilocin) does not appear to enhance delta MEG power23 or alter PCI values71 in human volunteers.
Other substances
Besides the substances reviewed above, we also wish to highlight possible effects of other miscellaneous substances on delta EEG activity, albeit with caveats.
Subanesthetic doses of ketamine, a dissociative drug72, are known to induce a 1–3 Hz rhythm in layer 5 of retrosplenial cortex in mice (also demonstrated with the dissociative compound phencyclidine; here, the term “dissociative” refers to detachment or dissociation from one’s body or surroundings, rather than a PPD)73. Similarly, a 3–4 Hz rhythm in posterior cingulate and isthmus cingulate cortex was recently demonstrated using subanesthetic ketamine in human epilepsy patients74. Note that, as with ketamine, posterior cingulate cortex has also been identified as a plausible source of delta activity induced by GHB37,38. Delta frequency rhythms induced by ketamine, while linked to dissociation in both mice73 and humans74, appear limited to deep posteromedial areas observable only with invasive recordings—no enhancement of delta activity is seen in humans with subanesthetic doses of ketamine using noninvasive recordings, e.g., EEG75,76 or MEG23. In fact, at least one study reported a significant delta power decrease caused by subanesthetic ketamine in human EEG77. Studies which do report delta EEG activity in scalp recordings under ketamine have used anesthetic doses78,79,80,81.
Next, basmisanil, a selective GABAA-α5 negative allosteric modulator, enhances 4 Hz EEG power during wakefulness in patients with Down syndrome, while also suppressing power in the beta band82. However, in healthy participants, EEG power enhancement with basmisanil occurs more so in the theta band (6–9 Hz)83. By the same token, benzodiazepines (i.e., GABAA positive allosteric modulators) generally have the opposite EEG effects—EEG suppression at delta/theta frequencies83,84,85,86 (but see an exception for delta here77) and enhancement at beta frequencies77,83,84,87,88—accompanied by partial or full sedation89. This demonstrates a very different PPD in which depressant and hypnotic effects occur without delta oscillations and are instead marked by high frequency activity more typical of cortical activation.
Another relevant substance is the acetylcholinesterase inhibitor donepezil. In healthy elderly adults, one study reported that an acute dose (5 mg) of donepezil caused a significant increases in EEG delta power alongside memory impairments90. On the other hand, chronic treatment with donepezil improves cognition and memory91,92 and reduces delta power during both wakefulness93 and REM sleep94 in patients with Alzheimer’s disease. While donepezil may have no obvious effect on waking consciousness, both it95 and another acetylcholinesterase inhibitor, galantamine96, affect sleeping consciousness by stimulating lucid dreaming, but this effect has not yet been studied in relation to EEG delta activity using acetylcholinesterase inhibitor compounds.
Near the end of our list are two sedating compounds, gabapentin and gaboxadol. Gabapentin, an analgesic and antiepileptic drug, is known to influence delta EEG activity. In rats, gabapentin normalizes deficits in delta power during slow-wave sleep induced by ethanol97. In humans, chronic daily use of gabapentin increases the percentage of delta power relative to a control group that received no drug or placebo98. However, it remains unknown whether gabapentin acutely enhances delta EEG power during wakefulness, especially at lower doses that do not require gradual titration. Next, gaboxadol is a muscimol derivative99 that has, like gabapentin, been shown to enhance low-frequency EEG power during NREM sleep100. In mice, gaboxadol administration leads to slow wave activity during wakefulness and REM sleep101. In humans, gaboxadol has been shown to enhance low-frequency power (including delta) during overnight wakefulness in the context of sleep restriction102. Delta enhancement has also been noted alongside enhancement of the theta and alpha bands in resting-state MEG recordings from male volunteers challenged with gaboxadol22. However, the amplitude of this gaboxadol-induced delta activity in humans is unclear and should be characterized by further studies.
