Abstract
Mesenchymal stem/Stromal cells (MSCs) have great therapeutic potentials, and they have been isolated from various tissues and organs including definitive endoderm (DE) organs, such as the lung, liver and intestine. MSCs have been induced from human pluripotent stem cells (hPSCs) through multiple embryonic lineages, including the mesoderm, neural crest, and extraembryonic cells. However, it remains unclear whether hPSCs could give rise to MSCs in vitro through the endodermal lineage. Here, we report that hPSC-derived, SOX17+ definitive endoderm progenitors can further differentiate to cells expressing classic MSC markers, which we name definitive endoderm-derived MSCs (DE-MSCs). Single cell RNA sequencing demonstrates the stepwise emergence of DE-MSCs, while endoderm-specific gene expression can be elevated by signaling modulation. DE-MSCs display multipotency and immunomodulatory activity in vitro and possess therapeutic effects in a mouse ulcerative colitis model. This study reveals that, in addition to the other germ layers, the definitive endoderm can also contribute to MSCs and DE-MSCs could be a cell source for regenerative medicine.
Similar content being viewed by others
Introduction
Mesenchymal stem/stromal cells (MSCs) are multipotent stem cells that can be obtained from diverse human tissues and organs1,2. MSCs demonstrate great therapeutical values for various diseases, such as cardiovascular diseases3,4,5,6, respiratory diseases7,8, and gastrointestinal diseases9,10. MSCs from different organs display unique genetic features that are associated with their lineage origins11,12,13. MSCs can also be generated from human pluripotent stem cells (hPSCs), which emerges as a valuable source to produce MSCs for translational applications.
hPSCs include embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), and they can theoretically differentiate into all cell types in the body14. hPSCs can also differentiate from MSCs in vitro through multiple lineages, such as the ectoderm15, mesoderm16, neuromesoderm17, and extraembryonic lineages18. However, there is no report on whether hPSCs could generate MSCs through the endoderm lineage. In contrast, MSCs have been successfully derived from endodermal organs, such as the lung, intestine, and liver19,20. Although it is generally believed that MSCs in endodermal organs come from the mesodermal origin, this concept has been challenged. For example, pericytes are a clear source for MSCs isolated from various organs21,22,23,24,25, and MSCs located in the cephalic region are of the neuroectodermal origin (via pericytes), but not the mesodermal origin26.
Here, we hypothesize that MSCs can be generated from hPSCs also through the endodermal lineage. We show that, indeed endoderm progenitors can give rise to MSCs in vitro. Like MSCs from the other sources, the endoderm-derived MSCs also displayed anti-inflammatory activity and ameliorated dextran sulfate sodium (DSS)-induced colitis in ulcerative colitis mouse model.
Results
MSC generation from hPSCs via definitive endoderm progenitors
To investigate whether MSCs could be induced from endoderm lineage in vitro, we decided to learn more about MSCs derived from endoderm organs. We examined the gene expression of MSCs derived from endoderm tissues. First, we derived MSCs from human endodermal tissues, including the colon and liver, and we also cultured MSCs originated from mesoderm adipose and extraembryonic umbilical cord as control (Supplementary Fig. 1a). We demonstrated that all these MSCs had the multipotency to become adipocyte, chondrocyte and osteocyte in cell culture (Supplementary Fig. 1b, c). We then compared the RNA-seq profile of these MSCs along with H9 hESCs. Based on transcriptome analysis, all the MSCs were clustered together against hESCs, and they all expressed genes that were specifically enriched in stromal cells according to Enrichr Cell Type Analysis (Supplementary Fig. 1d).
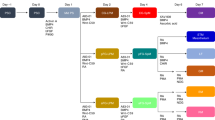
Next, we developed a method that could generate MSCs from hPSCs through definitive endoderm in cell culture. In order to track the emergence of definitive endoderm progenitors, SOX17 promoter-driven-GFP H9 hESCs were used in this project27. Five stages of treatments were applied on hESCs to induce MSCs through endoderm lineage (Fig. 1a). Stage 1 (day 0–1): Exit from pluripotency by WNT activation (GSK3 inhibitor CHIR99021) and Activin A in a defined medium; Stage 2 (day 1–3): The induction of definitive endoderm progenitors by Activin A in a defined medium; Stage 3 (day 3–5): MSC induction with MSC medium or serum-free condition; Stage 4 (day 5–15): MSC commitment with MSC medium or serum-free condition; Stage 5 (day 15–24): MSC enrichment and expansion in MSC medium.
a Stage-wise differentiation strategy to induce DE-MSCs with definitive endoderm (DE) origin from hESCs. DE-MSCs were obtained on day 24. b Cell morphology (upper panel) and expression of SOX17-GFP (lower panel) from day 0 to day 24. Spindle-like cells were observed on day 24 and they were SOX17-GFP negative on day 24. Scale bar = 100 μm. c RT-qPCR analysis for mRNA level of SOX17, CD44, NT5E (CD73), and ENG (CD105) on day 3, 5, 10, 15 (Passage 0, P0), 18 (Passage 1, P1), 21 (Passage 2, P2), 24 (Passage 3, P3) (n > 3), *p < 0.05, **p < 0.01. d Flow cytometry analysis of percentage of CD44+, CD73+, CD105+, CD45+ cells. The blue line represented IgG isotype control for the gating strategy, the red line represented the percentage of CD44+, CD73+, CD105+, and CD45+ cells. e Flow cytometry analysis of the percentage of SOX17+ cells on day 0, 3, 5, 10, 15, 18 (P1), 21 (P2), 24 (P3) (left panel), and flow cytometry analysis of the percentage of CD44+, CD73+, and CD105+ cells on day 18 (P1), 21 (P2), 24 (P3) (right panel) (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001. f Total cell number of DE-MSCs from passage 3 (P3) to passage 6 (P6) (n = 3). g RT-qPCR analysis was used to detect adipogenic (C/EBPβ), chondrogenic (COL2A1) and osteogenic (RUNX2) marker genes (n = 3), **p < 0.01, ***p < 0.001. h DE-MSCs were induced into adipocytes (stained with Oil Red O), chondrocytes (stained with Alcian Blue), and osteocytes (stained with Alizarin Red). Scale bar = 150 μm for an upper panel, scale bar = 75 μm for lower panel.
