Abstract
Human and animal studies have reported widespread reductions in cerebral blood flow associated with chronic cocaine exposures. However, the molecular and cellular mechanisms underlying cerebral blood flow reductions are not well understood. Here, by combining a multimodal imaging platform with a genetically encoded calcium indicator, we simultaneously measured the effects of acute cocaine on neuronal and astrocytic activity, tissue oxygenation, hemodynamics and vascular diameter changes in the mouse cerebral cortex. Our results showed that cocaine constricted blood vessels (measured by vessel diameter Φ changes), decreasing cerebral total blood volume (HbT) and temporally reducing tissue oxygenation. Cellular imaging showed that the mean astrocytic Ca2+ dependent fluorescence \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}})\) increase in response to cocaine was weaker but longer lasting than the mean neuronal Ca2+ dependent fluorescence \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}})\) changes. Interestingly, while cocaine-induced \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) increase was temporally correlated with tissue oxygenation change, the \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) elevation after cocaine was in temporal correspondence with the long-lasting decrease in arterial blood volumes. To determine whether the temporal association between astrocytic activation and cocaine induced vasoconstriction reflected a causal association we inhibited astrocytic Ca2+ using GFAP-DREADD(Gi). Inhibition of astrocytes attenuated the vasoconstriction resulting from cocaine, providing evidence that astrocytes play a critical role in cocaine’s vasoconstrictive effects in the brain. These results indicate that neurons and astrocytes play different roles in mediating neurovascular coupling in response to cocaine. Our findings implicate neuronal activation as the main driver of the short-lasting reduction in tissue oxygenation and astrocyte long-lasting activation as the driver of the persistent vasoconstriction with cocaine. Understanding the cellular and vascular interaction induced by cocaine will be helpful for future putative treatments to reduce cerebrovascular pathology from cocaine use.
Similar content being viewed by others
Introduction
Cocaine is a highly rewarding and addictive drug1, whose misuse is associated with significant morbidity and mortality. Among the most serious adverse effects from cocaine are cerebrovascular complications that result in transient ischemia and strokes2,3,4. Indeed, numerous clinical brain imaging studies have reported widespread reductions in cerebral blood flow and cerebral blood volume in individuals with cocaine use disorder5,6, and preclinical optical imaging studies have reported similar findings in rodent models7,8,9. However, the molecular and cellular mechanisms underlying cerebral blood flow reductions are not well understood and could reflect (1) direct vasoconstrictive properties of cocaine in blood vessels and/or indirect vasoconstriction secondary to release of sympathomimetic amines10,11, or (2) neuronal deficits that decrease flow secondary to reduced neuronal activity12 and metabolic demand13,14, or (3) astrocyte deficits that impair cerebral blood flow homeostasis15,16,17.
Neurovascular coupling is involved in modulation of brain function18. Neuronal-vascular interactions are necessary to maintain an adequate supply of oxygen and glucose for proper neuronal function19,20 and until recently most studies on neurovascular coupling focused on neurons. However, there is now increasing interest to study the interactions between neurons and glial cells and their role in neurovascular coupling. Astrocytes provide a cellular link between neuronal circuitry and blood vessels19,20,21, astrocytic processes wrap around neuronal synapses while astrocytic feet wrap around blood vessels22. This linkage suggests that astrocytes are not only essential for the supply of energy to neurons but can also transfer signals that regulate blood flow in response to neuronal activity23,24.
Astrocytes provide structural and nutritional support for neurons and participate in the maintenance of the blood brain barrier25,26. They also support brain function through their regulation of cerebral blood flow, neuromodulation, and balancing synaptic transmission27,28. How astrocytes respond to cocaine, which profoundly affects neurons29,30 and cerebral blood vessels17,31 is still not clear. It is likely that cocaine’s effects on brain function reflect the interaction between vascular systems, neurons and astrocytes, which may change as a function of time from cocaine exposure and chronicity. Distinguishing these effects would ideally require simultaneous multi parameter longitudinal measurements in vivo. In addition, studying the roles of astrocytes in brain function is difficult because they are essential for neuronal survival and their removal causes neuronal death. Thus, much of what we know about astrocyte function is derived from studies of isolated mammalian astrocytes in vitro27,32, which cannot inform us on how astrocytes interact with neurons and the surrounding vessels.
To address these challenges, in this study we simultaneously measured the effects of cocaine on neuronal or astrocytic activity, tissue oxygenation, vascular hemodynamics, and vascular diameter changes in the mouse cerebral cortex. To do so, we used a viral vector to express a genetically encoded Ca2+ indicator, GCaMP6f, into neurons33 or astrocytes34 within the somatosensory cortex of mice35. Our multimodality imaging platform through a cranial window36 enabled us to image cell-specific Ca2+ dependent fluorescence changes from astrocytes or neurons while concurrently measuring dynamic changes in cerebral blood volume (i.e., total hemoglobin concentration [HbT]) and tissue oxygenation with vascular changes separately for veins and arteries at high spatiotemporal resolution over a relatively large field of view (FOV). Meanwhile, we used a custom Matlab program to quantify changes in vessel diameter sizes after cocaine to provide a direct measurement of vascular responses. We show that the mean astrocytic Ca dependent fluorescence \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}})\) response to cocaine was weaker, and longer lasting than the mean neuronal Ca dependent fluorescence \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}})\) responses. Interestingly, while cocaine-induced \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) increases were temporally correlated with tissue oxygenation changes, the \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) elevation after cocaine was in temporal correspondence with the long-lasting decrease in HbT in arteries. To evaluate whether the temporal association between astrocytic activation and cocaine induced vasoconstriction reflects a causal association, we used GFAP-DREADD(Gi) to inhibit astrocytic Ca2+-G(A). Inhibition of astrocytes attenuated the vasoconstriction from cocaine, thus providing evidence that astrocytes play a critical role underlying cocaine’s vasoconstrictive effects in cerebral blood vessels.
Results
GCaMP6f-expressed Ca2+ Fluorescence in Astrocytes/Neurons
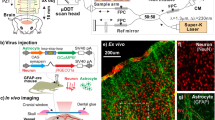
To track neuronal or astrocytic activation in response to cocaine, we delivered GCaMP6f, a genetically encoded Ca2+ indicator, into the cortex of C57BL/6 wild type and GFAP-Cre mice (Fig. 1c), respectively. For example, Fig. 1b illustrates the astrocytic GCaMP6f Ca2+ fluorescence image (i) along with the hemodynamic images (ii, iii) in the mouse cortex acquired with the multimodality imaging platform in vivo. To corroborate that GCaMP6f was specifically expressed in astrocytes or neurons, we used immunohistochemistry to label either neurons with NeuN or astrocytes with GFAP antibodies for brain sections from wild type and GFAP-Cre animals, respectively. Figure 1d shows representative ex vivo coronal sections of cortex with GCaMP6f-expressing neurons (i) and astrocytes (ii) visualized by anti-GFP staining in wild type and GFAP-Cre mice, respectively. Their ‘zoom-in’ images within the white boxes in Fig. 1d show that GFP + cells were all neurons in wild-type mice (iii) and astrocytes in GFAP-Cre mice (iv). These ex vivo images confirmed that GCaMP6f fluorescence in wild type mice was from neuronal Ca2+ and that in GFAP-Cre mice was from astrocytic Ca2+. Quantification in Supplementary Fig. 1j shows that levels of GCaMP6f expression in neurons (55.6% ± 8.9%, n = 5, wild type mice) and in astrocytes (56.4% ± 4.5%, n = 5, GFAP-Cre mice) did not differ (p = 0.94), indicating similar cortical Ca2+ dependent fluorescence signaling from neurons in wild type mice and from astrocytes in GFAP-Cre mice.