Finally, opioids have been shown to increase delta EEG power103,104, but it is unclear to what extent this occurs in humans outside of anesthetic doses, and, moreover, this effect is inconsistent, with some studies reporting attenuation of delta activity by opioids105,106.
The effects of these and other substances on delta oscillations and consciousness warrant further investigation, as in many of the above cases, delta enhancement appears limited to a particular anatomical region, arousal state, or chronic dosing.
Outlook and perspectives
Until recently, large delta and slow waves were commonly interpreted as evidence of unconsciousness. This interpretation is challenged by many documented exceptions, both from neurological disorders (reviewed previously5) and pharmacological manipulations. At first glance, some studies we have reviewed may appear to show that delta-enhancing drugs (e.g., tiagabine, GHB, scopolamine) simply induce drowsiness and partial-sedation. However, this seemingly uncomplicated relationship is contradicted by studies of psychedelic tryptamines such as DMT, which induce hypervivid experiences59 that often feel “more real than everyday normal consciousness”107,108. Future work is necessary to determine under what circumstances a gradual increase in delta activity may also indicate a gradual loss of consciousness and why this is not always the case (Box 1).
Although most pharmaco-EEG/MEG studies we cited used small samples (N ≤ 20, see Table 1), their findings are nonetheless sufficient to demonstrate that delta waves are in principle possible during consciousness. Because these substances allow PPDs to be observed in healthy volunteers, they may prove invaluable for future pharmaco-EEG experiments that seek to validate EEG biomarkers of conscious state (e.g., PCI) in neurologically-typical adults. In particular, GABABergic substances such as GHB may prove to be safe and well-tolerated models for abnormal cortical dynamics manifesting in the delta band. Following recent work that has used rare neurogenetic disorders to model abnormal cortical dynamics for the purpose of validating EEG biomarkers of consciousness14, such substances could be administered in healthy adult individuals to further validate EEG markers of consciousness such as the entropy of spontaneous EEG signals or perturbational complexity.
Data availability
Fig. 1 is based on previously published tiagabine MEG data which are publicly available through the Harvard Dataverse repository: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/9Q1SKM. The source data used to plot Fig. 1b are also provided in Supplementary Data 1.
References
Brown, E. N., Lydic, R. & Schiff, N. D. General anesthesia, sleep, and coma. N. Engl. J. Med. 363, 2638–2650 (2010).
Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 (2008).
Murphy, M. et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep 34, 283–291 (2011).
Nilsen, A. S., Juel, B. E., Thürer, B., Aamodt, A. & Storm, J. F. Are we really unconscious in” unconscious states”? Common assumptions revisited. Front. Hum. Neurosci. 16, 678 (2022).
Frohlich, J., Toker, D. & Monti, M. M. Consciousness among delta waves: a paradox? Brain 144, 2257–2277 (2021).
Frohlich, J. et al. High-voltage, diffuse delta rhythms coincide with wakeful consciousness and complexity in Angelman syndrome. Neurosci. Conscious. 2020, niaa005 (2020).
Buzsaki, G. Rhythms of the Brain (Oxford university press, 2006).
Nir, Y. et al. Regional slow waves and spindles in human sleep. Neuron 70, 153–169 (2011).
Compte, A., Sanchez-Vives, M. V., McCormick, D. A. & Wang, X.-J. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 89, 2707–2725 (2003).
Sanchez-Vives, M. V. Origin and dynamics of cortical slow oscillations. Curr. Opin. Physiol. 15, 217–223 (2020).
Tononi, G. Phi: A Voyage from the Brain to the Soul (Pantheon, 2012).
Oizumi, M., Albantakis, L. & Tononi, G. From the phenomenology to the mechanisms of consciousness: integrated information theory 3.0. PLoS Comput. Biol. 10, e1003588 (2014).
Mediano, P. A., Rosas, F. E., Bor, D., Seth, A. K. & Barrett, A. B. The strength of weak integrated information theory. Trends Cogn. Sci. 26, 646–655 (2022).
Frohlich, J. et al. Neural complexity is a common denominator of human consciousness across diverse regimes of cortical dynamics. Commun. Biol. 5, 1–17 (2022).