During the MSC induction process, SOX17-GFP was not observed in hESCs on day 0, but most cells become SOX17-GFP positive at the end of Stage 2 on day 3, indicating the emergence of definitive endoderm progenitors. We also showed that most cells were positive for both endoderm markers SOX1727 and CXCR428, and the percentage of SOX17+/ CXCR4+ cells was above 90%. It suggested that definitive endoderm progenitors were effectively induced from hESCs in 3 days (Supplementary Fig. 2a, b). At stage 3, the culture medium was switched to MSC medium, and SOX17-GFP positive percentage was maintained on day 5, but they gradually decreased afterward (Fig. 1b). RT-qPCR assay showed that the expression of SOX17 started to decrease after day 3, while the expression of MSC classical markers, including CD44, NT5E (CD73) and ENG (CD105), were detected on day 15 (Fig. 1c). The expression of MSC markers further increased after subsequent passages (Fig. 1c). Flow cytometry analysis demonstrated that almost all cells became CD44+/CD73+/CD105+, while remained CD45- after three passages (Fig. 1d). More detailed analysis showed that SOX17-GFP+ cells gradually decreased after day 3 (left panel of Fig. 1e) while cells with positive MSC markers increased (right panel of Fig. 1e). These data indicated that definitive endoderm progenitors could be induced to cells expressing classical MSC markers.
The proliferation ability of in vitro generated MSCs was confirmed (Fig. 1f). MSCs are usually defined by their multipotency to differentiate into multiple cell types in vitro. We treated the above endoderm-originated cells with specific differentiation conditions. We showed that those cells could be further induced into adipocytes, chondrocytes, and osteocytes (Fig. 1g, h). Considering that these cells fit the criteria of MSCs based on marker gene expression, morphology, and multipotency, we named them definitive endoderm-originated MSCs (DE-MSCs). Furthermore, we showed that DE-MSCs could be induced to pancreatic cell fate with the formation of an islet-like structure and the elevated expression of pancreatic progenitor marker genes PDX129 and PTF1A30 (Supplementary Fig 2c and Fig. 2d). These results strengthened the argument that DE-MSCs had the multipotent potentials.
To confirm that DE-MSCs were indeed induced from definitive endoderm progenitors, we sorted SOX17-GFP positive DE progenitors on day 3 and cultured them in MSC medium (Supplementary Fig. 2e, f). SOX17-GFP DE progenitors survived well after sorting (Supplementary Fig. 2g), proliferated, and finally became fibroblast-like cells (Supplementary Fig. 2h). Flow cytometry demonstrated that those cells were positive for CD44, CD73, and CD105 (Supplementary Fig. 2i). These data confirmed that definitive endoderm progenitors could contribute to MSCs in cell culture.
To examine the efficiency of the DE-MSC induction protocol, we tried to generate DE-MSCs from the H1 hESC line and NL-1 hiPSC line. These cells were induced to definitive endoderm progenitors and were then treated in an MSC medium. Fibroblast-like cells were observed after three weeks with CD44+ and CD105+ expression (Supplementary Fig. 2j and Fig. 2k). We then demonstrated that these cells could differentiate into trilineage (Supplementary Fig. 2l). These results suggested that the DE-MSC induction method is robust for various hPSCs lines.
Stepwise emergence of DE-MSCs in vitro revealed via scRNA-seq
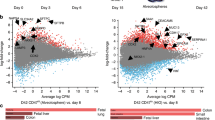
To understand how DE-MSCs emerge from definitive endoderm progenitors, scRNA-seq analysis was conducted after definitive endoderm was induced on day 3, day 5, and day 15. Based on the gene expression profile, cells were generally clustered according to their differentiation stages (Fig. 2a). On day 3 and day 5, most cells in these two stages were grouped together, respectively, indicating similar gene expression patterns. On day 15, two heterogeneous population emerge, implying cell type diversification (Fig. 2b). We showed that definitive endoderm marker genes (SOX17 and CXCR4) were highly expressed on day 3, but they decreased afterward (Fig. 2c). Meanwhile, some other endoderm marker genes were also expressed on both day 3 and day 5, such as FOXA231, OTX231, GATA432, and HHEX33 (Supplementary Fig. 3a). These results were consistent with Fig. 2 that most hESCs were effectively induced to definitive endoderm progenitors before MSC induction.
a Schematic figure showing the time points for collecting samples for single-cell RNA sequencing. b tSNE projection of day 3 (DE), day 5, and day 15 samples, and there were two clusters (cluster 1 and 2) in day 15 sample. c tSNE projection of definitive endoderm marker gene (SOX17 and CXCR4) expression on day 3 (DE), day 5, and day 15 clusters, indicating that SOX17 and CXCR4 were predominantly expressed in day 3 (DE) samples. d tSNE projection of gut (TTR), gut liver axis (AFP), and stromal (COL1A1 and LOX) marker gene expression on day 3 (DE), day 5, and day 15 clusters, showing that cells in cluster 2 of day 15 sample predominantly expressed AFP, COL1A1, and LOX. e Heatmap showing the column-scaled expression of DEG per cluster in day 3 (DE), day 5, and day 15 samples. Representative genes in each cluster were also shown. In addition, cell types were identified based on Enrichr. f Scatter plots showing the gene expression levels of CD44, NT5E (CD73), ENG (CD105), and NGFR(CD271) on day 3 (DE), day 5, and day 15 (cluster 1 and cluster 2) at single-cell resolution, indicating the emergence of MSCs in cluster 2 of day 15 sample.