a Multimodality imaging platform allows simultaneous imaging of cellular Ca2+ dependent fluorescence and hemodynamic changes in response to cocaine. LP: Long-pass filter >510 nm. b Cortical images acquired with the different channels including fluorescence image (i: λex = 488 nm, λem = 520 nm), reflected images at λ1 = 568 nm (ii) and λ2 = 630 nm (iii) to separate arteries (red tracks) from veins (blue tracks) and to retrieve hemodynamics (HbO2 and HbR) in brain tissue. c Animal models for viral delivery of GCaMP6f to neurons and astrocytes to mouse somatosensory cortex in vivo. d Ex-vivo confocal fluorescence images to show GCaMP6f distribution in cortical neurons (i) and astrocytes (ii), respectively. Zoomed-in views to confirm GCaMP6f-expressions in neurons (iii) and astrocytes (iv) within the white boxes. Green: GCaMP6f-expressing neurons and Red: NeuN (iii), Green: GCaMP6f-expressing astrocytes and Red: GFAP stains (iv) to represent neurons and astrocytes, respectively.
Vascular [HbT] and [ΔΦ] decrease due to cocaine-induced vasoconstriction
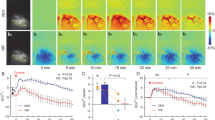
Figure 2 summarizes cocaine’s effects on the cortical vasculature including changes in total hemoglobin (Δ[HbT]) and in vascular diameter (ΔΦ) in veins and arteries. Δ[HbT] and Δ[Φ] represent the changes in total blood volume and vessel morphology, respectively, due to vasoconstriction from cocaine. Both wild type and GFAP-Cre mice were included, and their vascular changes indicated as Δ[HbT-G(N)], Δ[Φ-G(N)] and Δ[HbT-G(A)], Δ[Φ-G(A)], respectively. Figure 2a shows the absorption spectra of oxygenated- (HbO2, red curve) and deoxygenated- (HbR, blue curve) hemoglobin at different wavelengths, which can be used to calculate HbO2 and HbR (Eq.1), and to estimate HbT within vessels and tissue (Eq. 2). This is because λ1 = 568 nm is an isosbestic point of HbO2 and HbR spectra with high absorbance, enabling imaging blood absorption in both arteries (HbO2 dominant) and veins (HbR dominant). The specific wavelengths of λ1 = 568 nm and λ2 = 630 nm were used for imaging since at λ2 = 630 nm HbR absorption is higher than HbO2, which allows to distinguish veins from arteries in the cortex of mice from either HbT-G(N) (i neuronal) or HbT-G(A) (ii astrocytic) groups (Fig. 2b). Regions of interest (ROIs) were selected from arteries (e.g., red traces) and veins (e.g., blue traces) for each animal and their temporal changes with cocaine extracted. Figure 2c, d show Δ[HbT-G(N)] and Δ[HbT-G(A)] changes with time after cocaine (1 mg/kg, i.v.), respectively, which reveals that total blood volumes in arteries and veins decreased immediately (Δt = 2 min) after cocaine to ~−20%(t = 5–10 min) below their baseline followed by gradual recovery. Meanwhile, Fig. 2e, f show Δ[Φ-G(N)] and Δ[Φ-G(A)] changes as a function of time from cocaine injection (1 mg/kg, i.v.), respectively, which indicate that vessel sizes of arteries and veins decreased to ~−10% (t = 0–20 min) and slowly returned to baseline within t = 60 mins. The mean Δ[HbT] decay rates (Δ[HbT]%/min) are summarized in Fig. 2g for neuronal- (Group N) and astrocytic- GCaMP6f expressing (Group A) mice. For Group N, Δ[HbT-G(N)]%/min in arteries and veins were −15.66 ± 2.464%/min and −12.26 ± 2.491%/min, respectively; for Group A, Δ[HbT-G(A)]%/min in arteries and veins were −17.15 ± 5.041%/min and −16.68 ± 5.351%/min, respectively. The arteries’ changes from cocaine did not differ between Group N and Group A (p = 0.992), which indicates that viral GCaMP6f delivery did not affect vascular reactivity to cocaine. Comparison of cocaine-induced integrative Δ[Φ] changes in Group A and Group N is summarized in Fig. 2h. For Group N, Δ[Φ-G(N)]%/min in arteries and veins were −5.544 ± 1.210%min and −6.818 ± 2.110%min; for Group A, Δ[Φ-G(A)]%min in arteries and veins were −6.437 ± 1.702%/min and −5.402 ± 2.520%min. Cocaine induced changes in integrative Δ[Φ] did not differ between Group A and Group N nor between arteries and veins.
a Absorption spectra of oxygenated-hemoglobin (HbO2, red curve) and deoxygenated-hemoglobin (HbR, blue curve). Besides λex = 488 nm for excitation of cellular GCaMP6f Ca2+ fluorescence, λ1 = 568 nm and λ2 = 630 nm were selected in multimodality imaging platform to retrieve changes in HbO2 and HbR, thus HbT in cortical tissue as well as within vessels. b Spectral images of the cortex at λ1 = 568 nm in WT mouse (i) and GFAP-Cre mouse (ii) with separation of arteries (red traces) and veins (blue traces). Regions of interest (ROIs) were selected from arteries (e.g., pink dots), veins (e.g., blue dots) and tissue (e.g., yellow dots) from each animal. c, d Time courses of Δ[HbT] in arteries (red) and veins (blue) in response to cocaine (1 mg/kg, i.v.) in neuronal (c) and astrocytic (d) GCaMP6f-expressing animals (n = 5/group), both showing long-persistent decreases in arteries and veins by cocaine. e, f Vascular diameter (ΔΦ) changes in arteries (red) and veins (blue) as a function of time after cocaine injection (1 mg/kg, i.v.) in neuronal and astrocytic groups, respectively. g Comparisons of Δ[HbT]% decrease per minute between arteries and veins in the neuronal and astrocytic GCaMP6f-expressing animals (n = 5/group), showing no significant difference between arteries and veins in either group. h Comparisons of ΔΦ% decrease per minute between arteries and veins in neuronal and astrocytic GCaMP-expressing groups, showing no significant difference of ΔΦ% in response to cocaine. All error bars are presented as means ± SEM.
Cocaine induces a transient decrease in tissue oxygenation
Figure 3 shows cocaine-induced changes in tissue oxygen hemoglobin (HbO2), i.e., [HbO2]-G(N) and [HbO2]-G(A) in the cortices of Group N (neuronal) and Group A (astrocytic) mice. Figure 3a, b show representative baseline raw image at λ1 = 568 nm and time-lapse Δ[HbO2] ratio images after cocaine (1 mg/kg, i.v., t = 0 min). Figure 3c show the time courses of mean Δ[HbO2] changes after cocaine in Group N (solid line, ROIs=3/animal, n = 5) and Group A (dashed line, ROIs=3/animal, n = 5) mice. Cocaine immediately reduced Δ[HbO2] to −12.09 ± 3.777% (Group N) and −9.467 ± 2.612% (Group A) at ~10 min after cocaine that gradually recovered at 24.2 ± 3.625 min in Group N and at 25 ± 5.692 min in Group A, followed by ~10–15% overshoot over baseline at t = 60 min. Comparisons of peak decrease and reduction duration between Group N and Group A showed no differences (Fig. 3d, e), indicating that viral GCaMP delivery did not affect cortical tissue function in response to cocaine.
a, b Time lapse Δ[HbO2] images in response to cocaine (1 mg/kg, i.v) in a Group N animal (ii–v) and a Group A animal (ii–v) at t = 0, 5, 25, 40 min, respectively. Three ROIs were selected in tissue (e.g., yellow dots) from each animal. c Mean Δ[HbO2] changes with time in response to cocaine (1 mg/kg, i.v) in Group N (solid line, n = 5) and Group A (dashed line, n = 5), showing cocaine-induced Δ[HbO2] decrease in cortical tissue over 20–25 min followed by an overshoot over baseline about 10–15% at t = 60 min after cocaine. d Comparison of peak Δ[HbO2] decreases between G(N) and G(A), showing no difference (n = 5, p = 0.584). e Comparison of Δ[HbO2] return time to baseline between G(N) and G(A), showing no difference (n = 5, p = 0.909). All error bars are presented as means ± SEM.
