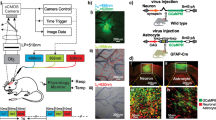
Abstract
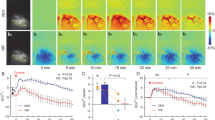
Cocaine affects both cerebral blood vessels and neuronal activity in brain. Cocaine can also disrupt astrocytes, which modulate neurovascular coupling—a process that regulates cerebral hemodynamics in response to neuronal activation. However, separating neuronal and astrocytic effects from cocaine’s direct vasoactive effects has been challenging, partially due to limitations of neuroimaging techniques able to differentiate vascular from neuronal and glial effects at high temporal and spatial resolutions. Here, we used a newly-developed multi-channel fluorescence and optical coherence Doppler microscope (fl-ODM) that allows for simultaneous measurements of neuronal and astrocytic activities (reflected by the intracellular calcium changes in neurons Ca2+N and astrocytes Ca2+A, respectively) alongside their vascular interactions in vivo to address this challenge. Using green and red genetically-encoded Ca2+ indicators differentially expressed in astrocytes and neurons, fl-ODM enabled concomitant imaging of large-scale astrocytic and neuronal Ca2+ fluorescence and 3D cerebral blood flow velocity (CBFv) in vascular networks in the mouse cortex. We assessed cocaine’s effects in the prefrontal cortex (PFC) and found that the CBFv changes triggered by cocaine were temporally correlated with astrocytic Ca2+A activity. Chemogenetic inhibition of astrocytes during the baseline state resulted in blood vessel dilation and CBFv increases but did not affect neuronal activity, suggesting modulation of spontaneous blood vessel’s vascular tone by astrocytes. Chemogenetic inhibition of astrocytes during a cocaine challenge prevented its vasoconstricting effects alongside the CBFv decreases, but it also attenuated the neuronal Ca2+N increases triggered by cocaine. These results document a role of astrocytes both in regulating vascular tone and consequently blood flow, at baseline and for modulating the vasoconstricting and neuronal activation responses to cocaine in the PFC. Strategies to inhibit astrocytic activity could offer promise for ameliorating vascular and neuronal toxicity from cocaine misuse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Correspondence and requests for additional materials should be addressed to Yingtian Pan.
References
Liu Y, Hua Y, Park K, Volkow ND, Pan Y, Du C. Cocaine’s cerebrovascular vasoconstriction is associated with astrocytic Ca(2+) increase in mice. Commun Biol. 2022;5:936. https://doi.org/10.1038/s42003-022-03877-w.
Wang J, Holt LM, Huang HH, Sesack SR, Nestler EJ, Dong Y. Astrocytes in cocaine addiction and beyond. Mol Psychiatry. 2022;27:652–68. https://doi.org/10.1038/s41380-021-01080-7.
Weis S, Buttner A. Neurotoxicology and drug-related disorders. Handb Clin Neurol. 2017;145:181–92. https://doi.org/10.1016/B978-0-12-802395-2.00014-6.
Mishra A. Binaural blood flow control by astrocytes: listening to synapses and the vasculature. J Physiol. 2017;595:1885–902. https://doi.org/10.1113/JP270979.
Allen NJ, Barres BA. Neuroscience: Glia—more than just brain glue. Nature. 2009;457:675–7. https://doi.org/10.1038/457675a.
Buttner A. Review: the neuropathology of drug abuse. Neuropathol Appl Neurobiol. 2011;37:118–34. https://doi.org/10.1111/j.1365-2990.2010.01131.x.
Niciu MJ, Henter ID, Sanacora G, Zarate CA Jr. Glial abnormalities in substance use disorders and depression: does shared glutamatergic dysfunction contribute to comorbidity? World J Biol Psychiatry. 2014;15:2–16. https://doi.org/10.3109/15622975.2013.829585.
Toth AB, Hori K, Novakovic MM, Bernstein NG, Lambot L, Prakriya M. CRAC channels regulate astrocyte Ca(2+) signaling and gliotransmitter release to modulate hippocampal GABAergic transmission. Sci Signal. 2019;12. https://doi.org/10.1126/scisignal.aaw5450.
Accorsi-Mendonca D, Almado CE, Bonagamba LG, Castania JA, Moraes DJ, Machado BH. Enhanced firing in NTS induced by short-term sustained hypoxia is modulated by Glia-neuron interaction. J Neurosci. 2015;35:6903–17. https://doi.org/10.1523/JNEUROSCI.4598-14.2015.