We then showed that some gut/liver axis genes were expressed on day 5, and were further elevated on day 15. Some gut marker genes, such as TTR34, AFP35, APOA136, APOB36, and H1937 were expressed in both clusters on day 15, and most of them had higher expression in cluster 1. The shared endoderm gene expression indicated that both clusters came from the definitive endoderm progenitors. Meanwhile, stromal genes38 (COL1A1, LOX, COL3A1, CCDC80, and THY1) were highly expressed on day 15, especially in cluster 2 (Fig. 2d and Supplementary Fig. 3a). The current evidence suggested that MSCs emerged with some endoderm gene expression.
We then analyzed the gene expression profiles according to differentiation stages. Cells from day 3 and day 5 had more uniform gene expression profiles, while cells from day 15 could be split into two clusters (cluster 1 and 2). Enrichr cell type analysis shows that cluster 1 were more associated with hepatocyte, liver (bulk tissue), and gastric tissue (bulk), while cluster 2 were associated with stromal cell type fibroblast (Fig. 2e). We then demonstrate that cells in cluster 2 had higher expression of MSC markers such as CD44, NT5E (CD73), ENG (CD105), and NGFR (CD271) (Fig. 2f). These scRNA-seq results indicated that DE-MSCs emerged from definitive endoderm between day 5 and day 15.
We further examined DE-MSC induction under mitogenic regulation from day 3 to day 5. WNT pathway activator CHIR99021 (CHIR)39 significantly elevated the expression level of CD44, NT5E (CD73), and ENG (CD105). TGFβ / Activin pathway inhibitor SB43154240 and CHIR together also promoted MSC markers. In contrast, the combination of WNT inhibitor XAV93939 and SB431542 significantly suppressed MSC gene expression (Supplementary Fig. 4a). Further study showed that DE-MSCs can be induced under CHIR or CHIR/SB short-term treatment (Supplementary Fig. 4b). And these DE-MSCs could also be induced to adipocytes, chondrocytes and osteocytes (Supplementary Fig. 4c, d). These results indicate that WNT and TGFβ pathways play important roles in DE-MSC induction.
Till now, all DE-MSCs were induced with an MSC medium that contained bovine fetal serum (FBS), so we examined whether MSCs could be induced under serum-free conditions (Supplementary Fig. 5a). In serum-free chemically defined medium, the expression of MSC marker genes was elevated on day 15 (Supplementary Fig. 5b). MSC differentiation was enhanced by WNT activation (CHIR99021) and CHIR99021/SB431542 treatment (Supplementary Fig. 5c). scRNA-seq also showed that stromal population emerged without serum (Supplementary Fig. 5d). However, those cells from serum-free conditions could not be effectively expanded (data is not shown), suggesting that more studies are necessary to expand DE-MSCs in serum-free conditions.
Expression of origin-specific markers in DE-MSCs
In order to evaluate whether DE-MSC induction history could lead to any molecular signatures in MSCs, we compared DE-MSCs with MSCs that were induced from other lineages, including mesoderm-originated MSCs (Meso-MSCs)41, neural crest-originated MSCs (NC-MSCs)42, and trophoblast-originated MSCs (Troph-MSCs)43 (Fig. 3a). The lineage specificity was demonstrated by the expression of specific progenitor marker genes such as MESP1 (mesoderm), SOX10 (neural crest), and CGB (trophoblast) (Supplementary Fig. 6a). Meso-MSCs, NC-MSCs, and Troph-MSCs were then induced in MSC medium, and they were CD44+ and CD105+ (Supplementary Fig. 6b). We further demonstrated that all these cells had trilineage differentiation capacity (Supplementary Fig. 6c, d).
a Schematic flow of in vitro MSC generation from mesoderm (Meso-MSCs), neural crest (NC-MSCs), and trophoblast (Troph-MSCs) origins, scale bar = 400 μm. b Heatmap of DEG in NC-MSCs, Troph-MSCs, Meso-MSCs, DE-MSCs (CHIR/SB), DE-MSCs (CHIR), and DE-MSCs (FBS) compared with hESCs and cell type prediction by Enrichr database, indicating that all these MSC highly expressed the genes related to fibroblast rather than hESCs. c Heatmap of DEG in DE-MSCs (CHIR/SB), DE-MSCs (CHIR), DE-MSCs (FBS), Meso-MSCs, NC-MSCs, and Troph-MSCs and cell type prediction by Enrichr database, showing that MSCs originated from different developmental origin has organ-specific gene expression signatures. d Heatmap of DEG in DE-MSCs (CHIR/SB), DE-MSCs (FBS), and DE-MSCs (CHIR) and cell type prediction by Enrichr database, indicating CHIR or CHIR/SB treatment from day 3 to day 5 modified the gene signatures of DE-MSCs.