Cocaine induces a robust transient increase in neuronal Ca2+ but a weak and persistent increase in astrocytic Ca2+
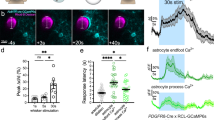
Figure 4 shows Ca2+ dependent fluorescence changes in neurons \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}})\) and astrocytes \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}})\) in response to cocaine (1 mg/kg, i.v). The spatiotemporal evolutions of cocaine-induced \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) and \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) at t = 0, 5, 25, and 40 min are illustrated in Fig. 4a, b, respectively; their mean increases with time for Group N (n = 5) and Group A (n = 5) mice are plotted in Fig. 4c. Both \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) and \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) fluorescence responded promptly to a cocaine injection. However, the neuronal reaction \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) was larger (>6% increase over baseline) and recovered within 30–40 min with a downshoot afterwards, whereas the astrocytic reaction in \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) was weaker, only 2%, but was longer lasting (>50 min) after cocaine. The peak increases following cocaine injection of 6.065 ± 1.463% for \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) (n = 5, ROIs = 5/animal) were significantly higher (p*=0.032) than 2.106 ± 0.4333% for \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) (n = 5, ROIs = 5/animal) (Fig. 4d). Their recovery time of 32.2 ± 1.933 min for \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) was significantly faster than 54.6 ± 4.167 min for \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) (Fig. 4e), indicative of longer-lasting cocaine effects on astrocytic than neuronal Ca2+ signaling (p* < 0.001).
a, b Time lapse images of Ca2+ fluorescence ratio changes \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) (ii-v) and \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) (ii–v) in response to cocaine (1 mg/kg, i.v) superposed on their baseline spectral images (i) obtained at λ1 = 568 nm. Five Regions of interest (ROIs) were selected from fluorescence expressing regions (e.g., pink dots) and one ROI from outside expressing area (e.g., white triangle), that was used to correct for confounding artifacts. c Cocaine-induced mean Ca2+ fluorescence changes in neurons (\(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\), solid line, n = 5), and astrocytes (\(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) dash line, n = 5), both showing immediate ΔF/FCa2+ increases after cocaine (1 mg/kg, i.v). The \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) increase was robust and returned to baseline within ~30 min, whereas the \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) increase was smaller (~2%) but longer lasting. d Comparison of peak increases in \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) vs \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\), show that the astrocytic response to cocaine was lower than the neuronal response (p*=0.032). e Comparison of recovery time between \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) and \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) indicated that the astrocytic response was longer lasting than the neuronal response (p*<0.001). All error bars are presented as means ± SEM.
Cocaine-induced Neuronal \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) fluorescent change correlates with tissue ΔHbO2
Figure 5a shows simultaneous changes in neuronal Ca2+ dependent fluorescence \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}})\) and oxygenated-hemoglobin (ΔHbO2-G(N)) within cortical tissue and blood volume (ΔHbT-G(N)) in arteries before and after cocaine (Group N, n = 5). \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) increased 6.065 ± 1.463% at 8–10 min after cocaine recovering to its baseline value at 32.2 ± 1.933 min followed by a downshoot till the end of recording (t = 60 min, green curve). Meanwhile, ΔHbO2 decreased −12.09 ± 3.777% at 8–10 min after cocaine and recovered at 24.2 ± 3.625 min followed by an overshoot (orange curve). Comparison of recovery time to the baseline between ΔCa2+-G(N) of 32.2 ± 1.93 min and Δ[HbO2]-G(N) of 24.2 ± 3.625 min revealed no difference (p = 0.087). In contrast, Δ[HbT]-G(N)) in arteries decreased −22.56 ± 4.496% at ~10 min after cocaine, remained low and then slightly recovered to −12.31 ± 6.122% at t = 60 min after cocaine (red curve).
a Mean \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) (green), Δ[HbO2]-G(N) (orange), and Δ[HbT]-G(N) (red) changes with time in cortex in response to cocaine (1 mg/kg, i.v.) (n = 5). b Correlation between cocaine-induced response in \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) and Δ[HbO2]-G(N) showed a strong inverse linear association (R = −0.984, p < 0.01). All error bars are presented as means ± SEM.
The correlation analysis between cocaine-induced \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) and Δ[HbO2]-G(N) for the time periods of peak response (t = 0–30 min, ‘cocaine quadrant region’) and overshoot (t = 30–60 min, ‘overshoot quadrant region’) is shown in Fig. 5b. The linear regression plot revealed a strong inverse correlation between neuronal Ca2+ and tissue ΔHbO2 (r = −0.984, p < 0.01), where colored dots represent different time periods from t = −10 min baseline to t = 60 min after cocaine. Though our findings cannot establish causality they suggest that cocaine-induced reduction in tissue oxygen content in the peak response period might reflect increased neuronal activation, whereas the increase in tissue oxygen content that followed might reflect decreased neuronal activity during the overshoot. In contrast, cocaine induced neuronal Ca2+ did not correlate with arterial ΔHbT (Supplementary Fig. 2), indicating that arterial volume changes with cocaine were not driven by changes in neuronal activity.
Cocaine-induced astrocytic \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) fluorescent change correlates with HbT in arteries
Cocaine-induced simultaneous changes in astrocytic Ca2+ \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}})\) dependent fluorescence and oxygenated-hemoglobin (Δ[HbO2]-G(A)) within cortical tissue and Δ[HbT]-G(A) in arteries (n = 5) showed \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) increased 2.106 ± 0.4333% at t ≈ 3 min after cocaine and plateaued till t ≈ 18 min followed by a gradual slow recovery till t ≈ 60 min (green curve in Fig. 6a). Changes in Δ[HbT]-G(A) showed a similar temporal pattern to that of \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\), which decreased −24.96 ± 8.415% at t = 2 min, remained largely unchanged till t = 35 min followed by a slow recovery to −3.634 ± 4.544% at t = 60 min after cocaine (red curve). In contrast, Δ[HbO2]-G(A) decreased −9.467 ± 2.612% after cocaine and recovered at t = 25 ± 5.692 min with an overshoot (orange curve).
a Mean \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) (green), Δ[HbO2]-G(A) (orange), and Δ [HbT]-G(A) (red) changes with time in cortex in response to cocaine (1 mg/kg, i.v.) (n = 5). b Correlation analysis between cocaine-induced response in astrocytic \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) and Δ[HbT] in arteries, showing a strong inverse correlation (R = −0.786, p < 0.01). All error bars are presented as means ± SEM.
Correlation analysis between the temporal changes in astrocytic \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) and Δ[HbT]-G(A) in arteries from baseline (t = −10–0 min) to 60 min after cocaine showed a strong inverse correlation (R = −0.786, p < 0.01) as shown in Fig. 6b, where colored dots represent different time periods of recording. This finding suggests that astrocytic activation by cocaine underlie cocaine induced vasoconstriction. In contrast, there was no association between \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) and tissue Δ[HbO2]-G(A) (Supplementary Fig. 3).