Bray JG, Reyes KC, Roberts AJ, Gruol DL. Altered hippocampal synaptic function in transgenic mice with increased astrocyte expression of CCL2 after withdrawal from chronic alcohol. Neuropharmacology. 2018;135:113–25. https://doi.org/10.1016/j.neuropharm.2018.02.031.
Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–31. https://doi.org/10.1152/physrev.00049.2005.
Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 2011;31:2607–14. https://doi.org/10.1523/JNEUROSCI.5319-10.2011.
Nimmerjahn A, Bergles DE. Large-scale recording of astrocyte activity. Curr Opin Neurobiol. 2015;32:95–106. https://doi.org/10.1016/j.conb.2015.01.015.
Gu X, Chen W, You J, Koretsky AP, Volkow ND, Pan Y, et al. Long-term optical imaging of neurovascular coupling in mouse cortex using GCaMP6f and intrinsic hemodynamic signals. Neuroimage. 2018;165:251–64. https://doi.org/10.1016/j.neuroimage.2017.09.055.
Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman, JP, et al. Sensitive red protein calcium indicators for imaging neural activity. Elife. 2016;5. https://doi.org/10.7554/eLife.12727.
Dana H, Novak O, Guardado-Montesino M, Fransen JW, Hu A, Borghuis BG, et al. Thy1 transgenic mice expressing the red fluorescent calcium indicator jRGECO1a for neuronal population imaging in vivo. PLoS One. 2018;13:e0205444. https://doi.org/10.1371/journal.pone.0205444.
Park K, Liyanage AC, Koretsky AP, Pan Y, Du C. Optical imaging of stimulation-evoked cortical activity using GCaMP6f and jRGECO1a. Quant Imaging Med Surg. 2021;11:998–1009. https://doi.org/10.21037/qims-20-921.
Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express. 2007;15:4083–97. https://doi.org/10.1364/oe.15.004083.
Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15:1219–23. https://doi.org/10.1038/nm.1971.
Srinivasan VJ, Jiang JY, Yaseen MA, Radhakrishnan H, Wu W, Barry S, et al. Rapid volumetric angiography of cortical microvasculature with optical coherence tomography. Opt Lett. 2010;35:43–45. https://doi.org/10.1364/OL.35.000043.
Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21:1361–7. https://doi.org/10.1038/nbt892.
Yuan Z, Luo Z, Volkow ND, Pan Y, Du C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. Neuroimage. 2011;54:1130–9. https://doi.org/10.1016/j.neuroimage.2010.08.045.
Ren H, Du C, Yuan Z, Park K, Volkow ND, Pan Y. Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol Psychiatry. 2012;17:1017–25. https://doi.org/10.1038/mp.2011.160.
You J, Li A, Du C, Pan Y. Volumetric Doppler angle correction for ultrahigh-resolution optical coherence Doppler tomography. Appl Phys Lett. 2017;110:011102. https://doi.org/10.1063/1.4973367.
Fellin T, D’Ascenzo M, Haydon PG. Astrocytes control neuronal excitability in the nucleus accumbens. ScientificWorldJournal. 2007;7:89–97. https://doi.org/10.1100/tsw.2007.195.
Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–8. https://doi.org/10.1192/bjp.152.5.641.
Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–7. https://doi.org/10.1126/science.aan2475.
Roalf DR, Gur RC. Functional brain imaging in neuropsychology over the past 25 years. Neuropsychology. 2017;31:954–71. https://doi.org/10.1037/neu0000426.
Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78. https://doi.org/10.1038/nature06976.
Morone KA, Neimat JS, Roe AW, Friedman RM. Review of functional and clinical relevance of intrinsic signal optical imaging in human brain mapping. Neurophotonics. 2017;4:031220. https://doi.org/10.1117/1.NPh.4.3.031220.
Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–6. https://doi.org/10.1073/pnas.0914722107.
Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–70. https://doi.org/10.1523/JNEUROSCI.4048-05.2006.
Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–9. https://doi.org/10.1038/nature02827.
Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–7. https://doi.org/10.1038/nn1623.
Farhy-Tselnicker I, Allen NJ. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13:7. https://doi.org/10.1186/s13064-018-0104-y.
Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev. 2018;98:239–389. https://doi.org/10.1152/physrev.00042.2016.