We then compared the transcriptomes of hESCs, DE-MSCs, Meso-MSCs, NC-MSCs, and Troph-MSCs. Compared to hESCs, all MSCs expressed genes that were enriched in fibroblast cell types by Enrichr analysis (Fig. 3b). DE-MSCs were clustered together, and NC-MSCs and Troph-MSCs were more closely associated. Further comparison among MSCs showed that each MSC type expressed genes associated with the specific lineage that were associated with the induction methods. DE-MSCs expressed genes enriched in endoderm organs, including the intestine, lung, and stomach. Meso-MSCs were related to myofibroblast and cardiovascular cells, NC-MSCs were associated with the fetal brain and spinal cord, and Troph-MSCs were related to the human placenta (Fig. 3c). We then showed that DE-MSCs derived with FBS alone were more associated with omentum and lung, CHIR treatment drove gene expression to more related to intestine, while CHIR/SB dual treatment led to upregulation of colon and pancreas related genes (Fig. 3d). These results indicated that MSC differentiated from endoderm progenitors could lead to endoderm signatures.
DE-MSC-mediated modulation of inflammatory responses in cell culture and mouse model
We next examined the immunoregulatory ability of DE-MSCs. MSCs were first exposed to proinflammatory factors IFN-γ, and we then checked the expression of proinflammatory cytokines (IL-6, IL-8, and CCL2) and anti-inflammatory cytokines (IDO1, PD-L1, and TSG6). RT-qPCR showed that IFN-γ upregulated the expression of proinflammatory IL-6, IL-8, and CCL2 in both DE-MSCs and UC-MSCs. For the anti-inflammatory genes, IFN-γ increased the expression of IDO1 and PD-L1 in both DE-MSCs and UC-MSCs, but TSG6 expression was not significantly changed (Fig. 4a and Supplementary Fig. 7a). We also showed that DE-MSCs (FBS) could upregulate anti-inflammatory cytokine TGF-β production in spleen cells (Fig. 4b). These findings suggested that DE-MSCs might have the potential to modulate inflammatory response through multiple pathways.
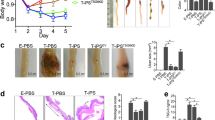
a mRNA levels of proinflammatory cytokines (IL-6 and IL-8) and anti-inflammatory cytokines (IDO1 and PD-L1) were analyzed by RT-qPCR assay in DE-MSCs (FBS), DE-MSCs (CHIR), DE-MSCs (CHIR/SB), and UC-MSCs that were exposed to IFN-γ (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. b Effect of DE-MSCs (FBS) and UC-MSCs on anti-inflammatory cytokine TGF-β level in spleens of colitis mice, showing the anti-inflammatory effect of DE-MSCs (FBS) (n = 3). **P < 0.01. c Average changes in body weight of each group from day 0 to day 14 (3–5 mice in each group). The unit of body weight was the gram. d Images of colons dissected from mice in healthy mice, DSS + PBS, DSS + PBS + DE-MSCs (FBS), DSS + PBS + DE-MSCs (CHIR), DSS + PBS + DE-MSCs (CHIR/SB), and DSS + PBS + UC-MSCs groups on day 14 (n = 5). e The length of colons from multiple experiments described in (c) were measured and plotted (n = 5). **p < 0.01, ***p < 0.001. f Hematoxylin and eosin (H&E) staining of distal colon sections from healthy mice, DSS + PBS, DSS + PBS + DE-MSCs (FBS), DSS + PBS + DE-MSCs (CHIR), DSS + PBS + DE-MSCs (CHIR/SB) groups, scale bar = 100 μm.
We then examined the effect of DE-MSCs in DSS-induced colitis in mice, which resembles human ulcerative colitis44. DSS treatment caused a significant decrease in weight in 14 days (Fig. 4c). However, peritoneal injection of DE-MSCs significantly alleviated the loss of body weight. After DE-MSC treatments, the body weight recovered close to that of a healthy animal after 14 days. We then demonstrated that DSS shortened colon length, but the symptom was suppressed by DE-MSCs (Fig. 4d, e). The analysis of the distal colon sections demonstrated that DSS disrupted intestinal epithelium, but DE-MSCs helped to suppress the disruption (Fig. 4e). H&E staining showed that the mouse intestinal epithelium was disrupted under DSS treatment, but DE-MSCs (FBS), DE-MSCs (CHIR), or DE-MSCs (CHIR/SB) treatments ameliorated epithelium disruption (Fig. 4f). Another pathological manifestation of colitis was an increase of CD8+ cytotoxic T cells in colon mucosa45,46. The infiltration by CD8+ cytotoxic T cells in DSS-induced colitis mice was detected by immunostaining. DE-MSCs and UC-MSCs treatments significantly reduced CD8+ cytotoxic T-cell infiltration (Supplementary Fig. 7b). One pathological manifestation of colitis is the depletion of mucin-producing goblet cells47. AB/PAS staining demonstrated the loss of mucin-producing goblet cells (rose red color cells) in DSS-induced colitis mice, but DE-MSCs and UC-MSCs treatments rescued the phenotype and ameliorated pathological deterioration (Supplementary Fig. 7c). According to previous studies48,49, DE-MSCs might have brought beneficial effects through their interaction with intermediate cells in DSS-induced colitis model. These data suggested that DE-MSCs could have therapeutic effects on colitis.