Timmermann, C. et al. Human brain effects of DMT assessed via EEG-fMRI. Proc. Natl Acad. Sci. 120, e2218949120 (2023). Pioneering study with simultaneous EEG and fMRI found that frontal delta power induced by DMT correlates with increases in global fMRI connectivity.
Ambrósio, A. F., Soares-da-Silva, P., Carvalho, C. M. & Carvalho, A. P. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 27, 121–130 (2002).
Besser, R., Hornung, K., Theisohn, M., Rothacher, G. & Krämer, G. EEG changes in patients during the introduction of carbamazepine. Electroencephalogr. Clin. Neurophysiol. 83, 19–23 (1992).
Matsuura, M. et al. Clinical significance of diffuse delta EEG activity in chronic schizophrenia. Clin. Electroencephalogr. 25, 115–121 (1994).
Koshino, Y. et al. Frontal intermittent delta activity in schizophrenic patients receiving antipsychotic drugs. Clin. Electroencephalogr. 24, 13–18 (1993).
Darmani, G., Nieminen, J., Bergmann, T., Ramezanpour, H. & Ziemann, U. A degraded state of consciousness in healthy awake humans? Brain Stimul.: Basic Transl. Clin. Res. Neuromodulation 14, 710–712 (2021). Participants given tiagabine show increases in broadband EEG power relative to baseline and a PCI value that is intermediate between the range of consciousness and unconsciousness.
Meldrum, B. S. & Chapman, A. G. Basic mechanisms of gabitril (tiagabine) and future potential developments. Epilepsia 40, S2–S6 (1999).
Nutt, D. et al. Differences between magnetoencephalographic (MEG) spectral profiles of drugs acting on GABA at synaptic and extrasynaptic sites: a study in healthy volunteers. Neuropharmacology 88, 155–163 (2015).
Barnett, L., Muthukumaraswamy, S. D., Carhart-Harris, R. L. & Seth, A. K. Decreased directed functional connectivity in the psychedelic state. NeuroImage 209, 116462 (2020). A clear demonstration of diffuse delta MEG power enhancement with tiagabine relative to placebo in healthy volunteers (data are shown in Figure 1).
Lancel, M., Faulhaber, J. & Deisz, R. A. Effect of the GABA uptake inhibitor tiagabine on sleep and EEG power spectra in the rat. Br. J. Pharmacol. 123, 1471–1477 (1998).
Mathias, S., Wetter, T. C., Steiger, A. & Lancel, M. The GABA uptake inhibitor tiagabine promotes slow wave sleep in normal elderly subjects. Neurobiol. Aging 22, 247–253 (2001).
Muthukumaraswamy, S. PharmacoMEG 1/f data https://doi.org/10.7910/DVN/9Q1SKM (2018).
Frohlich, J. et al. Electrophysiological phenotype in Angelman syndrome differs between genotypes. Biol. Psychiatry 85, 752–759 (2019).
Hamandi, K., Myers, J. & Muthukumaraswamy, S. Tiagabine-induced stupor—more evidence for an encephalopathy. Epilepsy Behavior 196–197 (2014).
Casali, A. G. et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 5, 198ra105–198ra105 (2013).
Comolatti, R. et al. A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain stimulation 12, 1280–1289 (2019).
Casarotto, S. et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 80, 718–729 (2016).
Sarasso, S. et al. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr. Biol. 25, 3099–3105 (2015).
Vienne, J. et al. Differential effects of sodium oxybate and baclofen on EEG, sleep, neurobehavioral performance, and memory. Sleep 35, 1071–1084 (2012). During waking from daytime naps, healthy male volunteers given baclofen showed enhancement of EEG power relative to placebo (~4–10 Hz).