Inhibition of astrocytic fluorescence \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\) activity with GFAP-DREADD (Gi) reduces cocaine’s vasoconstriction
Figure 7 shows the comparison of astrocytic \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\), vessels’ diameter and hemodynamic responses to cocaine in cortex of mice (n = 5) before and after DREADD(Gi) activation by clozapine. For ΔHbT, before DREADD (Gi) activation, cocaine immediately reduced ΔHbT to −18.84 ± 1.115% which did not return to baseline over 60 mins after cocaine. However, after 30 mins of clozapine’s activation of DREADD, cocaine-induced decrease in ΔHbT were much smaller (p < 0.05, maximum of −5.514 ± 0.5085% at t = 2–8 min) and rapidly returned to baseline after 8 min (p > 0.05). Vessel diameter size (ΔΦ) in response to cocaine, before clozapine decreased to −7.557 ± 2.463% gradually recovering to baseline at t = 60 min, whereas after clozapine there was no significant changes in Δϕ compared to baseline throughout the whole experiment time period (Fig. 7f). Persistent Δ [HbT] (Fig. 7c) and Δϕ (Fig. 7e) decreases reflected cocaine’s vasoconstriction effects along with the corresponding [Ca2+]-G(D) fluorescent increase (\(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\)), Fig. 7a). After astrocytic \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\) inhibition, \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\) was slightly increased (<1%) after cocaine for a short time period (2–5 min; Fig. 7b). Tissue ΔHbO2 after cocaine before clozapine, was reduced to −11.81 ± 1.047% and gradually recovered to baseline at 30.4 ± 8.853 min followed by an overshoot, consisted with observation shown in Fig. 3 above. After clozapine, tissue ΔHbO2 following cocaine injection was mildly reduced −5.118 ± 1.609% within t = 2–7 mins, with a fast recovery after 10 min.
Cocaine induced \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\) (a, b), HbT (c, d), vessel diameter size (e, f), and HbO2 (g, h) changes as function of time before and after astrocytic ΔCa2+ inhibition by clozapine. i–l Quantification analysis of average efficiency in \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\), vessels size, hemodynamic response respectively before DREADD(Gi) activation (Gray shadow in a, c, e, g) and after DREADD(Gi) activation by clozapine (Pink shadow in b, d, f, h). Meanwhile, yellow shadow indicates time periods with significant differences compared to baseline (p < 0.05), and green shadow indicate no-significant differences (p > 0.05). All error bars are presented as means ± SEM.
Comparisons of cocaine-induced mean fluorescent changes in \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\), ΔHbT, ΔHbO2, and Δ Φ before and after DREADD(Gi) activation by clozapine are summarized in Fig. 7i–l, showing that the cocaine-induced changes in astrocytic \({[{{{{\rm{Ca}}}^{2+}}]}{\mbox{-}{{{\rm{G(D)}}}}}}\) fluorescence, ΔHbT, Δϕ, and ΔHbO2, are significantly reduced after astrocytic ΔCa2+ inhibition. Specifically, without or with astrocytic ΔCa2+ inhibition, the mean \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(D)}}}}}}\) changed from 0.5046 ± 0.1169%/min to −0.1672 ± 0.1180%/min (p* = 0.004); ΔHbT changed from −9.668 ± 0.4799%/min to −0.6927 ± 0.0933%/min (P* < 0.001); Δϕ decreased from −3.413 ± 0.5230%/min to −0.4476 ± 0.3379%/min (P* = 0.009); and ΔHbO2 from −10.482 ± 3.8785%/min to −2.179 ± 0.3419 %/min, (P* = 0.048), respectively.
To determine whether clozapine would affect fluorescent neuronal Ca2+-G(C), and vessel diameters in the cortex, we administered clozapine (0.1 mg/kg, i.p.) to a group of animals (n = 3, shown in Table 1) during experiments and recorded changes in neuronal Ca2+ fluorescence \((\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(C)}}}}}})\) and vessel diameter size in at baseline (10 mins) and 30 mins after clozapine injection. Results are summarized in Supplementary Fig. 4. Supplementary Fig. 4a shows neuronal \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(C)}}}}}}\) changes as a function of time in response to clozapine. One-way repeated ANOVA showed a no significant time effect on neuronal \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(C)}}}}}}\) after clozapine injection. Quantification analysis of average efficiency in Supplementary Fig. 4b shows no significant differences before (0.2145 ± 0.1361%) and after (0.3940 ± 0.0940%) clozapine injection (p = 0.339). Supplementary Figure 4c shows the time traces of the vessel diameter change as a function of time in response to clozapine. One-way repeated ANOVA showed no significant differences on vessel size after clozapine injection. Quantification analysis of average efficiency Fig. S4d shows that there was no significant difference between before (0.2778 ± 0.4723%) and after (2.829 ± 1.458%) clozapine injection (p = 0.171).
Discussion
Here we show that acute cocaine triggered arterial and venous vasoconstriction and reduced tissue oxygenation. Whereas the later recovered within thirty minutes of injection followed by an overshoot, the vasoconstriction persisted throughout the measurement period. Simultaneous measures of the neuronal or the astrocytic Ca2+ dependent fluorescence responses to cocaine revealed a temporal association between the changes in tissue oxygenation and neuronal activation and between the persistent vasoconstriction and the long-lasting astrocytic activation. Specifically, the increased neuronal activation triggered by cocaine was associated with a parallel reduction in tissue oxygenation and the subsequent neuronal inhibition with increased tissue oxygenation despite persistent vasoconstriction, which was associated with astrocytic activation. Moreover, astrocytic inhibition with clozapine in GFAP-DREADD (Gi) expressed mice prevented cocaine-induced vasoconstriction, indicating that astrocytic activation mediated the vascular changes in response to cocaine.
Cocaine induced vasoconstriction reduces cerebral blood flow37 and can lead to neurological complications such as ischemia and stroke31,38,39. Here, we also documented a significant decrease in cerebral blood volume after acute cocaine that persisted 60 min after its administration. Though we also showed a parallel initial decrease in tissue oxygenation, the hypoxemia recovered by ~25 min followed by an overshoot over its baseline despite the persistent vasoconstriction. The divergence in their temporal sequence indicates that the coupling between tissue oxygenation and blood volume was disrupted by cocaine - an effect that, we postulate, reflects distinct effects of cocaine in neurons and in astrocytes. Cocaine-induced reduction in cerebral blood flow/volume in parallel with neuronal activation could render neuronal tissue particularly vulnerable to hypoxemia, facilitating neurotoxicity with repeated administration40,41.
Although cocaine’s vasoconstricting effects are well documented2,12,42, the cellular mechanisms that underlie it remain elusive. Accumulating evidence indicates that drug exposure can have dynamic and long-lasting effects on astrocytes and other glial cells43. However, the mechanism underlying the effects of cocaine on vasoconstriction as well as those underlying astrocytic ΔF/F [Ca2+]i increases are still not understood. Cocaine’s vasoconstriction effects are likely to reflect its sympathomimetic effects though its effects on L-type Ca channels in blood vessels are also likely to contribute44. Astrocytic ΔF/F [Ca2+]i accumulation associated with dopamine signaling involves Ca2+ release from internal stores via the Gq-PLC pathway45,46. Ionotropic receptors and voltage-gated Ca2+ channels also mediate Ca2+ influx into astrocytes47 and have been implicated in cocaine’s effects48. Recently, we showed49 that Ca2+ channel blockade reduced cocaine’s vasoconstriction and neurotoxicity in the prefrontal cortex. Others have also reported that Ca2+-channel blockers reduce negative outcomes from cocaine-induced cerebral ischemia and stroke by buffering cocaine-induced vasoconstriction50, thus indicating involvement of ionic homeostasis in cocaine-induced vasoconstriction. Additionally, cocaine triggers neuroadaptations48,51 in glutamate neurotransmission that is regulated by astrocytes that could further worsen cocaine induced neurotoxicity. These complex roles of astrocytes in brain under cocaine-induced pathophysiological condition require further investigation. Here, we show that astrocytic activation by cocaine underlies cocaine-induced vasoconstriction as evidenced by the temporal correspondence between astrocytic activation and the reduction in arterial blood volumes and the prevention of cocaine induced vasoconstriction with inhibition of astrocytic activation. These findings are clinically relevant for they suggest that interventions to reduce astrocyte activation by cocaine might help restore cerebral blood flow in cocaine users.