Discussion
MSCs are, in essence, cultured cells isolated from various tissues such as the bone marrow, skeletal muscles, placenta, and pancreas and have demonstrated remarkable effects in immune regulation and tissue regeneration as a therapy21,22. Whether identical cells exist naturally in vivo is unclear. Presumptive MSCs exist in various tissues and organs, however, the exact role of which in tissue turnover and repair is not known. MSCs have been induced from hPSCs through the mesoderm, neural crest, and extraembryonic lineages15,16,17,18. However, it is unclear whether the definitive endoderm has the potential to contribute to MSCs. In this study, we have demonstrated that definitive endoderm progenitors can be induced to generate MSCs (DE-MSCs) which possess multipotency and therapeutic effects.
Definitive endoderm progenitors have the potential to generate endodermal cell types such as hepatocytes, pancreatic islet cells, and pulmonary cells, given specific signaling modulators at proper times. The cell fate specification procedure can be diverted by exposing the progenitors to an MSC medium containing FBS, which leads to the emergence of MSCs. Apparently, some serum factors promote MSC fate against other more specialized endoderm cell types. We show that the induction of DE-MSCs is promoted by WNT activation but suppressed by WNT inhibition. Interestingly, WNT activation is also found to promote mesenchymal cell types related to epicardial and neural crest cells, which is consistent with previous reports50,51. It is possible that cells of all lineages have the plasticity to become MSCs before cell fate is finally determined. WNT activation is partially responsible for switching cell fate towards MSCs during organ formation52,53,54.
It has been reported that perivascular cells contribute to the isolation of MSCs from endodermal organs21,55,56. Perivascular cells, including pericytes and adventitial cells, have proved as native sources of MSCs in developing and adult human organs57. However, it awaits to be proved whether endodermal cells can be converted to MSCs in situ. In summary, this study demonstrates that definitive endodermal progenitors are a source of MSCs, which may be used for the treatment of degenerative diseases, especially, those in endodermal organs.
Methods
hPSC maintenance and differentiation to DE-MSCs
Under approval of the University of Macau’s Research Ethics Board, hESC lines H1 and H9 (from WiCell Research Institute, Inc., Madison, WI, http://www.wicell.org) and hiPSC line NL-1 (from NIH) were cultured in Matrigel-coated six-well plates in E8 medium with daily change, and cells were passaged every 3–4 days with EDTA when they reached 60–70% confluence58,59,60. For differentiation, 1 × 104 cells per well were seeded onto Matrigel-coated 24-well plate, and differentiation was initiated when cells reached about 40–50% confluence. hPSCs were induced into mesoendoderm under 5 μM CHIR99021 and 100 ng/ml Activin A in differentiation medium (DMEM/F12 supplemented with transferrin, chemically defined lipid concentrates, ascorbic acid, and sodium selenite) for 1 day, and definitive endoderm progenitors were patterned under 100 ng/ml Activin A treatment in differentiation medium for another 2 days. Subsequently, definitive endoderm progenitors were further differentiated in αMEM supplemented with 1 × MEM NEAA, 1 × GlutaMAXTM-I, 100 ng/ml FGF2, 10 μg/ml insulin, and 20% FBS (FBS condition) or without 20% FBS (serum-free condition) for another 12 days, and DE-MSCs (FBS condition) were generated after 2–3 passages (6–9 days). Optimally, 5 μM CHIR99021, 10 μM XAV939, 10 μM SB431542, and their combination can be applied from day 3 to day 5 under FBS condition or serum-free condition, and DE-MSCs (CHIR and CHIR/SB condition) can also be generated after 2–3 passages (6–9 days). FBS condition medium or serum-free condition medium was changed every 2 days. The information of chemicals and recombinant proteins was listed in Supplementary Table 1.
Isolation and expansion of MSCs from various human tissues
All primary cells were derived with ethics approval from Zhuhai People’s Hospital and the University of Macau. Colon-MSCs, liver-MSCs, and adipose-MSCs were isolated from the non-tumor colon, liver, and breast tissues, respectively. Umbilical cord-MSCs are isolated from umbilical cord tissue. The isolation and expansion of primary mesenchymal stem cells followed these steps: Briefly, the human tissues were cut into small pieces, and were then treated in a series of solutions at 37 °C, including Collagenase solution (DMEM/F12 supplemented with 5% FBS, 5 µg/ml insulin, 500 ng/ml hydrocortisone, 10 ng/ml EGF, 20 ng/ml cholera toxin, 300 U/ml collagenase III, 100 U/ml hyaluronidase) with intermittent pipetting for 2 h, Dispase solution (DMEM/F12 supplemented with 5 mg/ml Dispase II and 0.1 mg/ml deoxyribonuclease) for 5 min, and 0.25% trypsin solution for 2 min. The digestion was then stopped by DMEM/F12 medium with 5% FBS, and cells were washed with Hanks solution before treatment with RBC lysis buffer. After cells were washed with Hanks solution, they were cultured in a cell culture plate with MSC media. Cells were passaged 5 times before they were harvested for gene expression and functional analysis61.
Pancreatic induction from DE-MSCs
DE-MSCs were induced to pancreatic fate following these steps: Briefly, DM-MSCs were first cultured till 80% confluence, and were then differentiated towards β-cell-like cells in DMEM-low glucose medium, containing 5% platelet lysate, 10 µM retinoic acid (for 24 h only), 100 ng/ml Activin, 200 ng/ml glucagon-like peptide I (GLPI-1), 20 ng/ml epidermal growth factor (EGF), 10 ng/ml fibroblast growth factor (FGF), 10 ng/ml β-cellulin, 10 mM nicotinamide, and 2 mM glutamine. Cells were cultured in adherent conditions for the first 7 days and were then changed to ultra-low attachment dishes for another 2 weeks62.