Xu T, Pandey SC. Cellular localization of serotonin(2A) (5HT(2A)) receptors in the rat brain. Brain Res Bull. 2000;51:499–505. https://doi.org/10.1016/s0361-9230(99)00278-6.
Zhang X, Song D, Gu L, Ren Y, Verkhratsky A, Peng L. Decrease of gene expression of astrocytic 5-HT2B receptors parallels development of depressive phenotype in a mouse model of Parkinson’s disease. Front Cell Neurosci. 2015;9:388. https://doi.org/10.3389/fncel.2015.00388.
Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, et al. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell. 2019;177:1280–92.e1220. https://doi.org/10.1016/j.cell.2019.03.019.
Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int. 2010;57:411–20. https://doi.org/10.1016/j.neuint.2010.03.019.
Horvat A, Vardjan N. Astroglial cAMP signalling in space and time. Neurosci Lett. 2019;689:5–10. https://doi.org/10.1016/j.neulet.2018.06.025.
Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004;1029:120–3. https://doi.org/10.1016/j.brainres.2004.09.014.
Qiu J, Yan Z, Tao K, Li Y, Li Y, Li J, et al. Sinomenine activates astrocytic dopamine D2 receptors and alleviates neuroinflammatory injury via the CRYAB/STAT3 pathway after ischemic stroke in mice. J Neuroinflamm. 2016;13:263. https://doi.org/10.1186/s12974-016-0739-8.
Jones ME, Paniccia JE, Lebonville CL, Reissner KJ, Lysle DT. Chemogenetic manipulation of dorsal hippocampal astrocytes protects against the development of stress-enhanced fear learning. Neuroscience. 2018;388:45–56. https://doi.org/10.1016/j.neuroscience.2018.07.015.
Oe Y, Wang X, Patriarchi T, Konno A, Ozawa K, Yahagi K, et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat Commun. 2020;11:471. https://doi.org/10.1038/s41467-020-14378-x.
Eriksson PS, Hansson E, Ronnback L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain–coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–9. https://doi.org/10.1016/0028-3908(91)90170-g.
Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, et al. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex. 2014;24:2784–95. https://doi.org/10.1093/cercor/bht136.
Peakman MC, Hill SJ. Adenosine A1 receptor-mediated inhibition of cyclic AMP accumulation in type-2 but not type-1 rat astrocytes. Eur J Pharm. 1996;306:281–9. https://doi.org/10.1016/0014-2999(96)00202-6.
Woods MD, Freshney RI, Ball SG, Vaughan PF. Regulation of cyclic AMP formation in cultures of human foetal astrocytes by beta 2-adrenergic and adenosine receptors. J Neurochem. 1989;53:864–9. https://doi.org/10.1111/j.1471-4159.1989.tb11784.x.
Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, et al. alpha1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium. 2013;54:387–94. https://doi.org/10.1016/j.ceca.2013.09.001.
Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15:5535–50. https://doi.org/10.1523/JNEUROSCI.15-08-05535.1995.
Shao Y, McCarthy KD. Receptor-mediated calcium signals in astroglia: multiple receptors, common stores and all-or-nothing responses. Cell Calcium. 1995;17:187–96. https://doi.org/10.1016/0143-4160(95)90033-0.
Gould T, Chen L, Emri Z, Pirttimaki T, Errington AC, Crunelli V, et al. GABA(B) receptor-mediated activation of astrocytes by gamma-hydroxybutyric acid. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130607. https://doi.org/10.1098/rstb.2013.0607.
Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56:1127–37. https://doi.org/10.1002/glia.20684.
Nam MH, Han KS, Lee J, Won W, Koh W, Bae JY, et al. Activation of astrocytic Mu-opioid receptor causes conditioned place preference. Cell Rep. 2019;28:1154–66. https://doi.org/10.1016/j.celrep.2019.06.071.
Andersson M, Blomstrand F, Hanse E. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol. 2007;585:843–52. https://doi.org/10.1113/jphysiol.2007.142737.
Mariotti L, Losi G, Sessolo M, Marcon I, Carmignoto G. The inhibitory neurotransmitter GABA evokes long-lasting Ca(2+) oscillations in cortical astrocytes. Glia. 2016;64:363–73. https://doi.org/10.1002/glia.22933.
Perea G, Gomez R, Mederos S, Covelo A, Ballesteros JJ, Schlosser L, et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife. 2016;5. https://doi.org/10.7554/eLife.20362.
Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–82. https://doi.org/10.1523/JNEUROSCI.5255-05.2006.
Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron. 2017;95:531–49.e539. https://doi.org/10.1016/j.neuron.2017.06.029.
Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, Araque A. G(i/o) protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia. 2019;67:1076–93. https://doi.org/10.1002/glia.23589.
Kol A, Adamsky A, Groysman M, Kreisel T, London M, Goshen I. Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat Neurosci. 2020;23:1229–39. https://doi.org/10.1038/s41593-020-0679-6.
Van Den Herrewegen Y, Sanderson TM, Sahu S, De Bundel D, Bortolotto ZA, Smolders I. Side-by-side comparison of the effects of Gq- and Gi-DREADD-mediated astrocyte modulation on intracellular calcium dynamics and synaptic plasticity in the hippocampal CA1. Mol Brain. 2021;14:144. https://doi.org/10.1186/s13041-021-00856-w.
Buttner A, Mall G, Penning R, Sachs H, Weis S. The neuropathology of cocaine abuse. Leg Med (Tokyo). 2003;5:S240–242. https://doi.org/10.1016/s1344-6223(02)00122-0.
He GQ, Zhang A, Altura BT, Altura BM. Cocaine-induced cerebrovasospasm and its possible mechanism of action. J Pharm Exp Ther. 1994;268:1532–9.
Volkow ND, Ding YS, Fowler JS, Wang GJ. Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis. 1996;15:55–71. https://doi.org/10.1300/J069v15n04_04.
Reissner KJ, Pletnikov MV. Contributions of nonneuronal brain cells in substance use disorders. Neuropsychopharmacology. 2020;45:224–5. https://doi.org/10.1038/s41386-019-0494-5.
Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. https://doi.org/10.1038/nn2003.
Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. 2016;323:96–109. https://doi.org/10.1016/j.neuroscience.2015.03.064.
Wang JS, Holt LM, Huang HH, Sesack SR, Nestler EJ, Dong Y. Astrocytes in cocaine addiction and beyond. Mol Psychiatry. 2021. https://doi.org/10.1038/s41380-021-01080-7.
Wang JS, Li KL, Shukla A, Beroun A, Ishikawa M, Huang XJ, et al. Cocaine triggers astrocyte-mediated synaptogenesis. Biol Psychiatry. 2021;89:386–97. https://doi.org/10.1016/j.biopsych.2020.08.012.
London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, et al. Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–74. https://doi.org/10.1001/archpsyc.1990.01810180067010.
Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. https://doi.org/10.1176/appi.ajp.159.10.1642.
Allen CP, Park K, Li A, Volkow ND, Koob GF, Pan Y, et al. Enhanced neuronal and blunted hemodynamic reactivity to cocaine in the prefrontal cortex following extended cocaine access: optical imaging study in anesthetized rats. Addict Biol. 2019;24:485–97. https://doi.org/10.1111/adb.12615.
Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016;19:1619–27. https://doi.org/10.1038/nn.4428.
Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. 1995;52:456–63. https://doi.org/10.1001/archpsyc.1995.03950180042006.
Allain F, Minogianis EA, Roberts DC, Samaha AN. How fast and how often: the pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev. 2015;56:166–79. https://doi.org/10.1016/j.neubiorev.2015.06.012.
Du C, Tully M, Volkow ND, Schiffer WK, Yu M, Luo Z, et al. Differential effects of anesthetics on cocaine’s pharmacokinetic and pharmacodynamic effects in brain. Eur J Neurosci. 2009;30:1565–75. https://doi.org/10.1111/j.1460-9568.2009.06931.x.
Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Bridge P, et al. Cortical gray matter volumes are associated with subjective responses to cocaine infusion. Am J Addict. 2004;13:64–73. https://doi.org/10.1080/10550490490265352.
Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–31. https://doi.org/10.1523/JNEUROSCI.3612-06.2006.
Nicolas C, Tauber C, Lepelletier FX, Chalon S, Belujon P, Galineau L, et al. Longitudinal changes in brain metabolic activity after withdrawal from escalation of cocaine self-administration. Neuropsychopharmacol. 2017;42:1981–90. https://doi.org/10.1038/npp.2017.109.
Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–93. https://doi.org/10.1523/JNEUROSCI.1007-05.2005.
Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharm. 2014;69:1–69. https://doi.org/10.1016/B978-0-12-420118-7.00001-9.
Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–62. https://doi.org/10.1038/nature12024.