Snead, O. C. III Evidence for a G protein‐coupled γ‐hydroxybutyric acid receptor. J. Neurochem. 75, 1986–1996 (2000).
van Amsterdam, J. G., van Laar, M., Brunt, T. M. & van den Brink, W. Risk assessment of gamma-hydroxybutyric acid (GHB) in the Netherlands. Regulatory Toxicol. Pharmacol. 63, 55–63 (2012).
Metcalf, D. R., Emde, R. N. & Stripe, J. T. An EEG-behavioral study of sodium hydroxybutyrate in humans. Electroencephalogr. Clin. Neurophysiol. 20, 506–512 (1966).
von Rotz, R. et al. Neuronal oscillations and synchronicity associated with gamma-hydroxybutyrate during resting-state in healthy male volunteers. Psychopharmacology 234, 1957–1968 (2017). In the first study to apply source localization and connectivity analyses to pharmaco-EEG with GHB at low doses, posterior cingulate cortex appears to be a source of low frequency activity and GHB plasma concentrations correlate with delta-band lagged phase synchronization.
Dornbierer, D. A. et al. Neurophysiological signature of gamma-hydroxybutyrate augmented sleep in male healthy volunteers may reflect biomimetic sleep enhancement: a randomized controlled trial. Neuropsychopharmacology 44, 1985–1993 (2019).
Mamelak, M., Escriu, J. & Stokan, O. The effects of gamma-hydroxybutyrate on sleep. Biol. Psychiatry 12, 273–288 (1977).
Badr, G. G., Matousek, M. & Frederiksen, P. K. A quantitative EEG analysis of the effects of baclofen on man. Neuropsychobiology 10, 13–18 (1983).
Vienne, J., Bettler, B., Franken, P. & Tafti, M. Differential effects of GABAB receptor subtypes, γ-hydroxybutyric acid, and baclofen on EEG activity and sleep regulation. J. Neurosci. 30, 14194–14204 (2010).
Hodor, A., Palchykova, S., Gao, B. & Bassetti, C. Baclofen and gamma-hydroxybutyrate differentially altered behavior, EEG activity and sleep in rats. Neuroscience 284, 18–28 (2015).
Johnstone, A., Grigoras, I., Petitet, P., Capitão, L. P. & Stagg, C. J. A single, clinically relevant dose of the GABAB agonist baclofen impairs visuomotor learning. J. Physiol. 599, 307–322 (2021).
McDonnell, M. N., Orekhov, Y. & Ziemann, U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 173, 86–93 (2006).
Barbero-Castillo, A. et al. Impact of GABAA and GABAB inhibition on cortical dynamics and perturbational complexity during synchronous and desynchronized states. J. Neurosci. 41, 5029–5044 (2021).
Saper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J. & Scammell, T. E. Sleep state switching. Neuron 68, 1023–1042 (2010).
Wikler, A. Pharmacologic dissociation of behavior and EEG “sleep patterns” in dogs: morphine, N-allylnormorphine, and atropine. Proc. Soc. Exp. Biol. Med. 79, 261–265 (1952).
Ostfeld, A., Machne, X. & Unna, K. The effects of atropine on the electroencephalogram and behavior in man. J. Pharmacol. Exp. Therapeutics 128, 265–272 (1960).
Kikuchi, M. et al. EEG changes following scopolamine administration in healthy subjects. Neuropsychobiology 39, 219–226 (1999).
Sannita, W. G., Maggi, L. & Rosadini, G. Effects of scopolamine (0.25–0.75 mg im) on the quantitative EEG and the neuropsychological status of healthy volunteers. Neuropsychobiology 17, 199–205 (1987).
Sloan, E. P., Fenton, G. W. & Standage, K. P. Anticholinergic drug effects on quantitative electroencephalogram, visual evoked potential, and verbal memory. Biol. Psychiatry 31, 600–606 (1992).
Neufeld, M. et al. Effects of a single intravenous dose of scopolamine on the quantitative EEG in Alzheimer’s disease patients and age-matched controls. Electroencephalogr. Clin. Neurophysiol. 91, 407–412 (1994).