Trilineage differentiation of DE-MSCs
For osteogenic differentiation, DE-MSCs were cultured in high glucose DMEM containing 10% FBS, 1×l-glutamine, 0.1 μM dexamethasone, 10 mM β-glycerophosphate, 10 ng/ml BMP2, and 50 μg/ml ascorbic acid for 21 days with medium changes every 3 days. For chondrogenic differentiation, DE-MSCs were cultured in high glucose DMEM, 10% FBS, 2 mM l-glutamine, 0.1 μM dexamethasone, 50 μg/ml ascorbic acid, 1% sodium pyruvate, 10% insulin-transferrin-selenium, 10 ng/ml BMP2, and 10 ng/mL TGFβ for 21 days with medium changes every 3 days. For adipogenic differentiation, DE-MSCs were cultured in high glucose DMEM containing 10% FBS, 2 mM L-glutamine, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine, 50 μM indomethacin, and 10 μg/ml insulin for 21 days with medium changes every 3 days63,64,65.
Generation of MSCs from hPSCs via the mesoderm, neural crest, and trophoblast origins
The generation of mesoderm, neural crest, and trophoblast progenitors were first induced according to published protocols with minor modifications41,42,43. These progenitors were then cultured in an MSC medium for 14 days and were subsequently passaged three times to generate mesoderm-originated MSCs (Meso-MSCs), neural crest-originated MSCs (NC-MSCs), and trophoblast-originated MSCs (Troph-MSCs).
A mouse colitis model induced with DSS
Mice were administered with 2% DSS (molecular weight 36,000–50,000, MP Biomedical) in the drinking water for 6 days to establish the colitis mouse model18. Each mouse was injected intraperitoneally with 5 × 106 DE-MSCs (FBS, CHIR, or CHIR/SB condition) or 5 × 106 UC-MSCs in 1 × PBS or 1 × PBS alone (negative control) on day 2 and day 3 after the start of the DSS treatment. The body weight of mice were measured daily from day 0 to 14. On day 14, these mice were euthanized by CO2 asphyxiation.
Upon necropsy, each mouse’s colon was dissected and measured for its length. The colons were rinsed with sterile 1 × DPBS and fixed in 4% PFA at 48 °C for 48–72 h. The distal part of the colon was embedded in paraffin wax and sectioned at 5 μm in thickness, mounted to glass slides, and hematoxylin and eosin (H&E) staining and immunostaining with PE-conjugated Rat Anti-Mouse CD8a antibody (BD Biosciences, cat. No. 553032) were performed. In addition, Alcian blue/Periodic Acid-Schiff (AB/PAS) staining was used to detect mucin-producing goblet cells according to the manufacturer’s protocol (Sigma-Aldrich, 395B-1KT). Images of stained sections were acquired on Leica whole slides scanner SCN400F.
RT-qPCR
mRNA was extracted with RNAiso-plus (TAKARA, cat. No.108-95-2) and reverse transcription (RT) was performed with a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, cat. No. 4368813). qPCR was conducted with SYBR Premix Ex Taq (TAKARA, cat. No. RR420) and the Quantstudio-7 system (Applied Biosystems). The relative amounts of the amplificated nucleotide fragment were calculated by the 2^(-ΔCt) method. Primer sequences were listed in Supplementary Table 2.
Flow cytometry
The MSCs were evaluated according to the expression of CD44, CD73, CD105, and PDGFRβ by flow cytometer (Becton Dickinson C6). The antibodies used were CD44 (156-3C11) Mouse mAb (1:1000, CST), anti-CD73 antibody (ab54217) (1:1000, Abcam), anti-CD105 antibody (ab11414) (1:1000, Abcam), and anti-PDGFR β antibody (ab69506) (1:1000, Abcam).
ELISA assay for anti-inflammatory cytokines
Monocytes were isolated from spleens and cultured in RPMI 1640 medium (1 × 106 cells/well in a 24-well plate). The supernatants were collected 3 days later for ELISA assay66. The level of anti-inflammatory cytokine TGF-β was measured by a murine ELISA kit according to the manufacturer’s protocol (Solarbio).
scRNA-seq
Single cells were isolated and processed with the Nadia system (Dolomite Bio), and the sequencing libraries were prepared according to standard protocol67.
Bulk RNA-seq
Total mRNA was extracted by RNAiso-plus (TAKARA, cat. No.108-95-2). The RNA libraries were generated using the TruSeq RNA Sample Preparation kit (Illumina), and cDNA fragments were enriched by PCR using Illumina TruSeq PCR primers. Each library was sequenced as paired-end reads in HiSeq 2000/1000 (Illumina).
Bioinformatic analysis
The raw data of scRNA-seq was processed by the Drop-seq tools protocol (http://mccarrolllab.org/). Data normalization was conducted by R package “Seurat”. Seven hundred cells were chosen for each group and 2000 variable features were picked to cluster. The tSNE graphs for the FBS condition were set up according to the first 14 PCs (k = 20). The tSNE graphs for the serum-free condition were set up according to the first 13 PCs (k = 20). Cell types were identified with R package “SingleR” and Enrichr (https://maayanlab.cloud/Enrichr/).
Transcripts Per Million (TPM) was used to normalize gene read counts for bulk RNA-seq analysis. TPM values of corresponding genes were further normalized based on Z-score. R package ggplot2, as well as the Python package seaborn, was used to generate heatmaps. R package Deseq2 was used to pick differentially expressed genes (DEG) with q value <0.05, fold change >2 or <−2.