Neuronal responses to cocaine differed from those of astrocytes and had a distinct association with vascular changes. Specifically, cocaine initially triggered neuronal activation that lasted approximately 30 min and was associated with reduced tissue oxygenation subsequently followed by neuronal inhibition and increased tissue oxygenation. This indicates that the changes in oxygen metabolism due to cocaine effects on neuronal activation and inhibition underlie the changes in tissue oxygenation. The duration of neuronal activation that we observed with acute cocaine is consistent to the pharmacokinetics of cocaine in brain when given intravenously52,53 and to the duration of striatal dopamine increases3,31 and of locomotor activation. Neuronal activation with acute cocaine is also consistent with studies that used manganese-enhanced MRI54. The neuronal inhibition that follows the initial activation might underlie the reductions in brain glucose metabolism reported during cocaine withdrawal55,56.
The role of astrocytes in brain function including in neurovascular coupling is increasingly recognized57. Astrocytes contribute to cerebrovascular vasodilation during neurovascular coupling and to the vasoconstriction that subsequently restores vascular tone35,58. However studies on the effects of cocaine in astrocytes have mostly focused on synaptic and circuitry regulation associated with addiction-related behaviors59. Moreover, very few studies have investigated the effects of acute cocaine in astrocyte activity and to our knowledge no in vivo study has been published. An in vitro study done in slices from the nucleus accumbens incubated with cocaine reported increases in Ca2+ transients in astrocytes60. Though these results are consistent with our in vivo findings, comparisons are constrained by the fast pharmacokinetics of cocaine in vivo versus stable cocaine levels in in vitro preparations. Nonetheless both document the sensitivity of astrocytic Ca2+ transients to cocaine administration.
Our study showed that the astrocytic Ca2+ dependent fluorescent changes in response to cocaine (i.e., maximal \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(A)}}}}}}\) = 2.106 ± 0.4333%, n = 5) were lower than the neuronal Ca2+ fluorescent changes (maximal \(\Delta {F/F}_{{{{\rm{Ca}}}^{2+}}{\mbox{-}{{{\rm{G(N)}}}}}}\) = 6.065 ± 1.463%, n = 5, Fig. 4). To minimize expression difference between these two groups of animals, we delivered an identical viral volume into the cortex of all animals. Our ex vivo experiments (Supplementary Fig. 1) indicated that there were no significant differences of GCaMP6f expression into neurons and astrocytes (n = 3 animals/per group, ROIs=5/animal, p = 0.94). In addition, the Ca2+ dependent fluorescent time course was quantified as percent change relative to baseline to eliminate potential baseline variations between animals. Taken together, the amplitudes of fluorescent changes in G(N) and G(A) animals in response to cocaine injection should represent the cocaine-induced intracellular calcium changes in neuron and astrocytes, respectively. Indeed, we previously reported that cortical Ca2+N and Ca2+A fluorescent responses to sensory stimulation were stronger for neurons (ΔF/FN = 6.4 ± 0.29%) than astrocytes (ΔF/FA = 1.7 ± 0.1%), supporting difference in cellular Ca responses between these two cell types35.
One limitation of our study was that measurements were done in anesthetized mice using isoflurane. Thus, vasodilation from isoflurane might have accentuated the magnitude of cocaine induced vasoconstriction and its anesthetic effects might have attenuated the sensitivity of neurons and perhaps also of astrocytes to cocaine. Thus future studies should evaluate cocaine’s effects in awake animals. Another limitation was that we imaged neurons and astrocytes with GCaMP6f in separate group of animals, which was done to optimize detection of Ca2+ fluorescence changes as a function of cell type. In other words, it allowed us to avoid using the same gain setting fitted to the stronger fluorescence from one cell type (e.g., from neurons) relative to the weaker ones from another cell type (e.g., from astrocytes). Nevertheless, red-shifted genetically encoded calcium indicator such as jRGECO1a red fluorescence probe could be used with green probes such as GCaMP6f to image the activity and interactions of different cell types such as neurons and astrocytes simultaneously. To do so, custom-designed emission filters will be needed to synchronize with the excitation of GCaMP and jRGECO1a, respectively, so that it can detect images of green Ca2+ fluorescence from astrocytes and red Ca2+ fluorescence signal from neurons in the same field of view. Another limitation was the small sample size used in this study driven by laboriousness of the imaging experiments. Nevertheless, we were able to show significant effects of cocaine, which reflects the large effects being measured.
In summary, we simultaneously assessed neuronal/astrocytic Ca2+ dependent fluorescence, vascular diameter and hemodynamic changes in response to acute cocaine in the cortex of mice in vivo. We show that cocaine evoked neuronal activation and subsequent deactivation were accompanied by parallel decreases and increases in tissue oxygenation whereas long-lasting increases in astrocytic ΔF/F Ca2+ activity were associated with persistent vasoconstriction, which was blocked by astrocyte inhibition. This indicates that neurons and astrocytes play different roles in mediating neurovascular coupling in response to cocaine and implicate astrocytes in the long-lasting vasoconstriction associated with cocaine use. Understanding the cellular and vascular interaction induced by cocaine will be helpful for putative treatment strategies to reduce cerebrovascular pathology from cocaine use.
Methods
Animals and experimental design
Transgenic GFAP-Cre mice (Jackson Laboratory, https://www.jax.org/strain/024098) and C57BL/6 wild type (WT) mice (Jackson Laboratory) were used for this study when they reached ~8 weeks of age. Experimental mice were divided into four groups as shown in Table 1. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Stony Brook University and conducted according to the National Institutes of Health (NIH) Guidelines for Care and Use of Laboratory Animals.
Viral injection into mouse’s cortex
To detect neuron- and astrocyte-specific [Ca2+]i fluorescence response to acute cocaine challenge, we used the genetically encoded calcium indicator, GCaMP6f via viral delivery into mouse cortex. Specifically, 0.5 µl virus, AAV5.CAG.Flex.GCaMP6f.WPRE.SV40 (AV-5-PV2816, Penn Vector Core, Fig. 1c), was slowly infused at 0.2 µl/min into transgenic mice (GFAP-Cre, Group-A(b) in Table 1) to express GCaMP6f in astrocytes through a small hole predrilled in the skull overlaying the right somatosensory cortex [A/P: + 1.7; M/L: −1.5; D/V: −0.5 mm]; 0.5 µl virus, AAV5.Syn.GCaMP6f.WPRE.SV40 (AV-5-PV2822, Penn Vector Core), was infused into the same brain region of wild type mice (C57BL/6, Group-N(b) in Table 1) to express GCaMP6f into neurons. To examine whether astrocytic Ca2+ was involved in cocaine-induced vasoconstriction, a mixture of two viruses (AAV5.CAG.Flex.GCaMP6f.WPRE.SV40 with AAV5.GFAP. hM4D (Gi).mCherry 0.5 µl /each virus) was injected into the same region of cortex (GFAP-Cre, Group-D in Table 1). To assess clozapine’s effects on neuronal Ca dependent fluorescence signaling and vascular diameter changes, AAV5.Syn.GCaMP6f.WPRE.SV40 was infused into the cortex of wild type mice (C57BL/6, Group-C in Table 1) to express GCaMP6f into neurons. During viral injection, mice were anesthetized with inhalation of 2% isoflurane mixed with pure oxygen and their heads mounted on a stereotaxic frame while we monitored their physiology. After completion of procedure the mice were monitored daily for a few days to ensure they were healthy.