Ebert, U., Grossmann, M., Oertel, R., Gramatté, T. & Kirch, W. Pharmacokinetic‐pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J. Clin. Pharmacol. 41, 51–60 (2001).
Bajo, R. et al. Scopolamine effects on functional brain connectivity: a pharmacological model of Alzheimer’s disease. Sci. Rep. 5, 9748 (2015).
Castro-Zaballa, S. et al. EEG dissociation induced by muscarinic receptor antagonists: Coherent 40 Hz oscillations in a background of slow waves and spindles. Behavioural Brain Res. 359, 28–37 (2019). Based on intracranial recordings from cats challenged separately with atropine and scopolamine, this study speculates that animals maintain consciousness during cortical slow waves due to high levels of gamma synchronization.
Crick, F. & Koch, C. Towards a Neurobiological Theory of Consciousness Vol. 2, 263–275 (Saunders Scientific Publications, 1990).
Cantero, J. L., Atienza, M., Madsen, J. R. & Stickgold, R. Gamma EEG dynamics in neocortex and hippocampus during human wakefulness and sleep. Neuroimage 22, 1271–1280 (2004).
Bayne, T. & Carter, O. Dimensions of consciousness and the psychedelic state. Neurosci. Conscious. 2018, niy008 (2018).
Lawrence, D. W., Carhart-Harris, R., Griffiths, R. & Timmermann, C. Phenomenology and content of the inhaled N, N-dimethyltryptamine (N, N-DMT) experience. Sci. Rep. 12, 8562 (2022).
Lewis, R. Ayahuasca. Encyclopædia Britannica. Available at: https://www.britannica.com/science/ayahuasca (Accessed: 12 June 2023) (2017).
Dean, J. G. et al. Biosynthesis and extracellular concentrations of N, N-dimethyltryptamine (DMT) in mammalian brain. Sci. Rep. 9, 1–11 (2019).
Timmermann, C. et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci. Rep. 9, 16324 (2019).
Lempel, A. & Ziv, J. On the complexity of finite sequences. IEEE Trans. Inf. Theory 22, 75–81 (1976).
Pallavicini, C. et al. Neural and subjective effects of inhaled N, N-dimethyltryptamine in natural settings. J. Psychopharmacol. 35, 406–420 (2021).
Riga, M. S., Lladó-Pelfort, L., Artigas, F. & Celada, P. The serotonin hallucinogen 5-MeO-DMT alters cortico-thalamic activity in freely moving mice: regionally-selective involvement of 5-HT1A and 5-HT2A receptors. Neuropharmacology 142, 219–230 (2018).
Strassman, R. J., Qualls, C. R., Uhlenhuth, E. H. & Kellner, R. Dose-response study of N, N-dimethyltryptamine in humans: II. Subjective effects and preliminary results of a new rating scale. Arch. Gen. Psychiatry 51, 98–108 (1994).
Funk, C. M., Honjoh, S., Rodriguez, A. V., Cirelli, C. & Tononi, G. Local slow waves in superficial layers of primary cortical areas during REM sleep. Curr. Biol. 26, 396–403 (2016). Laminar recordings in mice demonstrate slow waves in primary sensory cortex and motor cortex during REM sleep, suggesting a role for slow waves during states of sensory disconnect during consciousness (e.g., dreaming).
Langille, J. J. Human REM sleep delta waves and the blurring distinction between NREM and REM Sleep. J. Neurosci. 39, 5244 (2019).
Bernardi, G. et al. Regional delta waves in human rapid eye movement sleep. J. Neurosci. 39, 2686–2697 (2019).
Thomas, C. W. et al. Psilocin acutely alters sleep-wake architecture and cortical brain activity in laboratory mice. Transl. Psychiatry 12, 1–13 (2022).
Ort, A. et al. TMS-EEG and resting-state EEG applied to altered states of consciousness: oscillations, complexity, and phenomenology. Iscience 26 (2023).
Frohlich, J. & Van Horn, J. D. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 28, 287–302 (2014).