Li H, Xie Y, Zhang N, Yu Y, Zhang Q, Ding S. Disruption of IP(3)R2-mediated Ca(2)(+) signaling pathway in astrocytes ameliorates neuronal death and brain damage while reducing behavioral deficits after focal ischemic stroke. Cell Calcium. 2015;58:565–76. https://doi.org/10.1016/j.ceca.2015.09.004.
Du C, Volkow ND, You J, Park K, Allen CP, Koob GF, et al. Cocaine-induced ischemia in prefrontal cortex is associated with escalation of cocaine intake in rodents. Mol Psychiatry. 2020;25:1759–76. https://doi.org/10.1038/s41380-018-0261-8.
Cheli VT, Santiago Gonzalez DA, Smith J, Spreuer V, Murphy GG, Paez PM. L-type voltage-operated calcium channels contribute to astrocyte activation In vitro. Glia. 2016;64:1396–415. https://doi.org/10.1002/glia.23013.
MacVicar BA, Hochman D, Delay MJ, Weiss S. Modulation of intracellular Ca++ in cultured astrocytes by influx through voltage-activated Ca++ channels. Glia. 1991;4:448–55. https://doi.org/10.1002/glia.440040504.
D’Ascenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C. Electrophysiological and molecular evidence of L-(Cav1), N- (Cav2.2), and R- (Cav2.3) type Ca2+ channels in rat cortical astrocytes. Glia. 2004;45:354–63. https://doi.org/10.1002/glia.10336.
Young SZ, Platel JC, Nielsen JV, Jensen NA, Bordey A. GABA(A) increases calcium in subventricular zone astrocyte-like cells through L- and T-type voltage-gated calcium channels. Front Cell Neurosci. 2010;4:8. https://doi.org/10.3389/fncel.2010.00008.
Park K, Chen W, Volkow ND, Allen CP, Pan Y, Du C. Hemodynamic and neuronal responses to cocaine differ in awake versus anesthetized animals: optical brain imaging study. Neuroimage. 2019;188:188–97. https://doi.org/10.1016/j.neuroimage.2018.11.062.
Du C, Park K, Allen CP, Hu XT, Volkow ND, Pan Y. Ca(2+) channel blockade reduces cocaine’s vasoconstriction and neurotoxicity in the prefrontal cortex. Transl Psychiatry. 2021;11:459. https://doi.org/10.1038/s41398-021-01573-7.
Wanat MJ, Bonci A. Dose-dependent changes in the synaptic strength on dopamine neurons and locomotor activity after cocaine exposure. Synapse. 2008;62:790–5. https://doi.org/10.1002/syn.20546.
Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, et al. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl). 1999;146:93–100. https://doi.org/10.1007/s002130051093.
Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, et al. A single amphetamine infusion reverses deficits in dopamine nerve-terminal function caused by a history of cocaine self-administration. Neuropsychopharmacology. 2015;40:1826–36. https://doi.org/10.1038/npp.2015.45.
Schindler CW, Justinova Z, Lafleur D, Woods D, Roschke V, Hallak H, et al. Modification of pharmacokinetic and abuse-related effects of cocaine by human-derived cocaine hydrolase in monkeys. Addict Biol. 2013;18:30–39. https://doi.org/10.1111/j.1369-1600.2011.00424.x.
Yuan Z, Luo ZC, Ren HG, Du CW, Pan Y. A digital frequency ramping method for enhancing Doppler flow imaging in Fourier-domain optical coherence tomography. Opt Express. 2009;17:3951–63. https://doi.org/10.1364/oe.17.003951.
Acknowledgements
This work was supported in part by National Institutes of Health (NIH) grants RF1DA048808 (YP, CD), 2R01 DA029718 (CD, YP), R21 DA057699 (YP, CD) and NIH’s Intramural Program of NIAAA (NDV). The authors would like to thank A. Li for participating in system development, K. Clair for immunostaining and discussion on mCherry control experiment, and also to NIDA’s Drug Supply Program for providing cocaine used in this study.
Author information
Authors and Affiliations
Contributions
CD, NDV, and YP designed the research; KP and YH carried out the in vivo experiments and participated in image processing and data analysis (equal contribution). CD, NDV, and YP contributed to data interpretation, result discussions, and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, C., Park, K., Hua, Y. et al. Astrocytes modulate cerebral blood flow and neuronal response to cocaine in prefrontal cortex. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-023-02373-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02373-9