Animal preparation for optical imaging
After 4–6 weeks of viral injection, a cranial optical window (~2 × 2 mm2) was created on the skull over the viral injection spot to allow for optical imaging61. Surgical procedures were performed under isoflurane anesthesia. The mouse head was placed on a stereotactic frame and the skull surrounding the viral injection spot was carefully thinned with a dental drill and then removed to expose the brain. Saline was applied to the brain tissue and the open cortex was immediately covered with a sterile coverslip (4 × 3 mm2) sealed with biocompatible glue. During surgery, the animal’s physiology was continuously monitored, including respiration and body temperature to ensure they were physiologically stable. After window implantation, a catheter was placed on a tail vein for drug administration. Then the animal was positioned on a custom head mount for in vivo image acquisition.
Simultaneous imaging of neuronal/astrocytic [Ca2+] fluorescence and cortical hemodynamic changes in response to cocaine
A multimodality imaging platform developed in our laboratory36,37,62,63 was used to simultaneously image astrocytic \(\Delta {F/F}_{({\Delta{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(A)}}}})}\) or neuronal \(\Delta {F/F}_{({\Delta{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(N)}}}})}\) fluorescence, oxygenated hemoglobin [HbO2], and deoxygenated hemoglobin [HbR] from the mouse cortex. As illustrated in Fig. 1a, the light beams at three wavelengths of 488 nm, 568 nm, and 630 nm were delivered to the cortex separately with a time-sharing mode (10 ms exposure time per channel). The GCaMP6f fluorescence was excited at λex = 488 nm with the emission peaked within 512–535 nm along with the reflectance (from λ1 = 568 nm, and λ2 = 630 nm) were detected by the sCMOS camera (synchronized with the illumination paradigm), respectively. The reflectance at λ1 = 568 nm and λ2 = 630 nm were used to extract changes in [HbO2] and [HbR] (Fig. 2a) in mouse cortex36,64. Also, the arteries and veins can be separately distinguished from the images obtained at λ1 = 568 nm and λ2 = 630 nm (Supplementary Fig. 5). To assess cocaine-induced dynamic changes in astrocytic or neuronal Ca2+ fluorescence and brain hemodynamics, we administered cocaine (1 mg/kg, i.v.) through the tail vein followed by 0.5 ml saline to ensure delivery of the cocaine in the catheter. A total of 70 min duration of images were acquired for each experiment, including 10 min baseline before cocaine and 60 min post cocaine administration.
To investigate astrocyte’s involvement in cocaine-induced vasoconstriction, animals in Experiment 3 (Table 1) underwent two sets of imaging session with at least 110 min between each cocaine infusion (a: VEH (saline) pretreatment followed by cocaine challenge; b: clozapine pretreatment (0.1 mg/kg;0.16 ml, i.p. 30 min) followed by cocaine challenge. During the experiment, astrocytic Ca2+ dependent fluorescence \((\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}]{\mbox{-}}{{{\rm{G(D)}}}}})\), vessels’ diameter and cortical hemodynamic changes at λ1 = 568 nm, and λ2 = 630 nm were detected before and after cocaine challenges. In addition, to access the effects of clozapine on neuronal Ca2+ fluorescence and vascular diameters, the neuronal Ca2+ dependent fluorescence \((\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}]{\mbox{-}}{{{\rm{G(C)}}}}})\) and cortical hemodynamic changes were recorded at baseline (10 mins) and after clozapine (30 mins) in Experiment 4 (n = 3).
Image and data processing
For each experiment, five data sets were obtained from each mouse cortex: (1) [Ca2+] dependent fluorescence intensity change from astrocytes \((\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(A)}}}}]})\) or neurons \((\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(N)}}}}]})\), which reflects astrocytic or neural activation, (2) changes in vessel diameter; (3) changes in total blood volume, ΔHbT within arteries and veins, (4) changes in oxygenated-hemoglobin ΔHbO2, and (5) deoxygenated hemoglobin ΔHbR to assess tissue oxygenation status in response to cocaine (1 mg/kg, i.v.). Data from different animals in each group were averaged and the amplitude of each signal and duration of responses to cocaine were quantified. We also compared these parameters between the astrocytic and neuronal expressed animal groups.
All multi-channel (i.e., multi-wavelength) images were acquired using the time-sharing strategy by multimodal imaging platform which were then regrouped to time-lapse image sets for each channel. For GCaMP6f-Ca2+ fluorescence imaging, five regions of interest (ROIs, pink dots as illustrated in Fig. 4a, b) were selected within the GCaMP6f-expressing cortical areas devoid of visible blood vessels to track the temporal changes of astrocytic or neuronal fluorescence induced by cocaine. To control for absorption differences due to hemodynamic changes, such as in HbT, values in regions expressing GCaMP6f were divided by the region of the cortex, where there was no expression (white triangle illustrated in Fig. 4a, b). The neuronal or astrocytic Ca fluorescent activity was quantified as ΔF/F (\(\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(N)}}}}]}\), \(\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(A)}}}}]}\), respectively). The relative changes of fluorescence signal over its baseline (before cocaine), ΔF/F(Δ[Ca2+])=[F(t)-Fbaseline]/Fbaseline) × 100]%were calculated for each animal to eliminate effects of variations in GCaMP6f expression on fluorescence intensity changes in response to cocaine.
To access cocaine-induced vasoconstriction and examine whether astrocytic Ca inhibition using GFAP-DREADD (Gi) (activated by clozapine before cocaine administration) would reduce the vasoconstriction effects of cocaine on cerebral vessels, the vessel diameters were quantified using a custom Matlab program. Specifically, multiple ROIs were selected across the blood vessels in different vessel types (e.g., veins and arteries to track vessel diameters starting from 10 min baseline period to 60 min post cocaine). The vessel diameter was estimated using images obtained from λ1 = 568 nm with a Gaussian process approach. The detail algorithm of computation has been published by Asl et al.,65. Briefly, it places x and y coordinates of a seed point at the center of a vessel at the selected location of the ROI. The centerline and direction of the vessel are then computed using the Radon transform, and train MATLAB to start tracking from the input seed point to the next 10 points (ϕi, i = 1–10, within ~60 μm) lying in the center of the blood vessel. The diameter ϕi at each point is then estimated using a separate Gaussian process. The mean volume of ϕi (i = 1–10) was calculated as the vessel diameters at the location selected ROIs. To track time course of vessel diameter changes, a while-loop was added to read from the first image to the last one (e.g., m = 4200 frames or based on our imaging time for each experiment above). As a result, there were 10*m data points computed to present the blood vessel change along the time course of imaging, which represents the vessel diameter changes as a function of time at the location of selected ROI. In other words, the vessel diameter at each ROI was continually tracked in each frame and its change was quantified over its baseline (before cocaine) as a function of time following cocaine injection.