Vesuna, S. et al. Deep posteromedial cortical rhythm in dissociation. Nature 586, 87–94 (2020). Foundational study demonstrating that ketamine induces delta rhythms in posteromedial cortex of mice, recently replicated by Tian et al. in humans.
Tian, F. et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat. Commun. 14, 1748 (2023).
Farnes, N., Juel, B. E., Nilsen, A. S., Romundstad, L. G. & Storm, J. F. Increased signal diversity/complexity of spontaneous EEG, but not evoked EEG responses, in ketamine-induced psychedelic state in humans. PLoS ONE 15, e0242056 (2020).
Vlisides, P. et al. Subanaesthetic ketamine and altered states of consciousness in humans. Br. J. Anaesth. 121, 249–259 (2018).
Forsyth, A. et al. Comparison of local spectral modulation, and temporal correlation, of simultaneously recorded EEG/fMRI signals during ketamine and midazolam sedation. Psychopharmacology 235, 3479–3493 (2018).
Dworak, M., McCarley, R. W., Kim, T. & Basheer, R. Delta oscillations induced by ketamine increase energy levels in sleep-wake related brain regions. Neuroscience 197, 72–79 (2011).
Akeju, O. et al. Electroencephalogram signatures of ketamine anesthesia-induced unconsciousness. Clin. Neurophysiol. 127, 2414–2422 (2016).
Rosen, I. & Hägerdal, M. Electroencephalographic study of children during ketamine anesthesia. Acta Anaesthesiologica Scandinavica 20, 32–39 (1976).
Domino, E. F. Neuronal mechanisms of ketamine-induced anesthesia. Int. J. Neuropharmacol. 7, 557–573 (1968).
Goeldner, C. et al. A randomized, double-blind, placebo-controlled phase II trial to explore the effects of a GABAA-α5 NAM (basmisanil) on intellectual disability associated with Down syndrome. J. Neurodev. Disord. 14, 1–14 (2022).
Hipp, J. F. et al. Basmisanil, a highly selective GABAA-α5 negative allosteric modulator: preclinical pharmacology and demonstration of functional target engagement in man. Sci. Rep. 11, 7700 (2021).
Nickel, B. & Szelenyi, I. Comparison of changes in the EEG of freely moving rats induced by enciprazine, buspirone and diazepam. Neuropharmacology 28, 799–803 (1989).
Buchsbaum, M. S. et al. Topographic EEG changes with benzodiazepine administration in generalized anxiety disorder. Biol. Psychiatry 20, 832–842 (1985).
van Lier, H., Drinkenburg, W. H., Van Eeten, Y. J. & Coenen, A. M. Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats. Neuropharmacology 47, 163–174 (2004).
Frohlich, J. et al. Mechanisms underlying the EEG biomarker in Dup15q syndrome. Mol. Autism 10, 1–15 (2019).
Jensen, O. et al. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage 26, 347–355 (2005).
Buffett-Jerrott, S. & Stewart, S. Cognitive and sedative effects of benzodiazepine use. Curr. Pharm. Des. 8, 45–58 (2002).
Balsters, J. H. et al. Donepezil impairs memory in healthy older subjects: behavioural, EEG and simultaneous EEG/fMRI biomarkers. PLoS ONE 6, e24126 (2011).
Matthews, H. P., Korbey, J., Wilkinson, D. G. & Rowden, J. Donepezil in Alzheimer’s disease: eighteen month results from Southampton Memory Clinic. Int. J. Geriatr. psychiatry 15, 713–720 (2000).
Seltzer, B. et al. Efficacy of donepezil in early-stage Alzheimer disease: a randomized placebo-controlled trial. Arch. Neurol. 61, 1852–1856 (2004).
Balkan, S. et al. Effect of donepezil on EEG spectral analysis in Alzheimer’s disease. Acta Neurologica Belgica 103, 164–169 (2003).
dos Santos Moraes, W. A. et al. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep 29, 199–205 (2006).