Statistics and reproducibility
The standard error of the mean (SEM) was calculated based on three or more independent experiments. Statistical significance was determined using a t-test.
Data transparency
The web-based tool Enrichr is available at https://maayanlab.cloud/Enrichr/.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The scRNA-seq and bulk RNA-seq data of this study have been deposited in the NCBI’s BioProject under accession code PRJNA709641. The numerical source data for RT-qPCR, flow cytometry, mouse weight, and mouse colon length are available in Figshare (https://doi.org/10.6084/m9.figshare.22229827). All other data are available from the corresponding author on reasonable request.
References
Andrzejewska, A., Lukomska, B. & Janowski, M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 37, 855–864 (2019).
Li, C., Zhao, H. & Wang, B. Mesenchymal stem/stromal cells: developmental origin, tumorigenesis and translational cancer therapeutics. Transl. Oncol. 14, 100948 (2021).
Tang, Y. L. et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann. Thorac. Surg. 80, 229–237 (2005).
Chen, J. L. et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 73, 778–786 (2003).
Murphy, J. M., Fink, D. J., Hunziker, E. B. & Barry, F. P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48, 3464–3474 (2003).
Rosova, I., Dao, M., Capoccia, B., Link, D. & Nolta, J. A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26, 2173–2182 (2008).
Lanzoni, G. et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 10, 660–673 (2021).
Bich, P. L. T. et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res. Ther. 11, 60 (2020).
Watanabe, Y. et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice. Stem Cells Transl. Med. 8, 271–284 (2019).
Lin, B. L. et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology 66, 209–219 (2017).
Baertschiger, R. M. et al. Mesenchymal stem cells derived from human exocrine pancreas express transcription factors implicated in beta-cell development. Pancreas 37, 75–84 (2008).
Herrera, M. B. et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 24, 2840–2850 (2006).
Walker, M. R., Brown, S. L., Riehl, T. E., Stenson, W. F. & Stappenbeck, T. S. Growth factor regulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) expression in colonic mesenchymal stem cells. J. Biol. Chem. 285, 5026–5039 (2010).
Snow, N. E. The human embryonic stem-cell debate: science, ethics, and public policy. Theol. Stud. 63, 650–651 (2002).
Isern, J. et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem-cell-niche function. Elife 3, e03696 (2014).
Vodyanik, M. A. et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7, 718–729 (2010).
Wang, H. Y. et al. Characterization and therapeutic application of mesenchymal stem cells with neuromesodermal origin from human pluripotent stem cells. Theranostics 9, 1683–1697 (2019).
Wang, X. F. et al. Immune modulatory mesenchymal stem cells derived from human embryonic stem cells through a trophoblast-like stage. Stem Cells 34, 380–391 (2016).
Hoffman, A. M. et al. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev. 20, 1779–1792 (2011).
Campagnoli, C. et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98, 2396–2402 (2001).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
da Silva Meirelles, L., Caplan, A. I. & Nardi, N. B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299 (2008).
Bexell, D. et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 17, 183–190 (2009).
Esteves, C. L. et al. Equine mesenchymal stromal cells retain a pericyte-like phenotype. Stem Cells Dev. 26, 964–972 (2017).
Ruiz-Magana, M. J. et al. Decidualization modulates the mesenchymal stromal/stem cell and pericyte characteristics of human decidual stromal cells. Effects on antigen expression, chemotactic activity on monocytes and antitumoral activity. J. Reprod. Immunol. 145, 103326 (2021).
Faal, T. et al. Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood-brain barrier interactions. Stem Cell Rep. 12, 451–460 (2019).
Wang, P., Rodriguez, R. T., Wang, J., Ghodasara, A. & Kim, S. K. Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 8, 335–346 (2011).
Cheng, X. et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 10, 371–384 (2012).
Hogrebe, N. J., Maxwell, K. G., Augsornworawat, P. & Millman, J. R. Generation of insulin-producing pancreatic beta cells from multiple human stem cell lines. Nat. Protoc. 16, 4109–4143 (2021).
Kawaguchi, Y. et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128–134 (2002).
Costello, I. et al. Lhx1 functions together with Otx2, Foxa2, and Ldb1 to govern anterior mesendoderm, node, and midline development. Genes Dev. 29, 2108–2122 (2015).
Thomas, T., Nowka, K., Lan, L. & Derwahl, M. Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid 16, 537–544 (2006).
Martinez Barbera, J. P. et al. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127, 2433–2445 (2000).
Jacobsson, B., Carlstrom, A., Platz, A. & Collins, V. P. Transthyretin messenger ribonucleic acid expression in the pancreas and in endocrine tumors of the pancreas and gut. J. Clin. Endocrinol. Metab. 71, 875–880 (1990).
Kwon, G. S. et al. Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Dev. Dyn. 235, 2549–2558 (2006).
Mokhtar, F. B. A., Plat, J. & Mensink, R. P. Genetic variation and intestinal cholesterol absorption in humans: a systematic review and a gene network analysis. Prog. Lipid Res. 86, 101164 (2022).
Brannan, C. I., Dees, E. C., Ingram, R. S. & Tilghman, S. M. The product of the H19 gene may function as an RNA. Mol. Cell Biol. 10, 28–36 (1990).
Liu, X. et al. Primary alcohol-activated human and mouse hepatic stellate cells share similarities in gene-expression profiles. Hepatol. Commun. 4, 606–626 (2020).