The hemodynamic responses of ΔHbO2 and ΔHbR were calculated from the acquired λ1 = 568 nm and λ2 = 630 nm images. To minimize the artifacts induced by the hemodynamic changes due to cocaine infusion, vehicle animals were used in Experiments1–2 in Table 1. The mean hemodynamic changes in response to saline (0.1 ml, i.v., Experiments 1a, 2a) were used to correct the temporal absorbance changes due to cocaine injection (e.g., Experiments 1b, 2b). Supplementary Figure 6 illustrates the correction procedures for one of the animals. The correction was conducted in the raw HbO2 (568 nm) and HbR (630 nm) channels for each animal. Meanwhile, Supplementary Fig. 7 shows the comparison of time courses of ΔHbT before correction (Supplementary Fig. 7a, c) and after correction (Supplementary Fig. 7b, d), it indicates the infusion correction slightly reduces ΔHbT changes within injection period (t < 2 mins). After correction, ΔHbO2 and ΔHbR were calculated based on the following equation:
where ελHbO2, ελHbR are the molar extinction coefficients for HbO2 and HbR, and Rλ1(t), Rλ2(t) are the measured diffuse reflectances at these wavelengths. Rλ1(0), Rλ2(0) are their baseline values before cocaine. Lλ1(t), Lλ2(t) are their pathlengths66,67. The total hemoglobin concentration change can be obtained by
which is also called the cerebral blood volume change within the cortex62.
Nine ROIs were selected from arteries, veins and tissue (three from each component as illustrated in Fig. 2b) to capture the hemodynamic responses to cocaine (ΔHbO2, ΔHbR, ΔHbT) from vascular and tissue compartments.
Immunohistochemistry and Ex-vivo Imaging
After in vivo imaging, the mouse was transcardially perfused with 0.1 M phosphate-buffered saline (PBS), then fixated with 4% paraformaldehyde (PFA) in 0.1 M PBS overnight. The cryoprotected brain was immersed in 30% sucrose solution and sectioned to 50 µm thick slices. For immunostaining, sectioned brain tissues were treated with the primary chicken anti-GFP antibody (1:200, Thermofischer) to enhance GCaMP6f fluorescence followed by specific secondary antibody 488 anti-chicken (1:200, Jackson Immunoresearch). After that, for GCaMP6f expressed in astrocytes brain slices, rabbit anti-GFAP (1:200, Millpore) was used meanwhile for GCaMP6f expressed in neurons samples, mouse anti-Neun (1:200, Millpore) was used. Both primary antibodies followed by their respective secondary antibodies (i.e., 594 anti-rabbit or 594 anti-mouse to visualize the location of astrocytes or neurons). Ex vivo Images were acquired with a confocal microscope.
Statistics and reproducibility
All data are presented as means ± SEM. Comparisons between two groups (e.g., astrocytic GCaMP6f-expressed group -Group A vs. neuronal GCaMP6f-expressed group -Group N) were analyzed using an unpaired t-test. Comparisons between multiple groups (>2 groups) were analyzed using One Way ANOVA. A P-value <0.05 was considered statistically significant for all cases. For cross-correlation between two temporal traces (e.g., (\(\Delta {F/F}_{[{{{\rm{Ca}}}^{2+}}{\mbox{-}}{{{\rm{G(N)}}}}]}\) vs ΔHbO2-G(N)), the Pearson correlation was calculated. A P-value <0.05 was considered significant. Sample size in each experiment are indicated in each figure legend and summarized in Table 1.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
References
Council, N. R. Understanding and Preventing Violence, Volume 3: Social Influences. (The National Academies Press, 1994).
Buttner, A., Mall, G., Penning, R., Sachs, H. & Weis, S. The neuropathology of cocaine abuse. Leg. Med. 5(Suppl 1), S240–S242 (2003).
Bartzokis, G. et al. Cortical gray matter volumes are associated with subjective responses to cocaine infusion. Am. J. Addiction 13, 64–73 (2004).
Bolouri, M. R. & Small, G. A. Neuroimaging of hypoxia and cocaine-induced hippocampal stroke. J. Neuroimaging 14, 290–291 (2004).
Volkow, N. D., Mullani, N., Gould, K. L., Adler, S. & Krajewski, K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br. J. Psychiatry 152, 641–648 (1988).
Bell, K. M., Milne, N. & Lyons, K. P. Regional cerebral blood-flow and cocaine abuse. West. J. Med. 161, 412–413 (1994).
Chen, W., Liu, P., Volkow, N. D., Pan, Y. & Du, C. Cocaine attenuates blood flow but not neuronal responses to stimulation while preserving neurovascular coupling for resting brain activity. Mol. Psychiatry 21, 1408–1416 (2016).
Du, C. et al. Cocaine-induced ischemia in prefrontal cortex is associated with escalation of cocaine intake in rodents. Mol. Psychiatry 25, 1759–1776 (2020).
Zhang, A. M., Cheng, T. P. O., Altura, B. T. & Altura, B. M. Acute cocaine results in rapid rises in intracellular free calcium concentration in canine cerebral vascular smooth muscle cells: Possible relation to etiology of stroke. Neurosci. Lett. 215, 57–59 (1996).
Kaufman, M. J. et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA 279, 376–380 (1998).
Zhang, Q. J. et al. Chronic cocaine disrupts neurovascular networks and cerebral function: optical imaging studies in rodents. J. Biomed. Opt. https://doi.org/10.1117/1.Jbo.21.2.026006 (2016).
Volkow, N. D., Ding, Y. S., Fowler, J. S. & Wang, G. J. Cocaine addiction: hypothesis derived from imaging studies with PET. J. Addict. Dis. 15, 55–71 (1996).
Sharma, H. S., Muresanu, D., Sharma, A. & Patnaik, R. Cocaine-Induced Breakdown of the Blood-Brain Barrier and Neurotoxicity. Int. Rev. Neurobiol. 88, 297-+ (2009).
Lammel, S., Ion, D. I., Roeper, J. & Malenka, R. C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70, 855–862 (2011).
Girouard, H. et al. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl Acad. Sci. USA 107, 3811–3816 (2010).
Malaplate-Armand, C. et al. Down-regulation of astroglial CYP2C, glucocorticoid receptor and constitutive androstane receptor genes in response to cocaine in human U373 MG astrocytoma cells. Toxicol. Lett. 159, 203–211 (2005).
Mulligan, S. J. & MacVicar, B. A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004).
Han, K. et al. Neurovascular coupling under chronic stress is modified by altered GABAergic interneuron activity. J. Neurosci. 39, 10081–10095 (2019).
Daneman, R. & Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015).
Vecino, E., Rodriguez, F. D., Ruzafa, N., Pereiro, X. & Sharma, S. C. Glia-neuron interactions in the mammalian retina. Prog. Retinal Eye Res. 51, 1–40 (2016).
Eleftherios, Z. et al. Architecture of the Neuro-Glia-Vascular System. bioRxiv https://doi.org/10.1101/2021.01.19.427241 (2021).
Abbott, N. J., Ronnback, L. & Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Attwell, D. et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010).
Gordon, G. R., Howarth, C. & MacVicar, B. A. Bidirectional control of arteriole diameter by astrocytes. Exp. Physiol. 96, 393–399 (2011).
Liu, C. Y., Yang, Y., Ju, W. N., Wang, X. & Zhang, H. L. Emerging roles of astrocytes in neuro-vascular unit and the tripartite synapse with emphasis on reactive gliosis in the context of Alzheimer’s disease. Front. Cell. Neurosci. 12, 193 (2018).
Kubotera, H. et al. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep. 9, 1263 (2019).
Fattore, L. et al. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience 110, 1–6 (2002).
Kimelberg, H. K. & Norenberg, M. D. Astrocytes. Sci. Am. 260, 66–72 (1989). 74, 76.