LaBerge, S. The Paradox and Promise of Lucid Dreaming: Research Update: Cholinergic Stimulation of Lucid Dreaming; Voluntary Control of Auditory Perception During Rem Lucid Dreams (International Association for the Study of Dreams, 2001).
LaBerge, S., LaMarca, K. & Baird, B. Pre-sleep treatment with galantamine stimulates lucid dreaming: A double-blind, placebo-controlled, crossover study. PLoS ONE 13, e0201246 (2018).
Ehlers, C. L., Sanchez-Alavez, M. & Wills, D. Effect of gabapentin on sleep and delta and theta EEG power in adult rats exposed to chronic intermittent ethanol vapor and protracted withdrawal during adolescence. Psychopharmacology 235, 1783–1791 (2018).
Salinsky, M. C. et al. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia 43, 482–490 (2002).
Wafford, K. A. & Ebert, B. Gaboxadol—a new awakening in sleep. Curr. Opin. Pharmacol. 6, 30–36 (2006).
Faulhaber, J., Steiger, A. & Lancel, M. The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology 130, 285–291 (1997).
Vyazovskiy, V. V., Kopp, C., Bösch, G. & Tobler, I. The GABAA receptor agonist THIP alters the EEG in waking and sleep of mice. Neuropharmacology 48, 617–626 (2005).
Younes, M., Schweitzer, P. K., Griffin, K. S., Balshaw, R. & Walsh, J. K. Comparing two measures of sleep depth/intensity. Sleep 43, zsaa127 (2020).
Volavka, J., Zaks, A., Roubicek, J. & Fink, M. Electrographic effects of diacetylmorphine (heroin) and naloxone in man. Neuropharmacology 9, 587–593 (1970).
Scott, J. C., Cooke, J. E. & Stanski, D. R. Electroencephalographic quantitation of opioid effect: comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology 74, 34–42 (1991).
Lelic, D., Hansen, T. M., Mark, E. B., Olesen, A. E. & Drewes, A. M. The effects of analgesics on central processing of tonic pain: a cross-over placebo controlled study. Neuropharmacology 123, 455–464 (2017).
Phillips, R. L., Herning, R. & London, E. D. Morphine effects on the spontaneous electroencephalogram in polydrug abusers: correlations with subjective self-reports. Neuropsychopharmacology 10, 171–181 (1994).
Shanon, B. The Antipodes of the Mind: Charting the Phenomenology of the Ayahuasca Experience (Oxford University Press on Demand, 2002).
Griffiths, R. R., Hurwitz, E. S., Davis, A. K., Johnson, M. W. & Jesse, R. Survey of subjective” God encounter experiences”: Comparisons among naturally occurring experiences and those occasioned by the classic psychedelics psilocybin, LSD, ayahuasca, or DMT. PLoS ONE 14, e0214377 (2019).
Darmani, G. et al. Effects of antiepileptic drugs on cortical excitability in humans: A TMS-EMG and TMS-EEG study. Hum. Brain Mapp. 40, 1276–1289 (2019).
Acknowledgements
The authors acknowledge support from the Open Access Publishing Fund of the University of Tuebingen. We also warmly thank Suresh Muthukumaraswamy for sharing tiagabine MEG data used to generate Fig. 1 in this manuscript.
Author information
Authors and Affiliations
Contributions
J.F. conceived of the idea for this mini-review, performed the literature review, wrote the first draft, and generated the display items; P.A.M.M. connected the project with external tiagabine data and gave conceptual input; F.B. provided expertise on neuropharmacology and guided the literature review; A.G. supervised the project. All authors helped edit the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.F. is a former employee of F. Hoffmann-La Roche Ltd. (October 2016–July 2017). All other authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: George Inglis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frohlich, J., Mediano, P.A.M., Bavato, F. et al. Paradoxical pharmacological dissociations result from drugs that enhance delta oscillations but preserve consciousness. Commun Biol 6, 654 (2023). https://doi.org/10.1038/s42003-023-04988-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04988-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.