Tran, F. H. & Zheng, J. J. Modulating the wnt signaling pathway with small molecules. Protein Sci. 26, 650–661 (2017).
Inman, G. J. et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharm. 62, 65–74 (2002).
Nguyen, M. T. X. et al. Differentiation of human embryonic stem cells to endothelial progenitor cells on laminins in defined and xeno-free systems. Stem Cell Rep. 7, 802–816 (2016).
Fukuta, M. et al. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE 9, e112291 (2014).
Li, E. et al. Generation of mesenchymal stem cells from human embryonic stem cells in a complete serum-free condition. Int. J. Biol. Sci. 14, 1901–1909 (2018).
Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 104, 15 25 11–15 25 14 (2014).
Ray, K. Deciphering the role of CD8(+) T cells in IBD: from single-cell analysis to biomarkers. Nat. Rev. Gastroenterol. Hepatol. 17, 595 (2020).
Nancey, S. et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology 131, 485–496 (2006).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Bazhanov, N. et al. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res. Ther. 7, 27 (2016).
Giri, J. & Galipeau, J. Mesenchymal stromal cell therapeutic potency is dependent upon viability, route of delivery, and immune match. Blood Adv. 4, 1987–1997 (2020).
Bao, X. et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat. Biomed. Eng. 1, 0003 (2016).
Menendez, L., Yatskievych, T. A., Antin, P. B. & Dalton, S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl Acad. Sci. USA 108, 19240–19245 (2011).
Cai, S. X. et al. Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res. Ther. 6, 65 (2015).
Song, L. et al. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J. Bone Min. Res. 27, 2344–2358 (2012).
Ling, L., Nurcombe, V. & Cool, S. M. Wnt signaling controls the fate of mesenchymal stem cells. Gene 433, 1–7 (2009).
Caplan, A. I. All MSCs are pericytes? Cell Stem Cell 3, 229–230 (2008).
Zimmerlin, L., Donnenberg, V. S. & Donnenberg, A. D. Pericytes: a universal adult tissue stem cell? Cytom. A 81, 12–14 (2012).
Crisan, M., Corselli, M., Chen, W. C. & Peault, B. Perivascular cells for regenerative medicine. J. Cell Mol. Med. 16, 2851–2860 (2012).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Chen, G., Hou, Z., Gulbranson, D. R. & Thomson, J. A. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 7, 240–248 (2010).
Zhang, Y. et al. Nicotinamide promotes pancreatic differentiation through the dual inhibition of CK1 and ROCK kinases in human embryonic stem cells. Stem Cell Res. Ther. 12, 362 (2021).
Bieback, K., Schallmoser, K., Kluter, H. & Strunk, D. Clinical protocols for the isolation and expansion of mesenchymal stromal cells. Transfus. Med Hemother. 35, 286–294 (2008).
Zanini, C. et al. Differentiation of mesenchymal stem cells derived from pancreatic islets and bone marrow into islet-like cell phenotype. PLoS ONE 6, e28175 (2011).
Ciuffreda, M. C., Malpasso, G., Musaro, P., Turco, V. & Gnecchi, M. Protocols for in vitro differentiation of human mesenchymal stem cells into osteogenic, chondrogenic and adipogenic lineages. Methods Mol. Biol. 1416, 149–158 (2016).
Debnath, T. & Chelluri, L. K. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol. Transfus. Cell Ther. 41, 7–16 (2019).
Xu, L. et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 8, 275 (2017).
Abbasi-Kenarsari, H. et al. Synergistic therapeutic effect of mesenchymal stem cells and tolerogenic dendritic cells in an acute colitis mouse model. Int. Immunopharmacol. 88, 107006 (2020).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Acknowledgements
We want to thank the technical support from the Bioimaging and Stem Cell Core Facility and Animal Facility at the University of Macau. We also thank the council members of the Macau Society for Stem Cell Research (MSSCR) for the constructive discussions. This project was funded by the University of Macau (File No. MYRG2018-00135-FHS and MYRG2019-00147-FHS) and by the Science and Technology Development Fund, Macau SAR (File No. 131/2014/A3, 056/2015/A2, 0059/2019/A1, 0123/2019/A3, 0011/2019/AKP, and 0002/2021/AKP).
Author information
Authors and Affiliations
Contributions
Y.Z., R.-H.X., and G.C. designed and planned the project; Y.Z. and W.L. conducted stem cell maintenance, differentiation, and fluorescence-activated cell sorting; Y.Z. characterized the DE-MSCs; Y.Z. and L.H. conducted the scRNA-seq on the Nadia instrument; Y.Z., X. Xiao, J.X., N.S., U.I.C., and E.C.W.C. conducted bioinformatics analysis; Y.Z., D.Z., and Y.Y. evaluated the therapeutic function of DE-MSCs on colitis mice model; Y.Z., Y.M., L.L., X. Xu, R.-H.X., and G.C. designed the experiments and analyzed data; Y.Z., H.C.K., W.L., and G.C. wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The use of hPSCs was approved by the University of Macau. The use of donor tissues and cells was approved by Zhuhai People’s Hospital’s Research Ethics Board.
Peer review
Peer review information
Communications Biology thanks Bruno Peault, Yo Mabuchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Edwina McGlinn and Manuel Breuer. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Yi, Y., Xiao, X. et al. Definitive Endodermal Cells Supply an in vitro Source of Mesenchymal Stem/Stromal Cells. Commun Biol 6, 476 (2023). https://doi.org/10.1038/s42003-023-04810-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04810-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.