Schulz, K. et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods 9, 597–602 (2012).
Wang, X. H. et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 9, 816–823 (2006).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267 (2006).
Badisa, R. B. & Goodman, C. B. Effects of chronic cocaine in rat C6 astroglial cells. Int. J. Mol. Med. 30, 687–692 (2012).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295 (2013).
Haustein, M. D. et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429 (2014).
Gu, X. et al. Synchronized astrocytic Ca(2+) responses in neurovascular coupling during somatosensory stimulation and for the resting state. Cell Rep. 23, 3878–3890 (2018).
Gu, X. et al. Long-term optical imaging of neurovascular coupling in mouse cortex using GCaMP6f and intrinsic hemodynamic signals. NeuroImage 165, 251–264 (2018).
Chen, W., Park, K., Volkow, N. D., Pan, Y. T. & Du, C. W. Cocaine-induced abnormal cerebral hemodynamic responses to forepaw stimulation assessed by integrated multi-wavelength spectroimaging and laser speckle contrast imaging. IEEE J. Sel. Top. Quant. https://doi.org/10.1109/Jstqe.2015.2503319 (2016).
Rapoport, R. M., Yoon, S. & Zuccarello, M. Cocaine constrictor mechanisms of the cerebral vasculature. J. Cardiovascular Pharmacol. 67, 442–450 (2016).
Nanda, A., Vannemreddy, P., Willis, B. & Kelley, R. Stroke in the young: relationship of active cocaine use with stroke mechanism and outcome. Acta Neurochir. Suppl. 96, 91-+ (2006).
George, O., Mandyam, C. D., Wee, S. & Koob, G. F. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacol 33, 2474–2482 (2008).
Roy, U. et al. DJ1 expression downregulates in neuroblastoma cells (SK-N-MC) chronically exposed to HIV-1 and cocaine. Front. Microbiol. 6, 749 (2015).
He, G. Q., Zhang, A., Altura, B. T. & Altura, B. M. Cocaine-induced cerebrovasospasm and its possible mechanism of action. J. Pharmacol. Exp. Ther. 268, 1532–1539 (1994).
Reissner, K. J. & Pletnikov, M. V. Contributions of nonneuronal brain cells in substance use disorders. Neuropsychopharmacology 45, 224–225 (2020).
Addy, N. A. et al. The L-type calcium channel blocker, isradipine, attenuates cue-induced cocaine-seeking by enhancing dopaminergic activity in the ventral tegmental area to nucleus accumbens pathway. Neuropsychopharmacology 43, 2361–2372 (2018).
Jennings, A. et al. Dopamine elevates and lowers astroglial Ca2+ through distinct pathways depending on local synaptic circuitry. Glia 65, 447–459 (2017).
Wang, W., Shen, J., Lu, X., Hoi, S. C. H. & Ling, H. Paying attention to video object segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 43, 2413–2428 (2021).
Verkhratsky, A. & Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 369, 20130595 (2014).
Wang, J. S. et al. Astrocytes in cocaine addiction and beyond. Mol. psychiatry 27, 652–668 (2022).
Du, C. & Park, K. Ca(2+) channel blockade reduces cocaine’s vasoconstriction and neurotoxicity in the prefrontal cortex. Transl. Psychiatry 11, 459 (2021).
Kosten, T. R. Pharmacotherapy of cerebral ischemia in cocaine dependence. Drug Alcohol Depend 49, 133–144 (1998).
D’Souza, M. S. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 9, 404 (2015).
Chen, W., Volkow, N. D., Li, J., Pan, Y. T. & Du, C. W. Cocaine decreases spontaneous neuronal activity and increases low-frequency neuronal and hemodynamic cortical oscillations. Cereb. Cortex 29, 1594–1606 (2019).
Ren, H. et al. Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol. Psychiatry 17, 1017–1025 (2012).
Lu, H. et al. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI). Proc. Natl Acad. Sci. USA 104, 2489–2494 (2007).
Du, C. W. et al. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J. Neurosci. 26, 11522–11531 (2006).
Nicolas, C. et al. Longitudinal changes in brain metabolic activity after withdrawal from escalation of cocaine self-administration. Neuropsychopharmacology 42, 1981–1990 (2017).
Hwang, S. N., Lee, J. S., Seo, K. & Lee, H. Astrocytic regulation of neural circuits underlying behaviors. Cells-Basel https://doi.org/10.3390/Cells10020296 (2021).
Filosa, J. A., Morrison, H. W., Iddings, J. A., Du, W. & Kim, K. J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 323, 96–109 (2016).
Wang, J. S. et al. Astrocytes in cocaine addiction and beyond. Mol. Psychiatry https://doi.org/10.1038/s41380-021-01080-7 (2021).
Wang, J. S. et al. Cocaine triggers astrocyte-mediated synaptogenesis. Biol. Psychiat 89, 386–397 (2021).
Park, K., Volkow, N. D., Pan, Y. & Du, C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J. Neurosci. 33, 15827–15836 (2013).
Yuan, Z., Luo, Z., Volkow, N. D., Pan, Y. & Du, C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. NeuroImage 54, 1130–1139 (2011).
Allen, C. P. et al. Enhanced neuronal and blunted hemodynamic reactivity to cocaine in the prefrontal cortex following extended cocaine access: optical imaging study in anesthetized rats. Addict. Biol. 24, 485–497 (2019).
Du, C., Volkow, N. D., Koretsky, A. P. & Pan, Y. Low-frequency calcium oscillations accompany deoxyhemoglobin oscillations in rat somatosensory cortex. Proc. Natl Acad. Sci. USA 111, E4677–E4686 (2014).
Asl, M. E., Koohbanani, N. A., Frangi, A. F. & Gooya, A. Tracking and diameter estimation of retinal vessels using Gaussian process and Radon transform. J. Med. Imaging 4, 034006 (2017).
Dunn, A. K., Devor, A., Dale, A. M. & Boas, D. A. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. NeuroImage 27, 279–290 (2005).
Yuan, S., Devor, A., Boas, D. A. & Dunn, A. K. Determination of optimal exposure time for imaging of blood flow changes with laser speckle contrast imaging. Appl. Opt. 44, 1823–1830 (2005).
Acknowledgements
This work was supported in part by National Institutes of Health (NIH) grants 2R01 DA029718 (C.D. and Y.P.), RF1DA048808 (Y.P. and C.D.), R21DA042597 (C.D. and Y.P.) and NIH’s Intramural Program of NIAAA (N.D.V.). The authors would like to thank Y.Q. Yan for partially assisting KP on data process and NIDA’s Drug Supply Program for providing cocaine used in this study.
Author information
Authors and Affiliations
Contributions
C.D., N.D.V., and Y.P. designed the research; Y.L., Y.H., and K.P. carried out the in vivo experiments and conducted image processing and analysis. Y.L., C.D. N.V.D., and Y.P. contributed to data interpretation, result discussions, and manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Jianbo Tang, Christian Lohr and Cam Ha Tran for their contribution to the peer review of this work. Primary Handling Editors: Chao Zhou and George Inglis. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Hua, Y., Park, K. et al. Cocaine’s cerebrovascular vasoconstriction is associated with astrocytic Ca2+ increase in mice. Commun Biol 5, 936 (2022). https://doi.org/10.1038/s42003-022-03877-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-03877-w
This article is cited by
-
Voltage-gated potassium channels control extended access cocaine seeking: a role for nucleus accumbens astrocytes
Neuropsychopharmacology (2024)
-
Astrocytes modulate cerebral blood flow and neuronal response to cocaine in prefrontal cortex
Molecular Psychiatry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.